Abstract
OBJECTIVES
Third-generation ventricular assist devices (VADs) are associated with improved outcomes, though in recent clinical trials bridge-to-transplant (BTT) rates are ∼30% at 6 months, so that transplantation can be used as a ‘bail out’ for serious complications. In the UK, there was a significant reduction in heart transplantation rates over the last decade, so that transplantation from VADs is much less frequent. The objective of this study was to determine outcomes and their predictors in this situation of low BTT rates, and as patients were exposed to long-term support, the incidence and outcomes of VAD thrombosis.
METHODS
We analysed outcomes for 102 consecutive patients between 2009 and 2013 (mean age 47 ± 13; VentrAssist n = 6 and HeartWare n = 96). The median duration of support was 462 ± 426 days.
RESULTS
Survival rates on the device were 75 and 66% at 1 and 2 years, respectively. Older age and more acute INTERMACS groups were significantly related to reduced survival within the first 90 days (P = 0.030 and 0.010, respectively). Poor preoperative right ventricular (RV) function had a negative effect on survival after 1 year (P = 0.009), though not earlier. VAD thrombosis (n = 24 HeartWare and n = 1 VentrAssist) occurred at 0.18 events per patient-year for HeartWare and 0.07 for VentrAssist devices at a median time of onset at 404 ± 281 days. There was no significant effect of VAD thrombosis on survival. Only 14 of 102 patients were transplanted at a median of 334 ± 347 days, and only 3 were transplanted within the first 6 months.
CONCLUSIONS
Third-generation left ventricular assist device implants with a low rate of transplantation have similar survival to destination therapy, and are susceptible to long-term complications of VAD thrombosis and right heart failure.
Keywords: Ventricular assist device, Transplantation, Outcomes, Thrombosis
INTRODUCTION
The number of patients with heart failure that may benefit from transplantation has always been far in excess of that of the actual numbers of transplants performed, but over recent years that discrepancy has further widened. For instance, over the last decade, there has been a steady increase in the numbers of patients listed for transplantation in the USA while the numbers of patients transplanted there remains static [1].
However, in the UK, at least until recently, there was a near 50% decrease in numbers of adult heart transplants [2].
The bridge-to-transplant (BTT) indication for ventricular assist devices (VADs) aims to support patients until a suitable organ can be found. In the USA, there has been a prioritization of stable VAD patients on the transplant list, a concept that arose from earlier generations of VADs when complication rates were higher than with current devices [3]. In the recent US ADVANCE study of the HeartWare VAD, 28.3% of patients were transplanted 6 months after device implantation [4]. However, other countries including the UK do not prioritize stable VAD patients. In this setting, the UK is unique, with a striking fall in numbers of heart transplants and no prioritization of these patients. In the UK, the rate of bridging to transplantation is about 5 and 10% at 6 and 12 months, respectively [5]. The consequence of this low rate of transplantation is that those patients with device-related complications will not have access to timely transplantation, thereby potentially increasing the rate of adverse outcomes including death on the device. This is one explanation why destination therapy (DT) VADs have slightly worse outcomes compared with BTT [6]. The purpose of this study was to review outcomes of third-generation VADs implanted with the ultimate goal of transplantation, though in a setting of low rates of transplantation, and to determine factors that influence short- and long-term outcomes. Complications will accrue over time in VAD patients. Recently, VAD thrombosis has been highlighted as a particular issue that is difficult to treat [7]; we therefore analysed the rate of VAD thromboses in our centre, and its relation to outcomes.
MATERIALS AND METHODS
Patient demographics
In the UK, long-term mechanical circulatory support is only licensed as a BTT, which in practice has also meant that bridge to decision patients could also be implanted with a view to later transplant listing [8]. Patient characteristics are presented in Table 1. In patients with congenital heart disease, 5 of those had transposition of great arteries (2 were congenitally corrected), and 1 had coarctation of the aorta with dysplastic aortic valve and previous surgeries with resulting severe left ventricular (LV) dysfunction. One patient had undergone several procedures for congenital aortic valve and supravalvular stenosis, resulting in severe LV dysfunction.
Table 1:
Patient characteristics
| Total number | 102 |
| Age (years) | 47 ± 13 |
| Gender (n) | |
| Male | 89 |
| Female | 13 |
| Diagnosis (n) | |
| Ischaemic | 40 |
| Nonischaemic | 53 |
| Congenital | 7 |
| Restrictive | 2 |
| INTERMACS class (n) | |
| 1 | 6 |
| 2 | 37 |
| 3 | 25 |
| 4 | 34 |
| Preoperative echocardiogram | |
| LV end-diastolic dimension (cm) | 6.8 ± 1.0 |
| LV end-systolic dimension (cm) | 6.1 ± 1.1 |
| RV size (cm) | 4.6 ± 1.1 |
| RV function grade (1–6)a | 4.2 ± 1.5 |
LV: left ventricular; RV: right ventricular.
aRV function grade: 1, normal; 2, mild; 3, mild–moderate; 4, moderate; 5, moderate–severe; 6, severe dysfunction.
All patients were treated with standard heart failure therapies as previously described [9]. Anticoagulation regimes varied during the time course of the study. For the HeartWare device, initially the target international normalized ratio (INR) was 2–2.5; this was subsequently increased in response to VAD thromboses to 2.5–3.5 and then subsequently changed to a target INR of 2.7. After December 2012 in response to information from HeartWare about higher doses of antiplatelets reducing rates of VAD thrombosis, the standard antiplatelet regime was changed to aspirin 300 mg daily, unless contraindicated. Prior to that we had used antiplatelet doses guided by thrombo-elastrography. Patients who developed a VAD thrombosis would have a second antiplatelet agent such as clopidogrel or prasugrel added to the aspirin. For the VentrAssist, we used a target INR of 2.5–3.5 and antiplatelet agents guided by thrombo-elastrography.
This study adhered to the terms of the United Kingdom Data and Protection Act and Freedom of Information Act, and was approved to obtain confidential information by the local Caldicott Guardian.
Data and statistical analysis
Data are presented as mean ± standard deviation (SD) and duration of days as median. Outcome measures used were all-cause mortality and survival on the device using Kaplan–Meier survival curves, comparing above and below median values for each variable, and the Tarone–Ware test for significance. These analyses were carried out on the following parameters: age, gender, preoperative diagnosis, INTERMACS classification [10] and preoperative echocardiographic measurements of LV and right ventricular (RV) size and RV function. Echocardiograms were performed by experienced sonographers with LV dimensions determined from parasternal long axis views, and RV measurements from the basal four-chamber view. The RV function was categorized on a visual scale 1–6 (1 = normal, 2 = mild, 3 = mild–moderate, 4 = moderate, 5 = moderate–severe, 6 = severe dysfunction). Single unpaired comparisons were made with the Mann–Whitney U-test as data were not normally distributed. Cox regression analysis of parameters that showed significance in the Kaplan–Meier survival curves was performed. P < 0.05 was considered significant. The statistical software used for analyses was SPSS Statistics Version 21.
RESULTS
Outcomes
The study period was from the first third-generation device implanted at this centre in January 2009 to September 2013 consisting of 102 consecutive patients (median follow-up 628 ± 467 days and median duration of support on device 462 ± 426 days). Survival on the device was 75% at 1 year and 66% at 2 years (Fig. 1). In our cohort, 14 (13.7%) of 102 patients were transplanted at a median of 334 ± 347 days. Three (2.9%) were transplanted within the first 6 months. Four patients (3.9%) had the device explanted for myocardial recovery.
Figure 1:
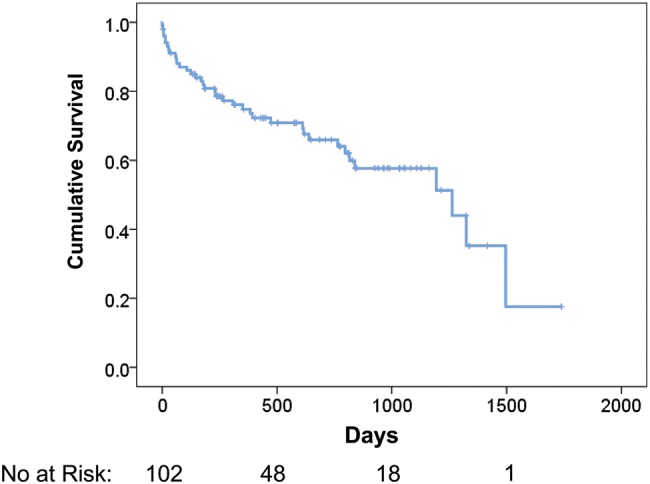
Survival on device with explant for recovery or transplantation censored [95% confidence interval (CI) of mean for survival time: 839–1157 days].
Causes of death of those who died on support were (total 38): 10 intracranial haemorrhage, 6 multiorgan failure, 7 VAD thrombosis, 5 sepsis, 4 right heart failure, 2 cerebral infarction, 1 pulmonary haemorrhage, 1 colon cancer, 1 sudden cardiac death and 1 gastrointestinal haemorrhage. Overall there were 9 thrombotic deaths (VAD thrombosis plus cerebral infarction) and 12 haemorrhagic deaths from all causes of haemorrhage.
At the time of implant, 30 patients were on the transplant waiting list and 38 were listed subsequently. Thirty-four patients were never listed—the reasons for these were: 17 died before they were medically fit to go on the list, 10 were assessed but felt unsuitable for transplantation (compliance, overweight, medical contraindications and patient choice), 6 were not listed as there were signs of LV recovery and 1 patient was awaiting assessment and likely to go on the list thereafter.
During the study period, 5 patients required temporary mechanical support of whom 3 required extracorporeal membrane oxygenator (ECMO) pre-LVAD and 2 were supported with a short-term RV device (CentriMag®) postoperatively.
Predictors of outcomes
Age significantly predicted early outcomes (defined as survival on device, Fig. 2). This included the overall follow-up time (P = 0.046, Fig. 2A), but the effect was primarily related to the first 90 days (all patients were discharged home within 90 days) (P = 0.030, Fig. 2B), and not in those that survived the first 90 days (deaths prior to 90 days censored, P = 0.51, Fig. 2C). A similar phenomenon was seen with the INTERMACS classification, in that more acute INTERMACS classes predicted outcomes (P = 0.046, Fig. 3A), 90-day outcomes (P = 0.010, Fig. 3B), but not outcomes in those that survived 90 days (P = 0.74, Fig. 3C). LV end-diastolic and end-systolic diameters and RV size had no significant effect on overall survival (all P = NS, data not shown). Poor preoperative RV function had a significant effect on survival in those who survived past 1 year (P = 0.009), though not earlier (Fig. 4). RV size had a borderline effect on survival in those who survived past 1 year (P = 0.067, data not shown). No significant effect on outcome was seen with gender, aetiology of heart failure or year of implant (all P = NS, data not shown).
Figure 2:
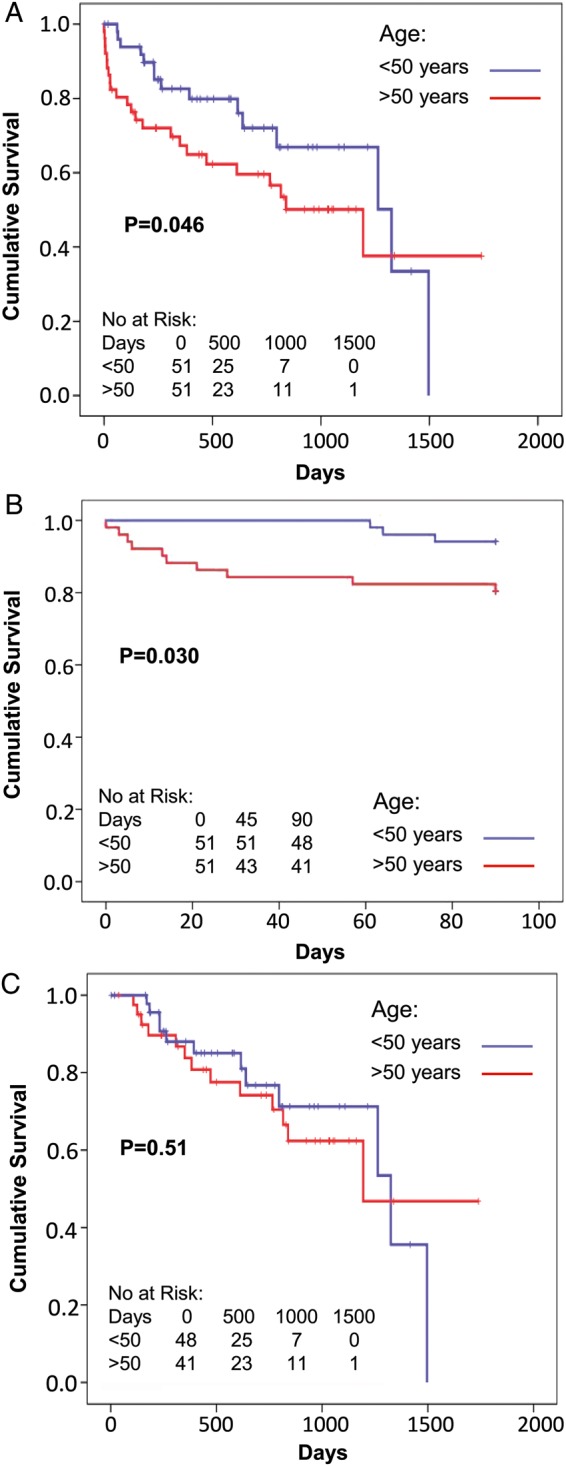
Survival on device of patients older or younger than 50 years. Overall survival (A) (95% CI: ≤50 years: 883–1242, >50 years: 715–1182), early (up to 90 days, B) (95% CI: ≤50 years: 87–90, >50 years: 69–85) and late (after 90 days with deaths before 90 days censored, C) (95% CI: ≤50 years: 951–1304, >50 years: 932–1420). Odds ratios for adverse outcomes for older age were 1.97 for A, 3.90 for B and 1.05 for C.
Figure 3:
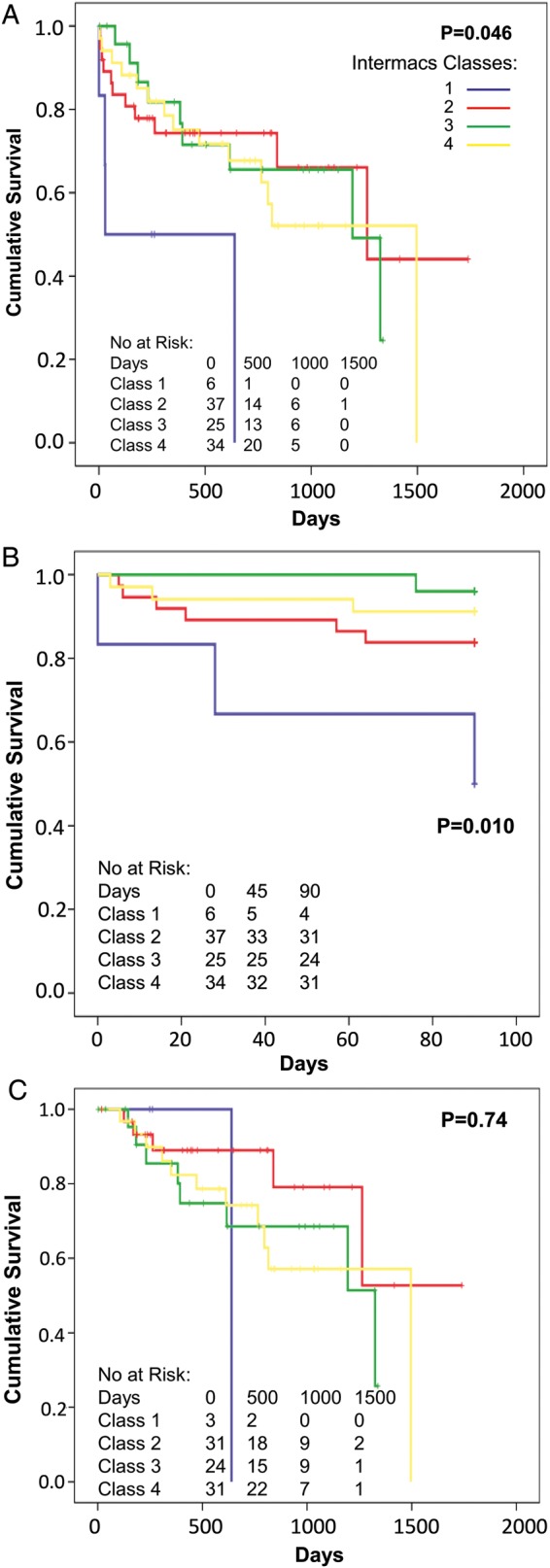
Survival on device of patients grouped into INTERMACS classes 1–4. Overall survival (A) [95% confidence interval (CI): INTERMACS 1: 42–616, INTERMACS 2: 848–1424, INTERMACS 3: 744–1168, INTERMACS 4: 764–1204], early (up to 90 days, B) (95% CI: INTERMACS 1–4: 29–101, 72–88, 88–91, 78–91) and late (after 90 days with deaths before 90 days censored, C) (95% CI: INTERMACS 1–4: 578–1016, 1011–1532, 765–1233, 885–1277).
Figure 4:
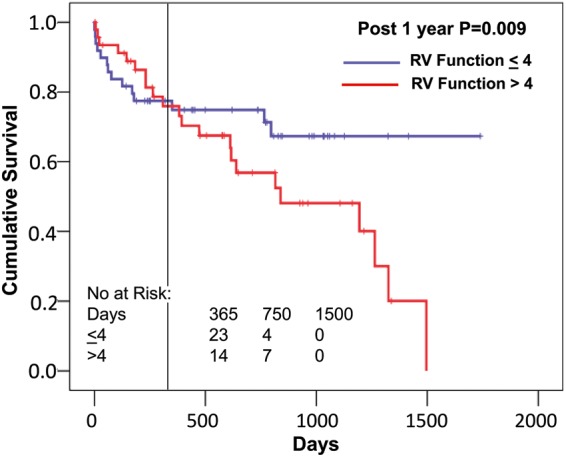
Relationship of preoperative right ventricular (RV) dysfunction to postoperative survival. Groups are divided into those greater than the median of right ventricular function score of 4, and those ≤4 [95% confidence interval (CI) ≤4: 1151–1405, >4: 552–900]. The vertical line indicates 1 year, and after that there is a clear separation of both groups, though not before. Censoring the survival before 1 year results in a significant difference between these groups after 1 year (odds ratio 10.8).
Cox regression analysis was performed with parameters found to be significant with survival curves: age (continuous variable) and INTERMACS class and RV function (both categorical) (Table 2). These parameters are all significantly related to outcomes (survival on device). Of note, the Exp(B) (the predicted change in the hazard for a unit increase in the predictor) for INTERMACS class 1 is particularly high (19.931), indicating a large effect of this parameter on outcomes, though there were only 6 patients in this category. With respect to preoperative RV function and outcomes, as noted above, right heart failure is a relatively rare cause of death in these patients. None of the 4 right heart failure deaths as noted above occurred after 1 year. In the 14 patients who died after 1 year, 12 had Grades 5 and 6 preoperative RV dysfunction. Causes of death in these patients were haemorrhagic cerebrovascular accident (n = 5), postoperative VAD exchange (n = 4), sepsis (n = 2) and gastrointestinal bleeding (n = 1).
Table 2:
Cox regression analysis: The model below includes age (continuous variable), INTERMACS (categorical variable) and right ventricular function (RV, categorical)
| P-value | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|
| Age | 0.001 | 1.079 | 1.031–1.130 |
| INTERMACS (overall) | 0.006 | ||
| INTERMACS (Class 1) | 0.001 | 19.931 | 3.375–117.688 |
| INTERMACS (Class 2) | 0.490 | 1.366 | 0.563–3.315 |
| INTERMACS (Class 3) | 0.526 | 0.743 | 0.296–1.864 |
| RV (overall) | 0.045 | ||
| RV (Grade 1) | 0.063 | 0.210 | 0.041–1.086 |
| RV (Grade 2) | 0.078 | 0.216 | 0.039–1.189 |
| RV (Grade 3) | 0.819 | 0.879 | 0.293–2.640 |
| RV (Grade 4) | 0.006 | 0.153 | 0.040–0.580 |
| RV (Grade 5) | 0.475 | 0.724 | 0.299–1.755 |
P-values, and Exp(B) are displayed. Exp(B) is the predicted change in the hazard for a unit increase in the predictor. For each categorical variable, the final category (INTERMACS 4 and RV function 6) are the reference categories and thus not shown in the table. 95% Confidence intervals (CIs) are shown for Exp(B).
Ventricular assist device thrombosis
A VAD thrombosis was usually diagnosed by an increase in power of the device, with associated increase in circulating markers of haemolysis, muffled quality sound on auscultation of the device and often haematuria. In 1 patient, there was a VAD thrombus seen on the inflow cannula on echocardiography with associated reduction in power, confirmed at the time of device explant for urgent transplantation. There were 25 first VAD thromboses in these patients over the study period (n = 1 VentrAssist, n = 24 HeartWare). Thirteen patients had recurrent events. The cumulative incidence of first VAD thromboses was 33% at 2 years (Fig. 5). The median time to the first VAD thrombus was 404 days (range 67–1261 days). The VAD thrombosis event rate was 0.18 per patient-year for HeartWare and 0.07 for VentrAssist. Those who had a VAD thrombus were supported on the device for longer periods than those who did not have a thrombus (median 685 ± 366 vs 354 ± 431 days, P = 0.007, 95% confidence intervals of the mean 572–875 and 393–589). The VAD thromboses had no significant impact on patient survival on the device or overall survival (P = NS, data not shown).
Figure 5:
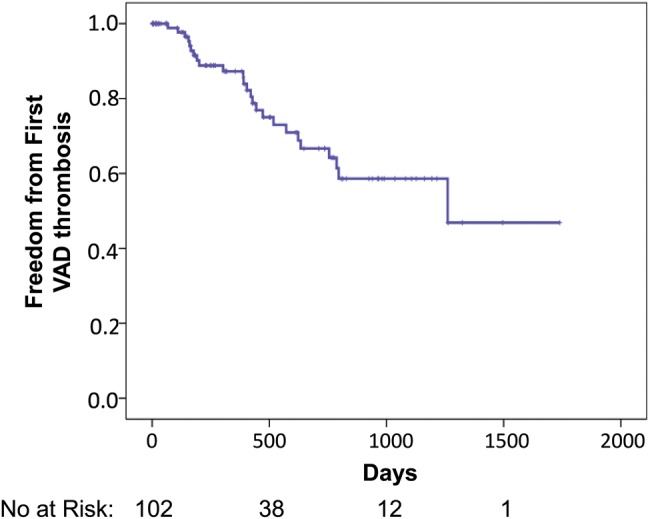
Cumulative freedom from first ventricular assist device (VAD) thrombosis [95% confidence interval (CI): 962–1335 days].
The timeline of the changes in the anticoagulation regime relative to the incidence of new VAD thromboses for HeartWare VADs only (divided into 6 monthly intervals noted as ‘a’ and ‘b’) is shown in Fig. 6. During the study period, there has been a change in the design of the HeartWare VAD inflow cannula in the form of a sintered area on the outer side facing the ventricle cavity to encourage epithelialisation that would reduce thrombus formation. The first of these sintered devices was implanted in September 2011 in this series. This had no significant antithrombotic effect in our cohort (P = NS, data not shown).
Figure 6:
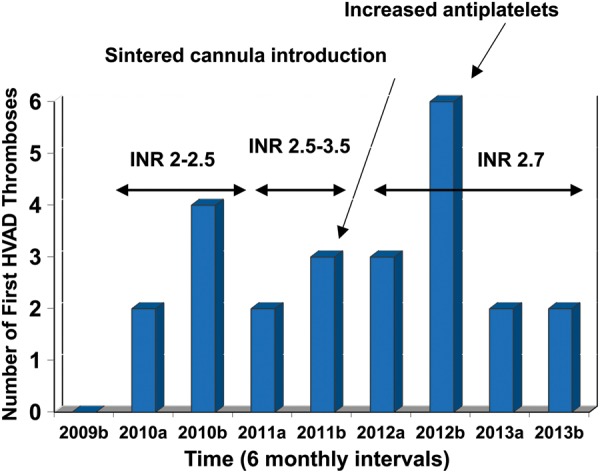
The timeline of HeartWare® VAD (HVAD) thromboses and number of 6 months new HVAD thromboses, with corresponding changes in the anticoagulation and antiplatelet management noted. Each year is divided into the first 6 months (a) and second 6 months (b). After 2012, a standard dose of aspirin 300 mg daily was introduced and subsequent rates of new HVAD thromboses apparently decreased. INR: international normalized ratio.
VAD thromboses were treated in 21 of these 25 patients with intravenous Alteplase 15 mg which by protocol could be repeated on one occasion and, if those two doses did not resolve the thrombus, an infusion of Alteplase 90 mg was given over 90 min. Patients were then treated with intravenous heparin for several days after thrombolysis. Four patients were never given thrombolysis: in 2 this was due to concomitant bleeding, and in 2 this was because there was a satisfactory response to more intensive anticoagulation and antiplatelets (includes the only VentrAssist patient with a VAD thrombus).
Of the 25 patients who had VAD thromboses, 9 were alive on support at the end of the study, 2 died due to the thrombus, 7 had device exchanges, 3 had urgent transplants, 1 had a device explant and 3 died of unrelated causes. In those 7 patients who had device exchanges, 4 died postoperatively, 1 died late after exchange with a recurrent device thrombosis and 2 subsequently had nonurgent transplants.
DISCUSSION
This study provides unique long-term data on BTT with third-generation VADs in patients where access to transplantation has not been readily available, resulting in exposure of these patients to complications of long-term support. Preoperative RV dysfunction is a risk factor for adverse outcomes with long-term support, while older age and INTERMACS class predict early but not late outcomes. VAD thrombosis is relatively common, which is a serious complication that also occurs in association with longer support on the device, though not associated with increased mortality. Overall, there is a balance of thrombotic and haemorrhagic complications, with death rates similarly due to both of these, so that a change in anticoagulation regimes may benefit either bleeding or thrombosis but adversely affect the other. These results reflect our total experience from the first third-generation device implanted in 2009 and, during the course of the study, there have been incremental changes in our management of these patients with changes in anticoagulation regimens and introduction of a comprehensive home-based care system.
Predictors of outcomes
Emin et al. [5] have recently published outcomes in the UK demonstrating improvements in survival over time with newer generation devices, and the results from this study reflect this improved survival. When compared with the INTERMACS registry outcomes, the results are slightly worse than the BTT outcomes (censored at transplantation), but very similar to the DT outcomes, and this difference is likely due to the availability of transplantation [6]. Risk factors for outcomes are also largely similar to those previously published—older age, more acute INTERMACS classification and poor preoperative RV function.
The one discrepancy is that whereas older age and INTERMACS classification are also early hazards in the INTERMACS registry, poor preoperative RV function only predicts poor outcomes after 1 year of support in this study, whereas in INTERMACS it is within 1–2 months. Quantifying RV function is prone to difficulties—particularly in this cohort of patients. We have used a visual assessment of RV function by experienced echocardiographers, while INTERMACS used other measures such as right atrial pressure, ascites or elevated bilirubin levels. Nevertheless, late right heart failure is well recognized [11]. Baumwol et al. have looked at failure to thrive and RV function late after left ventricular assist device implantation [12]. Those patients with failure to thrive over an average of 313 ± 254 days had a significant deterioration in RV function, whereas RV function did not change in the thriving group. Of note, whereas preoperative RV function is a predictor of adverse outcomes in this series, right heart failure is a relatively rare cause of death, suggesting that poor preoperative RV function predisposes to death from other complications.
Ventricular assist device thrombosis
VAD thrombosis rates of 0.18 per patient-year for the HeartWare devices in this series are higher than those recently published from the ADVANCE and Continued Access Protocol Trials (0.08) [13]. However, the study from Najjar et al. is over a significantly shorter time period (median 271 days for those with a VAD thrombosis and 289 days for those without, compared with this study of 462 days). The shorter duration of follow-up will likely underestimate the rate of VAD thrombosis, due to the infrequency of VAD thrombus events in the initial 6 months of support. Indeed, our data show that the median time of the first presentation of this event is greater than 1 year post implant. To illustrate this, when we compare our data restricted to the same time of follow-up with the study by Najjar et al., the VAD thrombosis rate is very similar at 0.09 events per patient-year (to 289 days). In our series, a VAD thrombosis was a marker of long-term support on the device compared with those without VAD thromboses—presumably because they survived the early postoperative hazards. Survival is not significantly different from those who did not have a VAD thrombus, though this does not mean that this is not a serious complication. If these patients who had a VAD thrombosis who had survived the early risks had not had a thrombus, their survival could potentially have been even greater.
Our data show that the rates of VAD thrombosis for the HeartWare are higher than that for the VentrAssist Device. Recently, the incidence of thrombosis of the HeartMate II device has been highlighted [7] with an increase in the rates of VAD thrombosis rates at 120 days from 2.2 to 8.4% from March 2011 to January 2013. This compares with the rate of VAD thrombosis in this series in the HeartWare device at 120 days of 6%. The median time to VAD thrombosis with the HeartMate II device has also decreased from 18.6 to 2.7 months, which compares with 13.5 months in this series for the HeartWare device.
It has to be noted that our VAD thromboses rates reflect the cumulative experience with the HeartWare device and our evolving understanding of an ideal anticoagulation and antiplatelet regimen. Najjar et al. have clearly shown that higher doses of antiplatelets prevent VAD thromboses, and our data from Fig. 6 support that. If our patients were treated with higher doses of antiplatelets throughout the series, rates of VAD thromboses may have been significantly less. Looking at the causes of death, there appears to be a fine balance of haemorrhagic and thrombotic deaths (12 vs 9, respectively) and so higher doses of anticoagulation or antiplatelets may benefit rates of VAD thrombosis though predispose patients to serious bleeding complications.
LIMITATIONS
This is a relatively small, single-centre study, with those inherent limitations despite the unique patient population and relatively long follow-up. The results reflect a cumulative experience over 4 years and, as mentioned above, during this time treatment protocols have changed—particularly with respect to anticoagulation. We have concentrated on device thrombosis and right heart failure as complications of long-term support, though other complications such as stroke and infection are equally important.
CONCLUSIONS
BTT outcomes with third-generation devices in the absence of meaningful rates of transplantation closely match those of published DT outcomes. Reducing complications of long-term support such as VAD thrombosis and right heart failure are necessary to improve overall outcomes.
Funding
Guy A. MacGowan is a recipient of a British Heart Foundation Clinical Leave Research Fellowship (FS/11/89/29162). Funding to pay the Open Access publication charges for this article was provided by the ‘British Heart Foundation’.
Conflict of interest: none declared.
REFERENCES
- 1.Stevenson LW. The urgent priority for transplantation is to trim the waiting list. J Heart Lung Transplant. 2013;32:861–7. doi: 10.1016/j.healun.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 2.MacGowan GA, Parry G, Schueler S, Hasan A. The decline in heart transplantation in the UK. BMJ. 2011;5:342. doi: 10.1136/bmj.d2483. [DOI] [PubMed] [Google Scholar]
- 3.Moazami N, Sun B, Feldman D. Stable patients on left ventricular assist device support have a disproportionate advantage: time to re-evaluate the current UNOS policy. J Heart Lung Transplant. 2011;30:971–4. doi: 10.1016/j.healun.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 5.Emin A, Rogers CA, Parameshwar J, MacGowan G, Taylor R, Yonan N, et al. Steering Group of the UK Cardiothoracic Transplant Audit, UK VAD Forum . Trends in long-term mechanical circulatory support for advanced heart failure in the UK. Eur J Heart Fail. 2013;15:1185–93. doi: 10.1093/eurjhf/hft127. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 8.MacGowan GA, Parry G, Hasan A, Schueler S. Editorial comment: ventricular assist devices for advanced heart failure: evidence that cannot be ignored. Eur J Cardiothorac Surg. 2013;43:1242–3. doi: 10.1093/ejcts/ezs636. [DOI] [PubMed] [Google Scholar]
- 9.McDiarmid A, Gordon B, Wrightson N, Robinson-Smith N, Pillay T, Parry G, et al. Hemodynamic, echocardiographic, and exercise-related effects of the HeartWare left ventricular assist device in advanced heart failure. Congest Heart Fail. 2013;19:11–5. doi: 10.1111/j.1751-7133.2012.00302.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–41. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 11.MacGowan GA, Schueler S. Right heart failure after left ventricular assist device implantation: early and late. Curr Opin Cardiol. 2012;27:296–300. doi: 10.1097/HCO.0b013e3283511e60. [DOI] [PubMed] [Google Scholar]
- 12.Baumwol J, Macdonald PS, Keogh AM, Kotlyar E, Spratt P, Jansz P, et al. Right heart failure and "failure to thrive" after left ventricular assist device: clinical predictors and outcomes. J Heart Lung Transplant. 2011;30:888–95. doi: 10.1016/j.healun.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]


