Abstract
Hyponatremia is associated with an increased risk of mortality on the liver transplant (LTx) waiting list. Though incorporation of the serum sodium into the MELD score may reduce wait list mortality, concerns remain about a potential association between pre-LTx hyponatremia and decreased post-LTx survival. Furthermore, the relationship between pre-LTx hypernatremia and post-LTx survival remains unexplored. The purpose of this study was to investigate the impact of the entire spectrum pre-LTx serum sodium (Na) on post-LTx outcomes. We identified 19,537patients with serum Na that was available immediately before LTx from 2003–20010. Patients were divided into three groups including hyponatremic (Na ≤ 130 mEq/L), normonatremic (Na=131–145mEq/L) and hypernatremic (Na >145 mEq/L) groups and their post-LTx outcomes compared. There was no difference in in-hospital mortality or 90-day survival between patients with hyponatremia and normonatremia. A fraction (2.4%) of patients had hypernatremia, which was associated with increased in-hospital death (11.2% vs. 4.2%, p<0.001) and diminished 90 day survival (86.4% vs. 94.0.%, p<0.001). After adjusting for important clinical variables, the association of preLTx hypernatremia with post-transplant mortality remained significant with a HR=1.13 for each unit increase in sodium >145mEq/L (p<0.001). Length of hospitalization after LTx was significantly longer in hypernatremic patients (p < 0.001). In conclusion, hyponatremia per se does not affect post-LTx survival. Pre-LTx hypernatremia is a highly significant risk factor for post-LTx mortality.
Keywords: liver transplantation, sodium, post-transplant mortality
INTRODUCTION
Hyponatremia is a common yet ominous sign in patients with end stage liver disease (ESLD). Nearly 50% of cirrhotic patients have serum sodium concentrations below the lower limit of the normal range (135–145 mmol/L).(1) Hyponatremia in cirrhosis has been defined as a serum sodium < 130 mEq/L.(2) Hyponatremia in cirrhosis results from antidiuretic hormone-mediated renal retention of free water despite increased total body sodium. Major complications of hepatic decompensation have been associated with hyponatremia including bacterial infections, ascites, renal failure, encephalopathy, and reduced quality of life.(3–6) It is now well established that hyponatremic patients with ESLD have increased mortality independent of other indicators of severity of liver disease, such as the MELD score.(3–10)
Liver transplantation (LTx) not only restores the functional reserve of the liver but also corrects hemodynamic derangements of end stage liver disease, such as splanchnic vasodilation and renal hypoperfusion associated with portal hypertension. To the extent that these factors drive hyponatremia in ESLD patients, successful LTx rapidly restores sodium homeostasis. Although the impact of hyponatremia on waitlist mortality has been studied widely, it remains unclear whether hyponatremia preceding liver transplantation affects post-transplantation outcomes.(11–14) Earlier European studies reported that hyponatremic patients had shorter survival and higher incidence of complications following LTx than those with normonatremia. In our previous work based on a US multicenter database, we found no association between hyponatremia and decreased survival postLTx, whereas certain complications such as central pontine myelinolysis were more common among hyponatremic patients. The importance of this debate has become even more important currently given the potential for US-wide implementation of an organ allocation system incorporating serum sodium.
Hypernatremia is the less common dysnatremia, yet in a very early publication it was associated with high mortality rates.(15) Hypernatremia is also associated with higher mortality in patients with chronic kidney disease or heart failure, patients admitted to the ICU, and geriatric patients admitted to the hospital.(16–19) There is a paucity of data on the relationship between pre-LTx hypernatremia and post-LTx outcomes
In this study, we utilize population-based data in the United States to address (1) whether hyponatremia prior to LTx is associated with diminished post-LTX survival and (2) whether hypernatremia has an impact on post-LTx outcome.
MATERIALS AND METHODS
Data Source
Data were obtained from the Organ Procurement and Transplantation Network for 47,254 patients who underwent liver transplantation (LTx) in the Unites States from 2003 to 2010. All adult patients (≥ 18 year) with chronic liver disease who received their first liver transplant were eligible for inclusion in the study. Reasons for exclusion included age under 18 years (n=4,232), listed as status 1 (n=3,071), prior liver transplantation (n=895), any malignancy (n=7,248), or incomplete data (n=12,271).
Demographic, clinical and laboratory data at the time of LTx were extracted from the Standard Transplant Analysis and Research (STAR) dataset file. All laboratory data, including serum sodium, were measured within 0–7 days prior to transplantation. Recipients were divided into three groups according to the serum sodium value: hyponatremic group (Na ≤ 130 mEq/L), normonatremic group (Na=131–145mEq/L) and hypernatremic group (Na >145 mEq/L). These sodium values were chosen based on currently accepted definitions of hyponatremia and hypernatremia as well as the visual inspection of a smoothing spline generated from the Cox proportional hazard regression analysis (see below) Underlying liver disease diagnosis was categorized into viral, cholestatic, alcohol, and other etiologies. Patients with more than one diagnosis were categorized under the etiology most likely to determine the post-LTx outcome. For example, patients with hepatitis C and alcohol were classified as hepatitis C.
Data Analysis
Descriptive analysis of the recipient characteristics included comparison of hypo-, normo- and hypernatremic patients with respect to demographic, clinical and laboratory data. Following LTx, the main outcome variable considered was survival, including post-LTx mortality within 30 and 90 days as well as in-hospital mortality. Patient survival was calculated by the interval between LTx and the last known follow up or death. In addition, graft survival was calculated by the interval between LTx and death, re-transplantation or last day of follow-up. The Kaplan-Meier method was used to estimate recipient survival after LTx using the log-rank test to determine statistical significance. The relationship between pre-LTx sodium and survival post-LTx was analyzed using the multivariable Cox proportional hazard regression analysis. The Cox model was used to generate a smoothing spline to determine the relationship between serum sodium, across the spectrum of values, and post-LTx transplant survival. The spline was evaluated visually and by formal test for linearity, which helped determine rational sodium ranges to evaluate. A number of covariates at the time of LTx, specifically age, gender, race, presence of ascites, etiology of liver disease, MELD, dialysis, and life support were considered as a potential confounding variable of serum sodium. A p-value less than 0.05 was used for statistical significance in all analyses.
RESULTS
Recipient Characteristics
There were 19,537primary transplant recipients that met the inclusion criteria. Table 1 describes patients’ characteristics at the time of LTx. A Majority (84.6%) of patients (n=16,525) had a serum sodium in the normal range (131–145 mEq/L) while 13.0.% (n=2,548) were hyponatremic (≤130 mEq/L) and 2.4% (n=464) were hypernatremic (>145 mEq/L). The median serum sodium for the entire population was 136 mEq/L and the median age 53 years. Viral hepatitis was the most common etiology of liver disease (46.8%). Of the patients with a diagnosis of ‘other’ (26.3%) liver disease, 8.4% had cryptogenic cirrhosis, and 6.7% had cirrhosis due to non-alcoholic steatohepatitis. Ascites was present in 86.8% of recipients and was generally graded as mild. Hepatic encephalopathy was present in 73.5%, most of whom had grade 1–2 HE. Dialysis and life support were necessary in 12.2% and 5.2% of recipients, respectively. The median calculated MELD score at the time of LTx was 22.
Table 1.
Recipient characteristics at the time of transplantation.
| Total (N=19537) | Na <=130 (N=2548) | Na =131–145 (N=16525) | Na >145 (N=464) | p value
|
|||
|---|---|---|---|---|---|---|---|
| 3-way | hypo vs normo | hyper vs normo | |||||
| Serum sodium* | 136 (132–139) | 127 (125–128) | 136 (134–139) | 148 (147–150) | NA | NA | NA |
| Age* | 53 (48–59) | 53 (48–58) | 54 (48–59) | 54 (49–60) | 0.002 | - | - |
| Gender (Male) | 13036 (66.7%) | 1787 (70.1%) | 10966 (66.4%) | 283 (61.0%) | <0.001 | <0.001 | 0.016 |
| Race | <0.001 | <0.001 | 0.003 | ||||
| White | 14529 (74.4%) | 2002 (78.6%) | 12207 (73.9%) | 320 (69.0%) | |||
| Black | 1734 (8.9%) | 155 (6.1%) | 1534 (9.3%) | 45 (9.7%) | |||
| Asian | 539 (2.8%) | 62 (2.4%) | 452 (2.7%) | 25 (5.4%) | |||
| Other/Unknown | 2735 (14.0%) | 329 (12.9%) | 2332 (14.1%) | 74 (15.9%) | |||
| Diagnosis | <0.001 | <0.001 | 0.072 | ||||
| Alcohol | 3390 (17.4%) | 509 (20.0%) | 2812 (17.0%) | 69 (14.9%) | |||
| Cholestatic | 1858 (9.5%) | 197 (7.7%) | 1629 (9.9%) | 32 (6.9%) | |||
| Other | 5138 (26.3%) | 574 (22.5%) | 4428 (26.8%) | 136 (29.3%) | |||
| Viral | 9151 (46.8%) | 1268 (49.8%) | 7656 (46.3%) | 227 (48.9%) | |||
| Ascites | <0.001 | <0.001 | 0.001 | ||||
| Absent | 2560 (13.4%) | 155 (6.2%) | 2351 (14.5%) | 54 (11.9%) | |||
| Mild | 10001 (52.2%) | 1188 (47.5%) | 8623 (53.2%) | 190 (42.0%) | |||
| Moderate+ | 6592 (34.4%) | 1157 (46.3%) | 5227 (32.3%) | 208 (46.0%) | |||
| Encephalopathy | <0.001 | <0.001 | <0.001 | ||||
| None | 5072 (26.5%) | 503 (20.1%) | 4499 (27.8%) | 70 (15.5%) | |||
| grade 1–2 | 11835 (61.8%) | 1695 (67.8%) | 9914 (61.2%) | 226 (50.0%) | |||
| grade 3–4 | 2241 (11.7%) | 301 (12.0%) | 1784 (11.0%) | 156 (34.5%) | |||
| Dialysis** | 2383 (12.2%) | 154 (6.0%) | 2145 (13.0%) | 84 (18.1%) | <0.001 | <0.001 | 0.001 |
| Life support | 1015 (5.2%) | 63 (2.5%) | 848 (5.2%) | 104 (22.5%) | <0.001 | <0.001 | <0.001 |
| Total Bilirubin* | 4.6 (2.4–10.8) | 6.8 (3.5–14.0) | 4.3 (2.2–10.0) | 7.6 (3.8–22.4) | <0.001 | <0.001 | <0.001 |
| INR* | 1.7 (1.4–2.2) | 1.9 (1.6–2.4) | 1.7 (1.4–2.2) | 2.0 (1.6–2.6) | <0.001 | <0.001 | <0.001 |
| Creatinine* | 1.2 (0.9–1.9) | 1.3 (0.9–2.0) | 1.2 (0.8–1.9) | 1.5 (1.0–2.5) | <0.001 | <0.001 | <0.001 |
| MELD score* | 22 (16–30) | 25 (20–30) | 21 (16–29) | 29 (22–35) | <0.001 | <0.001 | <0.001 |
median (interquartile range)
During week prior to transplant
In comparing the three groups according to serum sodium concentrations, the vast majority of parameters shown in Table 1 were statistically significantly different, although not all of the differences may be medically meaningful. In gender comparisons, male preponderance was the highest in the hyponatremia group and the lowest in the hypernatremia group. Compared to patients with normonatremia, hypo- and hypernatremic patients displayed evidence of more advanced hepatic decompensation. The proportion of patients with moderate to severe ascites was higher in hypo- and hypernatremic patients when compared to normonatremic patients. Grade 3 or 4 hepatic encephalopathy was most common among patients with hypernatremia. Similarly, the MELD score and its component laboratory data were the highest in hypernatremic patients, followed by the hyponatremic and then the normonatremic groups.
Serum Sodium and Post-LTx Survival
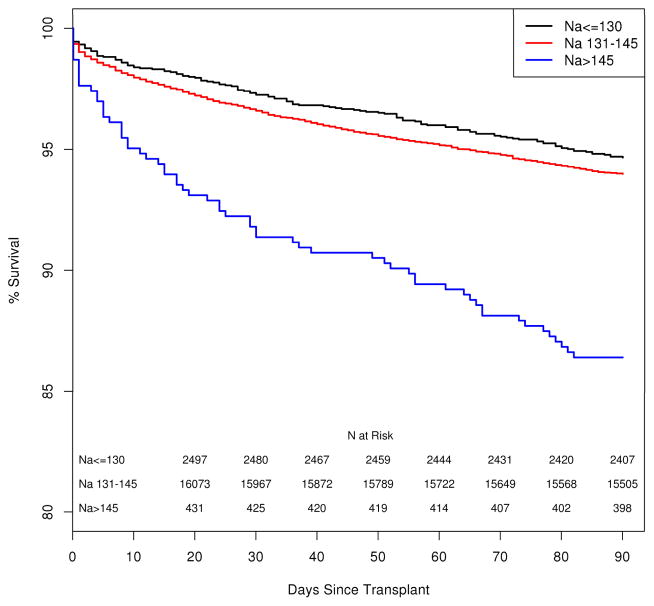
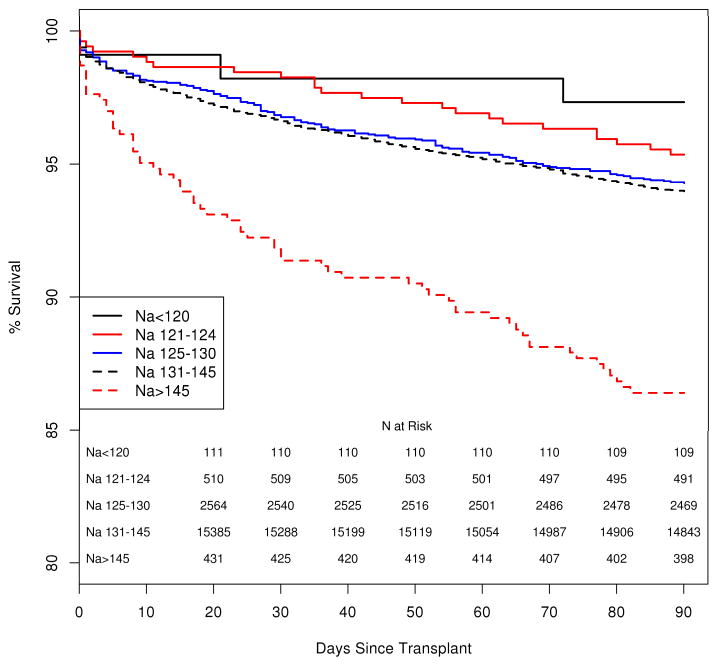
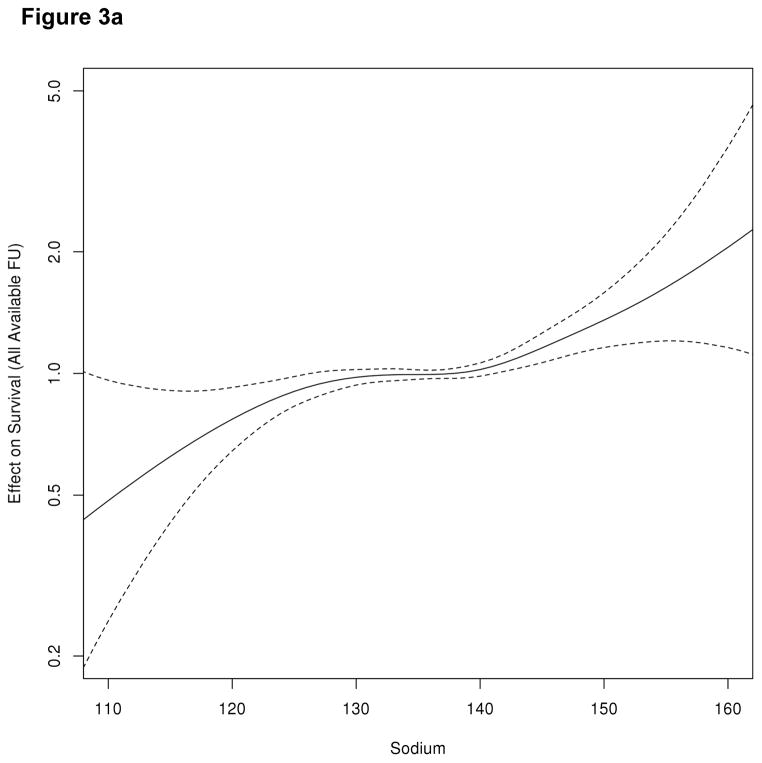
Following LTx, the median follow up after LTx was 1,063 days (interquartile range, IQR= 548–1,732). One thousand five hundred eighty-one (8.1%) of the 19,537 patients died within the first 90 days after LTx, including 673 (3.4%) who died within the first 30 days. Figure 1 illustrates patient survival up to 90 days following LTx. It is apparent that there is no difference in survival between hyponatremic and normonatremic patients, whereas patient survival in hypernatremic patients is significantly shorter (log-rank, p <0.001). The Kaplan-Meier probability of patient survival at 90 days was 94.0.% (95% confidence interval [CI]: 93.6–94.3%) for normonatremia, 94.7% (95%CI: 93.8–95.5%) for hyponatremia and 86.4% (95%CI: 83.3–89.9%) for hypernatremia. We considered post-LTx survival beyond the first week given the large drop in the hypernatremic group in the first week. While survival remained similar between hypo- and normonatremic patients, the curve (not shown) for hypernatremia patients continued to diverge from the others two groups and was statistically significant. (log rank, p<0.001) Figure 2 demonstrates survival of patients arbitrarily subcategorized as having mild (125–130), moderate (120–124) or severe (<120) hyponatremia. Of note, patients with any degree of hyponatremia did not have a statistically significant difference in the Kaplan Meier probability of survival relative to their counterparts with normal serum sodium. Figure 3a depicts a smoothing spline which demonstrates the relationship between pre-LTx serum sodium and post-LTx mortality, after adjusting for the MELD score. Between a serum sodium of 130 mEq/L and 145 mEq/L, the curve is flat indicating that there was no change in mortality. Below 130 mEq/L of sodium, the confidence band widened progressively indicating decreasing number of events in patients with severe hyponatremia; however, there was no evidence of increasing mortality as serum Na decreased. On the other hand, there was a dramatic increase in the risk of death associated with Na > 145 mEq/L. Here also, the confidence band widened; however, mortality risk with Na > 150 mEq/L rose steeply and was statistically significantly (p< 0.001). Figure 3b demonstrates the relationship between pre-LTx serum sodium, and post-LTx mortality after adjusting for multiple important clinical variables. With those adjustments, there was no significant change in the conclusion.
Figure 1.
Kaplan-Meier curve compares patient survival after LTx by the serum sodium level
Figure 2.
Kaplan-Meier curve compares patient survival after LTx by mild (Na 125–130), moderate (121–124), and severe hyponatremia (Na < 120) compared to normonatremia and hyponatremia
Figure 3.
Figure 3a. Association between serum sodium at LTx and post-LTx mortality, after adjusting for MELD score. FU=follow-up
Figure 3b. Association between serum sodium at LTx and post-LTx mortality, after adjustment for age, sex, gender, diagnosis, MELD, hepatic encephalopathy, ascites, dialysis, and life support. FU=follow-up
Hyponatremia and post-LTx Survival
Table 2 presents results of the multivariable regression analysis focusing on the impact of hyponatremia on post-LTx mortality. Serum sodium in the low and normal ranges had no clinically important effect on survival (after adjusting for known factors that affect post-LTx outcomes) at 1 month (hazard ratio, HR=1.017, 95% CI 1.001–1.034) or 3 months (HR=1.015 95% CI 1.003–1.027), again consistent with data presented in Figure 1. The p values were significant however, due to the large number of observations. Other covariates that were significant included MELD and ‘other’ liver disease. As there were patients with missing laboratory data within the predefined window (0–7 days prior to LTx), we conducted a sensitivity analysis by repeating the same multivariable regression analysis with sodium values from different time frames before transplantation. When serum sodium values from 0–30 days prior to transplant were included, there were an additional 1,820 patients that met inclusion criteria with a sodium value available within the 8–30 day time frame, providing a total of 20,514 with the necessary data for the sensitivity analysis. This did not result in any material change. The hazard ratio associated with serum sodium was 1.013(95% CI 0.998–1.02) and 1.014 (95%CI 1.002–1.026) at 30 and90 days, respectively. Similarly, the multivariable regression analysis was conducted for patients with serum sodium levels recorded within 48 hours (UNOS data 2005–2008) of the time of transplant (n=11,568) and the hazard ratios were not significant at 1 month (HR=1.007; 95% CI 0.968–1.027) or 3 months (HR=1.0; 95% CI 0.995–1.025). There were 4,166 (31% of total) patients with sodium values available within 24 hours of transplant; the 30 and 90 day hazard ratios were 1.03 (95% CI 0.996–1.066) and 1.02 (95% CI 0.995–1.049) for this smaller patient group, respectively. A separate Cox PH regression analysis was also performed for patients with serum sodium < 125 mEq/L (n=426) and < 120 mEq/L (n=80) at 0–7 days prior to transplant, which demonstrated no effect on 30 and 90 day post-LTx mortality.
Table 2.
Impact of serum sodium on mortality of non-hypernatremic patients* at 30 and 90 days, and 1 year
| variables | 30 day mortality | 90 day mortality | 1 year mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| MELD | 1.036 | 1.027–1.045 | <0.001 | 1.038 | 1.031–1.045 | <0.001 | 1.034 | 1.029–1.039 | <0.001 |
| Na | 1.017 | 1.001–1.034 | 0.035 | 1.015 | 1.003–1.027 | 0.012 | 1.012 | 1.004–1.021 | 0.004 |
| Ascites | 1.052 | 0.815–1.358 | 0.697 | 1.090 | 0.897–1.324 | 0.389 | 1.104 | 0.963–1.265 | 0.157 |
| Diagnosis | |||||||||
| Viral | Reference | -- | Reference | -- | -- | Reference | -- | -- | |
| Cholestatic | 0.897 | 0.657–1.225 | 0.194 | 0.855 | 0.675–1.083 | 0.193 | 0.696 | 0.589–0.822 | <0.001 |
| ALD | 1.018 | 0.812–1.277 | 0.877 | 1.026 | 0.868–1.213 | 0.760 | 0.730 | 0.645–0.826 | <0.001 |
| Other | 1.393 | 1.161–1.673 | <0.001 | 1.319 | 1.149–1.514 | <0.001 | 0.992 | 0.890–1.094 | 0.874 |
Excludes Na > 145 mEq/L
Hypernatremia and Post-LTx Survival
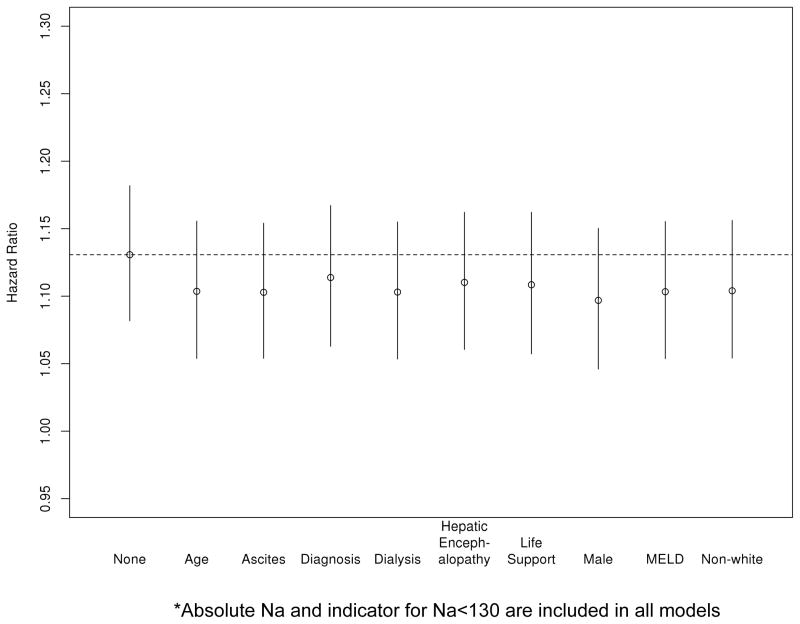
Given the fact that hypernatremic patients were generally sicker with high proportion of patients receiving dialysis and life support as well as having high MELD scores (median 29, IQR 22–35) we investigated whether the impact of hypernatremia on survival may be explained by those other variables that indicate the level of sickness. In Figure 4, one unit (mEq/L) increase in serum sodium above >145 was associated with a HR of 1.131 (95% CI: 1.082–1.182, p<0.001), when no other covariate was considered. This effect did not change meaningfully when other potential confounders were taken into account, namely recipient age, race, ascites, encephalopathy, dialysis, life support, and MELD score.
Figure 4.
Effect of excess sodium (number of points in excess of serum sodium of 145 mEq/L) with various adjustors from cox model* of 3 month survival after liver transplantation
Serum Sodium and Immediate Post-LTx Outcome
Table 4 demonstrates short term outcome of patients after liver transplantation. Approximately 4.3% (832/19,537) of the total population experienced an in-hospital death (death before or within 24 hours after hospital discharge). As can be expected from Figure 1, in-hospital mortality was significantly higher in hypernatremic patients than those with lower serum sodium (Table 3). The median length of hospitalization after LTx was 10 days (interquartile range: 7–18 days), which was significantly longer in hypernatremic patients than that in patients with lower serum sodium (p < 0.001).
Table 3.
Short-term outcome after liver transplantation.
| Na <=130 | Na =131–145 | Na >145 | Total | P value | |
|---|---|---|---|---|---|
|
| |||||
| (N=2548) | (N=16,525) | (N=464) | (N = 19,537) | ||
| In-hospital Death (n, %) | 92 (3.6) | 688 (4.2) | 52 (11.2) | 832 (4.3) | <0.001 |
| Days of hospital (median, Q1, Q3) | 11 (8–19) | 10 (7–17) | 18 (10–32) | 10 (7–18) | <0.001 |
DISCUSSION
Abnormal serum concentrations of sodium are common in patients with end stage liver disease and those undergoing LTx. The main observation in this study is that pre-LTx hyponatremia is not associated with decreased survival afterward, whereas there is a strong association between hypernatremia and diminished post-LTx survival. These relationships are clearly shown in Figure 1, in which the hyponatremic and normonatremic survival curves are very similar. Even patients with severe hyponatremia (Na<120 mEq/L) did not have diminished post-transplant survival, although there were a small number (n=111) of patients in this group (figure 2). In contrast, 90 day survival was 8% lower in patients with hypernatremia as compared to those with normal or low serum sodiums. The relationship between pre-transplant hypernatremia and decreased post-LTx survival persisted even after adjusting for MELD score and other markers of severity of illness.
There is a broad consensus that hyponatremia adversely affects survival of end stage liver patients including those on LTx waitlist. However, the impact of hyponatremia on post-LTx survival has been debated. European investigators have shown an association between hyponatremia and decreased post-LTx survival.(11, 12) Dawwas et al. conducted a multicenter study based on a registry of 5,152 LTx recipients in the UK and Ireland. In that study, serum sodium < 130 mEq/L was associated with 55% increase in 90-day mortality after adjusting for other factors that may affect post-LTx survival.(12) In another study of 241 patients from a single center in Spain, the 90-day mortality in hyponatremic (Na<130mEq/L, n=19) patients was 16% compared with 5% in those with normonatremia.(11) On the other hand, we have recently reported that hyponatremia did not have an impact on patient survival, based on databases obtained from networks of select academic LTx centers in the US.(13) Data in this study, in close agreement with the latter study, indicates that there is not a relationship between pre-LTx hyponatremia and post-LTx mortality. Hackworth et al. recently reported no difference in 6-month post-transplant survival in their U.S. cohort consisting of patients with pre-transplant hyponatremia (n=34) or resolved hyponatremia (n=56), compared to patients who never had hyponatremia (n=123).(14) In addition, we now have prospective data from a small number of patients (n=62) from the OPTN Region 11 in which the MELDNa was used experimentally.(20) The 6 month survival was the same in those in whom the MELDNa was utilized as compared to standard MELD.
The dichotomy between the two European and the three U.S. studies is intriguing. In all of these studies, hyponatremic patients clearly have more advanced liver disease, including a higher prevalence of ascites and encephalopathy and more severe abnormalities in biochemical decompensation (e.g., MELD). One potential mechanism by which hyponatremic patients may suffer poorer outcome is the use of less than ideal donors. At times, patients that are in an urgent need of LTx may be offered an organ that may be considered less than ideal. For example, in the UK study, donors for hyponatremic patients were more likely to be ABO-incompatible or older. Those organs also were judged to have suboptimal appearance by the surgeon, one of the strongest indicators of organ quality. Additionally, patients with severe hyponatremia were more likely to have hepatitis C and less likely to have PBC.(12) Thus, it is possible that recipient hyponatremia (accompanied by more severe hepatic decompensation) may be confounded by other baseline characteristics and donor factors that affected the outcome. Another explanation for the difference between the U.S. and European data may be the MELD-based organ allocation system. Under this system, patients with advanced liver disease are systematically recognized and given a higher priority. Much of the European data were generated under a system in which the priority of transplantation took into account other factors than disease severity, such as waiting time. In such a system, ill patients with high MELD and hyponatremia wait longer, allowing development of complications that affect postoperative outcome.
Hypernatremia occurs infrequently (1–2% of hospitalized patients), yet it has been associated with in-hospital mortality as high as 41–66%.(21–23) Mortality is reported to be significantly higher in patients who develop hypernatremia in the hospital than in those who present with hypernatremia.(24–26) It has been postulated that patients who develop hypernatremia (usually in the ICU setting) have multiple physiologic mechanisms that predispose them to hypernatremia including insensible free water loss, nasogastric suction, loop diuretics, gastrointestinal bleeding and parenteral nutrition.(22, 27) In patients with liver disease, lactulose therapy has been associated with a high incidence of hypernatremia.(28) Osmotic diuresis from increased nitrogen excretion in the kidneys has been correlated with GI hemorrhage in cirrhotic patients with ascites.(29) Our data amplify the message that hypernatremia is associated with complications of advanced liver disease.(15)
Published data are sparse about the impact of hypernatremia on post-LTx survival. In the UK study by Dawwas et al., hypernatremia (Na > 145mEq/L) was associated with a 2.3-fold increase in the risk of post-LTx mortality, which occurred mostly within the first 90 days.(12) Similar to our data, hypernatremia was not common (1.6%, n=81)), and it was associated with more advanced hepatic decompensation requiring dialysis and life support in a high proportion of patients. In this paper, we further demonstrate that adjusting for these pre-transplant variables did not attenuate the effect of hypernatremia (Figure 4).
In the intensive care arena, hypernatremia has been suggested as an indicator of quality of care.(30) This is especially true of iatrogenic hypernatremia that goes unnoticed or untreated, which has been associated with high mortality and may point to a failure for the medical staff to incorporate electrolyte balance in their patient management strategy. Moreover, some physicians may erroneously believe that a high serum sodium concentration helps to mobilize ‘third-space’ fluid. The fact that pre-LTx characteristics could not explain away the increased mortality associated with hypernatremia may suggest that hypernatremia may simply be a surrogate of the quality of care at the individual center, which deserves further exploration. Donor factors may also contribute though it has previously been demonstrated that the difference between donor and recipient sodium values at transplant does not affect post-LTx outcomes.(31).
The authors recognize some limitations of the study. One of the pitfalls of using registry data is the lack of detailed clinical information on the etiology and management of sodium abnormalities. The UNOS database was not specifically designed to study transplant outcomes in relation to pre-LTx sodium measurements. Serum sodium may be influenced by free water restriction, use of diuretics or hypertonic saline infusion. Conceivably, hyponatremia in some patients could have been corrected between the sodium data collection and LTx, or conversely, patients with normal serum sodium could develop hyponatremia. However, Hackworth et al. have recently shown that patients with hyponatremia that is corrected prior to liver transplantation have no survival difference when compared to patients with normal sodium homeostasis.(14) Less is known about how treatment of hypernatremia affects post-LTx outcomes. A second limitation of this study is the lack of accurate data on the cause of death, which prevented us from providing more information about how hypernatremia affects post-LTx mortality. In a recent study, the leading causes of death in hypernatremic patients were hypoxic respiratory failure, septic shock, neurologic, and cardiogenic shock.(27) Whether or not correction of hypernatremia in its early stages prevents the development of the ultimate cause of death in post-liver transplant patients is unknown. Lastly, data regarding neurologic consequences including central pontine myelinolysis were not available for this study.
In conclusion, the present study demonstrated pre-transplant hyponatremia, although a strong indicator of waitlist mortality, does not affect mortality following LTx. This is the largest study conducted on this issue. These data suggest that incorporation of serum sodium in determining organ allocation priority should not diminish survival after LTx. Although hypernatremia was uncommon, it appears to have a significant impact on post-LTx survival. The effect of hypernatremia was independent of indicators of advanced liver failure.
Acknowledgments
The study was funded by a grant from the National Institute of Diabetes, Digestive and Kidney Diseases (R01-DK34238 and DK-92336).
Abbreviations
- Cl
confidence interval
- ESLD
End Stage Liver Disease
- IQR
Interquartile Range
- LTx
liver transplantation
- MELD
Model for End Stage Liver Disease
- MELDNa
Model for End Stage Liver Disease - Sodium
- Na
sodium
- US
United States
Contributions and Disclosures
-
MD Leise
Contributed to the whole content.
Intellectual contributions: conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis.
No funding sources or conflict of interest.
-
BC Yun
Contributed to part of the content.
Intellectual contributions: conception and design, analysis and interpretation of data, drafting of the manuscript,
No funding sources or conflict of interest.
-
JT Benson
Contributed to part of the content.
Intellectual contributions: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis.
#x02022;No funding sources or conflict of interest.
-
Joseph J. Larson
Contributed to part of the content.
Intellectual contributions: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis.
No funding sources or conflict of interest.
-
JD Yang
Contributed to part of the content.
Intellectual contributions: conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
No funding sources or conflict of interest.
-
TM Therneau
Contributed to the whole content.
Intellectual contributions: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis.
No funding sources or conflict of interest.
Had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data.
-
CB Rosen
Contributed to part of the content.
Intellectual contributions: conception and design, critical revision of the manuscript for important intellectual content
No conflicts of interest.
-
JK Heimbach
Contributed to part of the content.
Intellectual contributions: conception and design, critical revision of the manuscript for important intellectual content, statistical analysis.
No funding sources or conflict of interest.
-
SW Biggins
Contributed to part of the content.
Intellectual contributions: conception and design, critical revision of the manuscript for important intellectual content, statistical analysis.
No funding sources or conflict of interest.
-
WR Kim
Contributed to the whole content.
Intellectual contributions: conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical, or material support, and supervision
No conflicts of interest.
Funding: DK34238 and DK-92336
References
- 1.Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–36. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 2.Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28(3):851–64. doi: 10.1002/hep.510280337. [DOI] [PubMed] [Google Scholar]
- 3.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11(3):336–43. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 4.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20(6):1495–501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 5.Guevara M, Baccaro ME, Torre A, Gomez-Anson B, Rios J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104(6):1382–9. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 6.Haussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43(6):1187–90. doi: 10.1002/hep.21235. [DOI] [PubMed] [Google Scholar]
- 7.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40(4):802–10. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 8.Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48(3):1002–10. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–60. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Londono MC, Guevara M, Rimola A, Navasa M, Taura P, Mas A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130(4):1135–43. doi: 10.1053/j.gastro.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Dawwas MF, Lewsey JD, Neuberger JM, Gimson AE. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl. 2007;13(8):1115–24. doi: 10.1002/lt.21154. [DOI] [PubMed] [Google Scholar]
- 13.Yun BC, Kim WR, Benson JT, Biggins SW, Therneau TM, Kremers WK, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. 2009;49(5):1610–5. doi: 10.1002/hep.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, Luketic VA, et al. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int. 2009;29(7):1071–7. doi: 10.1111/j.1478-3231.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 15.Warren SE, Mitas JA, 2nd, Swerdlin AH. Hypernatremia in hepatic failure. Jama. 1980;243(12):1257–60. [PubMed] [Google Scholar]
- 16.Shorr AF, Tabak YP, Johannes RS, Gupta V, Saltzberg MT, Costanzo MR. Burden of sodium abnormalities in patients hospitalized for heart failure. Congest Heart Fail. 2011;17(1):1–7. doi: 10.1111/j.1751-7133.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125(5):677–84. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waite MD, Fuhrman SA, Badawi O, Zuckerman IH, Franey CS. Intensive care unit-acquired hypernatremia is an independent predictor of increased mortality and length of stay. J Crit Care. 2013;28(4):405–12. doi: 10.1016/j.jcrc.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Arampatzis S, Frauchiger B, Fiedler GM, Leichtle AB, Buhl D, Schwarz C, et al. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 2012;125(11):1125, e1–e7. doi: 10.1016/j.amjmed.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Fisher RA, Heuman DM, Harper AM, Behnke MK, Smith AD, Russo MW, et al. Region 11 MELD Na exception prospective study. Ann Hepatol. 2012;11(1):62–7. [PubMed] [Google Scholar]
- 21.Long CA, Marin P, Bayer AJ, Shetty HG, Pathy MS. Hypernatraemia in an adult in-patient population. Postgrad Med J. 1991;67(789):643–5. doi: 10.1136/pgmj.67.789.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124(2):197–203. doi: 10.7326/0003-4819-124-2-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor KA, Cotter PE, Kingston M, Twomey C, O’Mahony D. The pattern of plasma sodium abnormalities in an acute elderly care ward: a cross-sectional study. Ir J Med Sci. 2006;175(3):28–31. doi: 10.1007/BF03169169. [DOI] [PubMed] [Google Scholar]
- 24.Mandal AK, Saklayen MG, Hillman NM, Markert RJ. Predictive factors for high mortality in hypernatremic patients. Am J Emerg Med. 1997;15(2):130–2. doi: 10.1016/s0735-6757(97)90082-6. [DOI] [PubMed] [Google Scholar]
- 25.Erasmus RT, Matsua TE. Frequency, aetiology and outcome of hypernatraemia in hospitalised patients in Umtata, Transkei, South Africa. East Afr Med J. 1999;76(2):85–8. [PubMed] [Google Scholar]
- 26.O’Donoghue SD, Dulhunty JM, Bandeshe HK, Senthuran S, Gowardman JR. Acquired hypernatraemia is an independent predictor of mortality in critically ill patients. Anaesthesia. 2009;64(5):514–20. doi: 10.1111/j.1365-2044.2008.05857.x. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw SM, Townsend DR, McDermid RC. Disorders of sodium and water balance in hospitalized patients. Can J Anaesth. 2009;56(2):151–67. doi: 10.1007/s12630-008-9017-2. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DC, McGrew WR, Jr, Hoyumpa AM., Jr Hypernatremia and lactulose therapy. Jama. 1983;249(10):1295–8. [PubMed] [Google Scholar]
- 29.Rodes J, Arroyo V, Bordas JM, Bruguera M. Hypernatremia following gastrointestinal bleeding in cirrhosis with ascites. Am J Dig Dis. 1975;20(2):127–33. doi: 10.1007/BF01072338. [DOI] [PubMed] [Google Scholar]
- 30.Polderman KH, Schreuder WO, Strack van Schijndel RJ, Thijs LG. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med. 1999;27(6):1105–8. doi: 10.1097/00003246-199906000-00029. [DOI] [PubMed] [Google Scholar]
- 31.Cywinski JB, Mascha E, Miller C, Eghtesad B, Nakagawa S, Vincent JP, et al. Association between donor-recipient serum sodium differences and orthotopic liver transplant graft function. Liver Transpl. 2008;14(1):59–65. doi: 10.1002/lt.21305. [DOI] [PubMed] [Google Scholar]