Abstract
Recombinant Newcastle disease virus (rNDV) have shown oncolytic therapeutic efficacy in preclinical studies and are currently in clinical trials. In this study, we have evaluated the possibility to enhance the cancer therapeutic potential of NDV by means of inserting both interleukin-2 (IL-2) and tumor necrosis factor-related apoptosis inducing ligand (TRAIL) delivered by rNDV. We demonstrated that rNDV expressing TRAIL (rNDV-TRAIL) or both human IL-2 and TRAIL (rNDV-IL-2-TRAIL) significantly enhanced inherent anti-neoplastic of rNDV by inducing apoptosis. And we showed that apoptosis-related genes mRNA expression was increased after treated with rNDV-TRAIL or rNDV-IL-2-TRAIL compared with rNDV and rNDV-IL-2. We also demonstrated that both rNDV-IL-2 and rNDV-IL-2-TRAIL induced proliferation of the CD4+ and CD8+ in treated mice and elicited expression of TNF-α and IFN-γ antitumor cytokines. These mice treated with oncolytic agents exhibited significant reduction in tumor development compared with mice treated with the parental virus. In addition, experiments in both hepatocellular carcinoma and melanoma-bearing mice demonstrated that the genetically engineered rNDV-IL-2-TRAIL exhibited prolonged animals’ survival compared with rNDV, rNDV-IL-2, and rNDV-TRAIL. In conclusion, the immunotherapy and oncolytic virotherapy properties of NDV can be enhanced by the introduction of IL-2 and TRAIL genes, whose products initiated a broad cascade of immunological affects and induced tumor cells apoptosis in the microenvironment of the immune system.
Keywords: rNDV, cancer therapy, IL-2, TRAIL, rNDV-IL-2-TRAIL, immunotherapy and oncolytic virotherapy, oncolytic agent
Introduction
NDV have shown oncolytic therapeutic efficacy in clinical study when genetically engineered and reverse genetics was used for constructing and rescuing viruses.1 Use of reverse genetics to enhance the oncolytic properties of NDV has been reported in many studies,2-14 and most of these NDV strains are mesogenic, and such highly infectious strains are problematic in clinical use due to possible unintentional release of highly infectious virus into the environment, which affects almost all species of domestic and wild birds. Mesogenic strains of NDV may cause losses to poultry industries.15 In addition, mesogenic NDVs have been forbidden in many countries for its high pathogenicity for chicken without maternal antibody. As a result, avirulent lentogenic strains may be a better alternative as cancer therapy vector.16 The lentogenic strain LaSota is safe to humans and animals and has been recommended by World Health Organization (WHO) as a NDV vaccine. The advantage of LaSota is that it has no geographical constraints for widely used in the world.15
TRAIL is a promising anticancer agent due to its ability to lyse tumors by inducing apoptosis.17 TRAIL receptors are attractive therapeutic targets in cancer therapy, which can induce tumor cell apoptosis and have minimal toxicity to normal tissues. TRAIL induces apoptosis in established tumor cell lines by binding with TRAIL receptors, but not transformed cells, which is a merit for anticancer agent. TRAIL-induced apoptosis by expression of “death-inducing” and “decoy” receptors on cells, apoptosis induced by TRAIL and other TNF members may be regulated by inhibitory proteins that bind to Fas-associated death domain or other proteins in the caspase pathway.18 Previous studies have proved that TRAIL is an efficient oncolytic agent for cancer therapy.19-21
IL-2 is a pleiotropic cytokine with important effects on cells of the innate and adaptive immune systems and plays a pivotal role in T-cell activation and effector functions, including T-cell proliferation, interferon-γ production, and cytotoxicity.22 IL-2 can stimulate the propagation of lymphocytes and induce the cytotoxic T lymphocytes and lymphokine-activated killer cells for tumor cells. IL-2 also influences memory T cells homeostasis through the regulation of memory T cell numbers and drives the generation of antigen-specific T cells, promotes the survival of memory CD8+ T cells.23 Therefore, it has been used for cancer immunotherapy.24 And previous study been reported that expressing therapeutic IL-2 gene by rNDV enhances therapeutic effects of NDV though stimulating body’s immune system to produce tumor-specific immune cells to attract tumors.7,8,25,26
In this study, combining selective replication of rNDV in tumor cells with the immunoregulatory ability of IL-2 and the oncolytic efficacy of TRAIL for cancer therapy was explored. We demonstrated that genetically-engineered Newcastle disease virus simultaneously expressing IL-2 and TRAIL genes significantly enhanced anti-cancer effects of NDV for murine hepatic carcinoma (HCC) and malignant melanoma models. Our results indicated that rNDV-IL-2-TRAIL may potentially be applied for clinical application for hepatoma and melanoma in patients.
Results
Generation of recombinant NDVs and expression of foreign genes in tumor cells
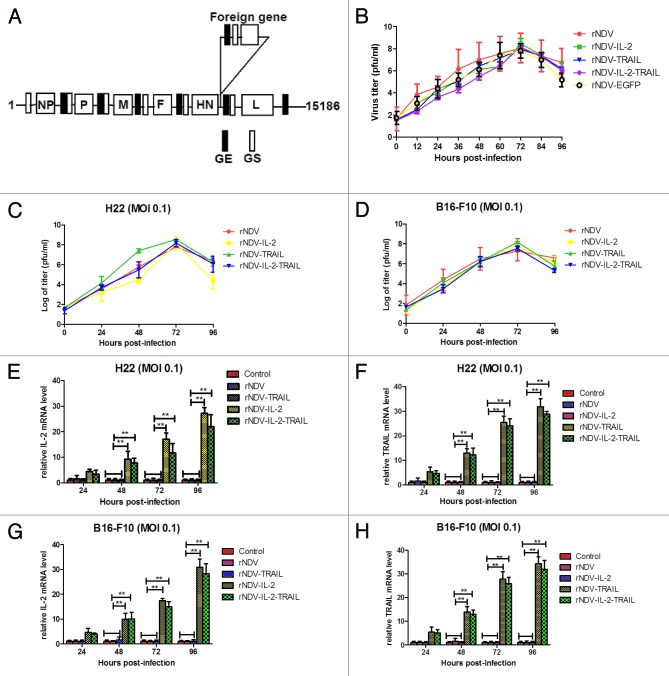
The cDNA clones containing the human IL-2 and TRAIL gene alone or in combination at position between HN and L were constructed as illustrated in Figure 1A, and rNDVs were rescued using reverse genetics technology. All recombinant viruses were grown to high titers in embryonated chicken eggs. The growth characteristics of the recombinant NDV viruses were examined in a single-step growth cycle in DF-1 cell lines. As shown in Figure 1B, the kinetics of replication of the recombinant viruses showed that insertion of the foreign gene resulted in a slightly delay in the onset of replication. However, after 72 h, virus titers reached to the same level as the parental LaSota strain. We also further investigated replication of rNDVs in H22 and B16-F10 cells. The results in Figure 1C and D indicated that viruses titer reach the highest after 72 h post infection.
Figure 1. Construction of rNDVs and foreign genes expressed in the recombinant virus-infected tumor cells. (A) Construction of the therapeutic rNDV vectors expressing hIL-2, TRAIL, or IL-2 and TRAIL protein. The small black boxes indicate conserved gene-end (GE) sequences. The small white boxes indicate conserved gene-start (GS) sequences. (B) Comparison of viral growth kinetics. (C and D) Viral proliferation was tested in H22 and B16-F10 cells. (E–H) mRNA Expression of the IL-2 (E and G) and TRAIL (F and H) genes in in H22 and B16-F10 cells infected with rNDV, rNDV-IL-2, rNDV-TRAIL, and rNDV-IL-2-TRAIL, respectively. One-way ANOVA revealed a significant effect. **P < 0.01, vs control.
To determine the expression of foreign genes, IL-2 and TRAIL mRNA expression in H22 and B16-F10 cells were detected by RT-PCR. The results in Figure 1E and H showed that tumor cells infected with the recombinant viruses expressed the desired mRNA. We also detected gene products by ELISA using anti-IL-2-specific and anti-TRAIL-specific antibodies, respectively. As shown in Tables 1 and 2, tumor cells infected with the recombinant viruses expressed the desired gene products, while no foreign genes were detected in the tumor cells infected with rNDV.
Table 1. IL-2 expression in rNDV-IL-2 and rNDV-IL-2-TRAIL-infected tumor cells.
| Hours | Cells | H22 | B16-F10 | A549 | CT-26 |
|---|---|---|---|---|---|
| 24 | rNDV-IL-2 | 285.68 | 378.46 | 321.74 | 329.18 |
| rNDV-IL-2-TRAIL | 226.32 | 350.86 | 316.22 | 326.72 | |
| 48 | rNDV-IL-2 | 574.28 | 836.92 | 684.62 | 548.98 |
| rNDV-IL-2-TRAIL | 486.12 | 782.64 | 598.88 | 522.48 | |
| 72 | rNDV-IL-2 | 1421.29 | 1178.13 | 1011.77 | 1129.17 |
| rNDV-IL-2-TRAIL | 1046.62 | 850.51 | 936.28 | 826.74 | |
| 96 | rNDV-IL-2 | 1746.48 | 1572.36 | 1342.66 | 1432.16 |
| rNDV-IL-2-TRAIL | 1353.77 | 1050.22 | 1236.36 | 1146.70 |
Expression of the IL-2 gene in tumor cell lines infected with rNDV-IL-2 and rNDV-IL-2-TRAIL. Tumor cells were infected with rNDV-IL-2 and rNDV-IL-2-TRAIL at 0.1 MOI. IL-2 was analyzed by ELISA.
Table 2. TRAIL expression in rNDV-TRAIL and rNDV-IL-2-TRAIL-infected tumor cells.
| Hours | Cells | H22 | B16-F10 | A549 | CT-26 |
|---|---|---|---|---|---|
| 24 | rNDV-TRAIL | 324.62 | 384.44 | 350.86 | 412.16 |
| rNDV-IL-2-TRAIL | 288.06 | 364.80 | 308.25 | 376.44 | |
| 48 | rNDV-TRAIL | 664.26 | 654.84 | 594.26 | 626.32 |
| rNDV-IL-2-TRAIL | 576.24 | 574.22 | 546.36 | 584.46 | |
| 72 | rNDV-TRAIL | 1450.89 | 1123.51 | 862.48 | 1169.24 |
| rNDV-IL-2-TRAIL | 1256.51 | 883.52 | 946.47 | 1149.06 | |
| 96 | rNDV-TRAIL | 1644.47 | 1488.72 | 1142.74 | 1502.08 |
| rNDV-IL-2-TRAIL | 1452.64 | 1108.12 | 1306.46 | 1348.35 |
Expression of the TRAIL gene in tumor cell lines infected with rNDV-TRAIL and rNDV-IL-2-TRAIL. Tumor cells were infected with rNDV-TRAIL and rNDV-IL-2-TRAIL at 0.1 MOI. TRAIL was analyzed by ELISA.
Characterization of biological activity for the IL-2 and TRAIL
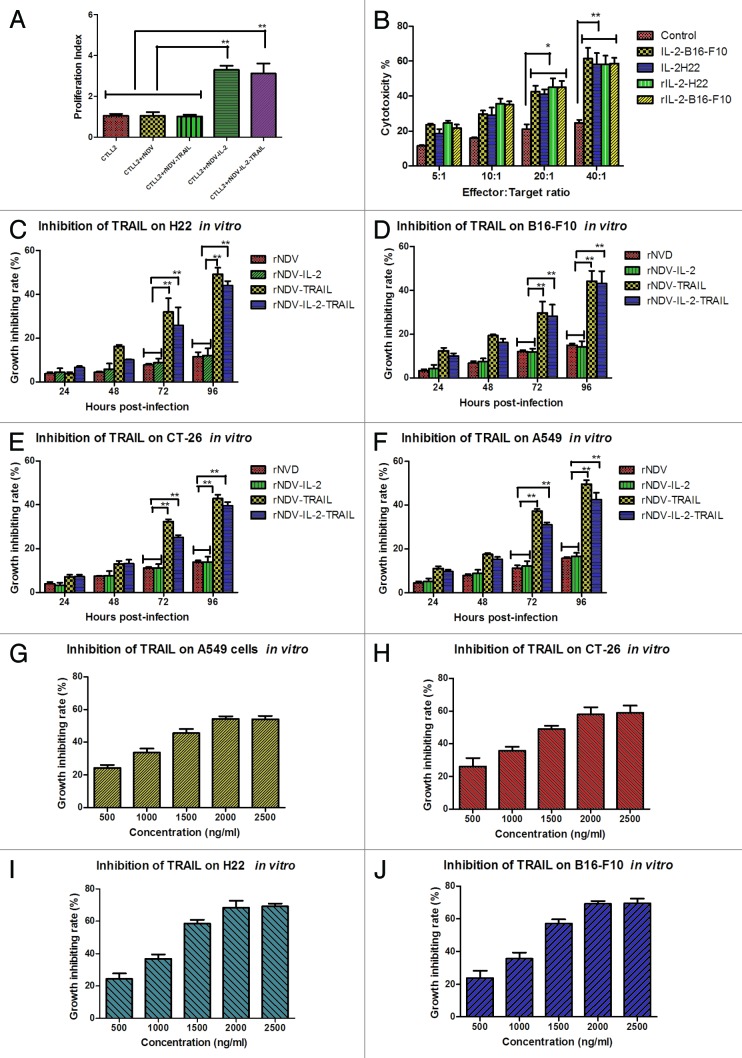
In order to prove the biological activity of IL-2 expressed by rNDV-IL-2, we examined the proliferation of T cells by adding the IL-2 from rNDV-IL-2 or rNDV-IL-2-TRAIL-infected DF1 cells. As shown in Figure 2A, the result showed the purified IL-2 from rNDV-IL-2 and rNDV-IL-2-TRAIL infected cells could stimulate proliferation of CTLL2 cells, while no proliferation was observed in the negative control. This result indicated that IL-2 protein expressed by rNDV-IL-2 and rNDV-IL-2-TRAIL was biological active. Also, we evaluated the cytotoxicity of lymphokine-activated killer (LAK) cells by stimulation of splenocytes with the IL-2 from rNDV-IL-2 or rNDV-IL-2-TRAIL-infected DF1 cells. The result in Figure 2B showed that the IL-2 from rNDV-IL-2 or rNDV-IL-2-TRAIL-infected DF1 cells stimulated T leukomonocyte to differentiate into LAK cells, which killed B16-F10 and H22 cells compared with control.
Figure 2. Identification of bioactivity for the IL-2 and TRAIL expressed by rNDV. (A) T (CTLL2) cells proliferation was stimulated by IL-2 expressed by rNDV-IL2 or rNDV-IL-2-TRAIL-infected DF1 cells. (B) The purified IL-2 enhanced cytotoxicity of immune cells for H22 and B16-F10 cells. Student’s paired two-tailed t test revealed a significant effect. **P < 0.01, vs control. (C–F) The purified TRAIL from rNDV-TRAIL or rNDV-IL-2-TRAIL-infected DF1 cells inhibited tumor cells growth. (G–J) TRAIL inhibited tumor cells growth in vitro in a concentration-dependent manner. ANOVA revealed a significant effect. *P < 0.05, **P < 0.01, vs control. All the values are the mean and SEM of triplicate samples.
To prove the biological activity of TRAIL expressed from the recombinant viruses, the TRAIL from rNDV-TRAIL or rNDV-IL-2-TRAIL-infected DF-1 cells was added directly into the culturing tumor cells and adjusted TRAIL final concentration was 1000 ng/mL. As shown in Figure 2C–F, TRAIL from the DF1 cells was cytotoxic for tumor cells at the appointed times. We also studied the effects of different concentrations of TRAIL on A549, CT-26, B16-F10, and H22 tumor cells after 72 h. As expected, TRAIL demonstrated an inhibitory effect on A549, CT-26, B16-F10, and H22 cells in vitro. We observed that TRAIL inhibited tumor cells in vitro when its concentration was more than 1000 ng/mL, and the inhibiting effect was concentration-dependent. Nevertheless, there was no significant difference in the growth-inhibiting rate when the concentration of TRAIL reached 2000 ng/mL, suggesting that TRAIL has achieved its saturated concentration at 2000 ng/mL (Fig. 3G–J).
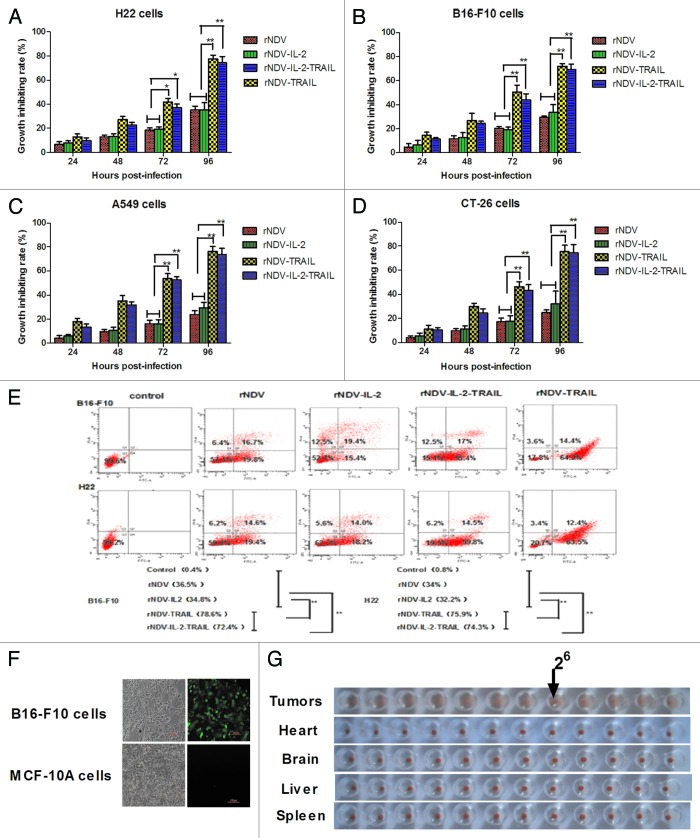
Figure 3. TRAIL enhanced NDV inhibition of tumor cells growth. (A–D) Expressing TRAIL enhanced the anti-tumor potency of rNDV in vitro. The Student paired two-tailed t test revealed a significant effect. *P < 0.05, **P < 0.01, vs control. (E) The rNDV-TRAIL and rNDV-IL-2-TRAIL enhanced the ability to induce tumor apoptosis for inserting TRAIL. Q1, death tumor cells; Q2, early apoptosis cells; Q3, survival cells; Q4, late apoptosis cells. (F and G) rNDV retains selective replication in tumor in vitro (F) and in vivo (G). All the values are the mean and SD of triplicate samples; the Student paired two-tailed t test revealed a significant effect. *P < 0.05, **P < 0.01, vs control.
Incorporation of TRAIL into rNDV enhances its apoptotic potency for tumor cells and retains its tumor-selectivity
As an anti-tumor drug, the cytotoxic effects for tumor cells are important for cancer therapy. Therefore, we investigated the anti-tumor potential of rNDVs in tumor cells. Cytotoxicity was assessed by MTT assay at 24, 48, 72, and 96 h post infection. Apoptotic rates of B16-F10 and H22 cells were detected by using flow cytometry. The results in Figure 3A–D showed that the insertion of TRAIL greatly enhanced the cytotoxic effects of the rNDV for tumor cells. As shown in Figure 3E, TRAIL enhanced rNDV to induce tumor cells apoptosis. Also, we further confirmed that the rNDVs selectively replicated in tumor cells. As shown in Figure 3F, enhanced green fluorescent protein detected in B16-F10 tumor cells, but not in MCF-10A normal cells, suggesting that the rNDV retained tumor-selectivity in vitro. We further confirmed the safety of rNDVs in vivo in rNDV-treated-mice, including heart, liver, brain, spleen. As shown in Figure 3G, HA titer was observed in tumor, but not in organizations. These results further tested that lentogenic NDV may be a safe antitumor drug for human use.
Analysis of the rNDV-infected tumor cells apoptosis by real-time PCR
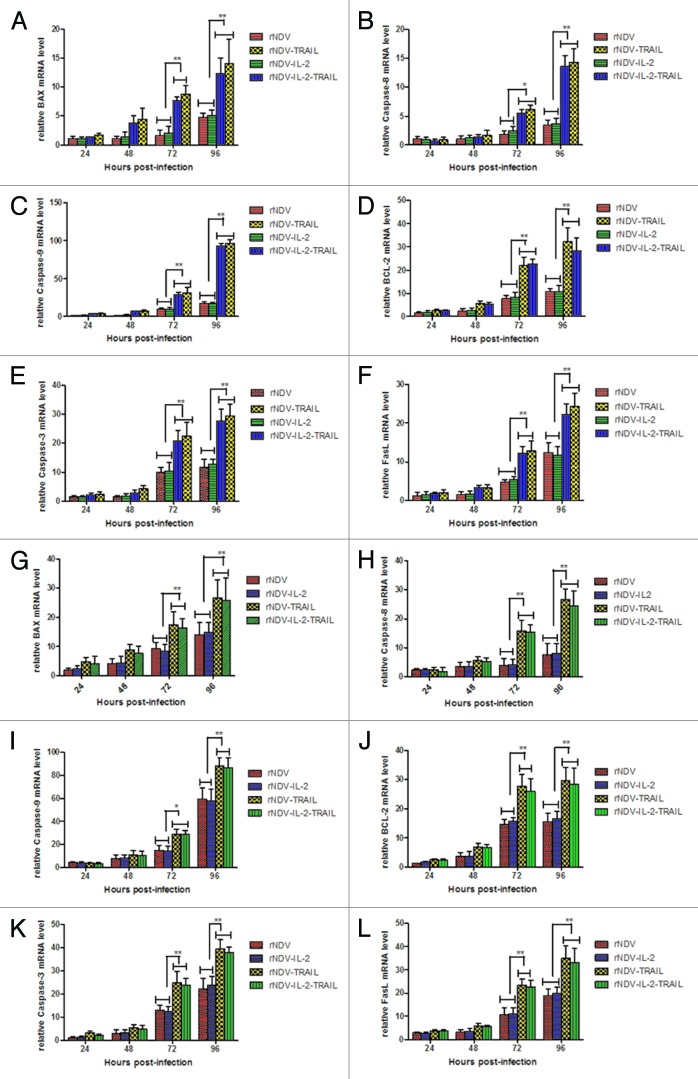
To confirm the mechanism of apoptosis induced by rNDV-TRAIL, we investigated the relative mRNA expression of apoptosis-related genes. BAX, BCL-2, Fas-L, caspase-8, caspase-3, and caspase-9 expression was analyzed by Real-Time PCR at different time after infection. As shown in Figure 4A–L, apoptosis-related genes mRNA expression were increased in B16-F10 (Fig. 4A–F) and H22 (Fig. 4G–L) cells after infected with rNDV-TRAIL or rNDV-IL-2-TRAIL. Previous study has shown that TRAIL induces apoptosis by both mitochondrial pathway and extrinsic apoptotic pathway.27 BAX and caspase-9 are TRAIL-induced apoptosis genes in mitochondrial pathway and caspase-8 is TRAIL-induced apoptosis gene in extrinsic apoptotic pathway. Fas-L and caspase-3 are shared by both mitochondrial pathway and extrinsic apoptotic pathway.28 These results indicated both the mitochondrial pathway and the extrinsic apoptotic pathway involved in rNDV-TRAIL or rNDV-IL-2-TRAIL-inducing apoptosis of B16-F10 and H22 cells.
Figure 4. rNDV inserted TRAIL enhanced induce-tumor apoptosis potency. (A–L) Regression analyzed the rNDVs-induced B16-F10 (A–F) and H22 (G–L) lines apoptosis. Expression of each gene was calculated relative to the expression of housekeeping gene β-actin and the results are expressed as the n-fold difference relative to β-actin. All the values are the mean and SD of triplicate samples One-way ANOVA revealed a significant effect. *P < 0.05, **P < 0.01, vs control.
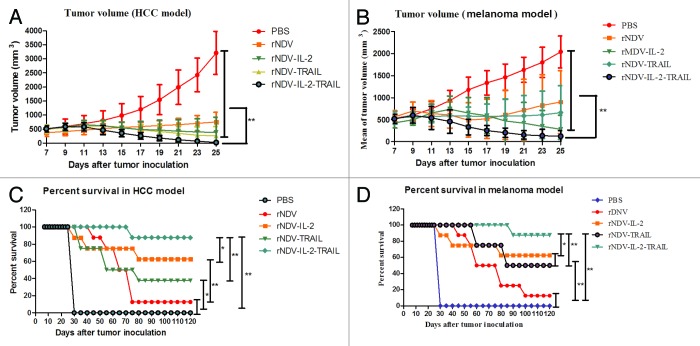
Recombinant NDVs inhibited tumor growth and prolonged animals’ survival
In order to prove the effects of rNDVs in cancer therapy, melanoma and HCC models were used to evaluate the anti-tumor efficacy of rNDVs. During the period of treatment, there were no several side effects except swelling at the injection site. We randomly selected 8/16 mice in each group to minor the tumor size. On day 25 after tumor implantation, all control (PBS group) mice in two models and 8 animals in virus-treated groups in melanoma and hepatic carcinoma models were sacrificed for further analysis. As shown in Figure 5A and B, the tumor size in the rNDVs-treated groups was significantly smaller than that in the PBS-treated group. Furthermore, Mice treated with rNDV-IL-2-TRAIL exhibited a significant change in tumor growth inhibition compared with rNDV, rNDV-IL-2 and rNDV-TRAIL. (The Student paired two-tailed t test revealed a significant effect, **P < 0.01.) This indicated rNDV-IL-2-TRAIL was more potent than other recombinant viruses in cancer therapy. As shown in Figure 5C and D, in the 120-d survival experiment, survival rate of mice in rNDV-IL-2-TRAIL-treated group in two tumor models was the highest. Also, mice in rNDV-IL-2-TRAIL-treated groups had a significant effect on the reduction of tumors with 7 of 8 mice undergoing complete regression in melanoma and HCC models compared with others experimental groups. The survival rate suggested that rNDV-IL-2-TRAIL will be an ideal drug for cancers therapy, which enhanced anti-cancer ability by combining virotherapy with immunotherapy.
Figure 5. rNDV-IL-2-TRAIL effectively suppressed tumor growth and prolonged animal’s survival. (A) Mean value of tumor volume in virus-treated and PBS-treated group in melanoma model. (B) Mean value of tumor volume in virus-treated and PBS-treated group in HCC model. (C) Survival of the melanoma model animals in 120 d period after treatment with the recombinant viruses. (D) Survival of the HCC model animals in 120 d period after treatment with the recombinant viruses. The tumor-bearing mice were sacrificed when the tumor volume developed to a significant size (diameter >18 mm). All the values are the mean and SD of triplicate samples. The Student paired two-tailed t test revealed a significant effect, *P < 0.05, **P < 0.01.
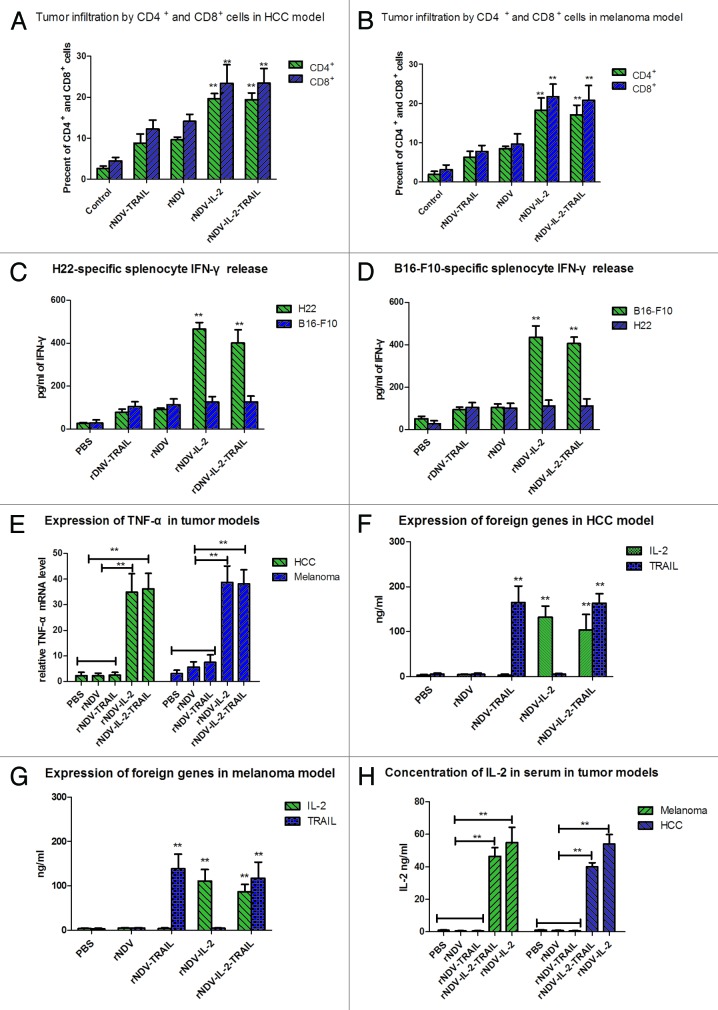
Tumors treated with rNDV-IL-2 and rNDV-IL-2-TRAIL resulted in immune cell accumulation
CD4+ and CD8+ T lymphocyte is the major cell types responding to IL-2 stimulation. IL-2 also stimulated proliferation, cytolytic activity, and cytokine secretion of T lymphocytes and natural killer cells. IL-2 was used for the generation of lymphokine-activated killer (LAK) cells from peripheral blood lymphocytes or for the expansion of tumor infiltrating lymphocytes (TIF) in cancer therapy. CTL responses play an important role in cancer therapy. In this study, we proceeded to analyze whether the rNDV-IL-2 or rNDV-IL-2-TRAIL-treated tumors demonstrated a higher level of immune cell infiltration to enhance CTL responses against tumor cells. As shown in Figure 6A and B, tumors from the animals treated with rNDV-IL-2 and rNDV-IL-2-TRAIL exhibited an increased number of both CD4+ and CD8+ cells compared with PBS, rNDV, rNDV-TRAIL groups (*P < 0.05, **P < 0.01). These results suggested that the IL-2 protein had a beneficial effect on the immune response against melanoma and hepatic carcinoma. Also, the expression level of INF-γ and TNF-α in spleen were measured as an indication of T-cell function. The results in Figure 6C–E suggested that treatment with rNDV-IL-2 or rNDV-IL-2-TRAIL exhibits an anti-tumor effect though stimulating immune system compared with PBS, rNDV, rNDV-TRAIL groups. Our results also indicated that IFN-γ release was tumor specific, because splenocytes from rNDV-IL-2 or rNDV-IL-2-TRAIL group in HCC (melanoma) model cultured with B16-F10 (H22) cells did not enhance IFN-γ release compared with control. These data suggested that IL-2 expressed by rNDV contributed strong immune response by LAK and TIF in rNDV-IL-2-TRAIL and rNDV-IL-2-treated mice. In order to assess potential toxicity associated with high-dose IL-2 administration in vivo, ELISA was performed to detect IL-2 and TRAIL expression in tumors in HCC and melanoma models. As shown in Figure 6F and G, IL-2 and TRAIL was >100 ng/mL in the tumors of rNDVs-infected mice. In addition, in consideration of high concentration of IL-2 was harmful for human beings and animals, we analyzed the concentration of human IL-2 in serum in tumor-bearing mice on day 25 after inoculation in vivo. The results in Figure 6H indicated that systemic IL-2 concentration in serum was not high and it could not induce toxicities in vivo during the period of experiments.
Figure 6. IL-2 enhanced immune responses against tumor cells in vivo. (A and B) Percent of the CD4+ and CD8+ cells on tumors from HCC (A) and melanoma (B) mice was analyzed by FACS. (C and D) Tumor-specific IFN-γ released from stimulated splenocytes in HCC (C) and melanoma model (D) was analyzed by ELISA. (E) Expression of TNF-α was examined in melanoma and HCC models animals. (F and G) TRAIL and IL-2 in tumor homogenates was analyzed in HCC (F) and melanoma (G) models. (H) Concentration of human IL-2 in serum was analyzed by ELISA in HCC and melanoma models. All the values are the mean and SEM of triplicate samples. One-way ANOVA revealed a significant effect. *P < 0.05, **P < 0.01, vs control.
Discussion
TRAIL is a member of the TNF superfamily, interacts with its functional death receptors, and induces apoptosis in a wide range of cancer cell types. Therefore, TRAIL has been considered as an attractive agent for cancer therapy. TRAIL induced apoptosis in a wide range of tumor cell lines but not normal cells, which make it another ideal oncolytic drug for anticancer therapy.29-31 Nevertheless, some studies have found that tumors easily build resistance to TRAIL-based therapies.32,33 Tumor cell resistance to TRAIL seemed to occur through the modulation of various molecular targets, including differential expression of death receptors, overexpression of anti-apoptotic molecules, mutations in apoptotic genes, defected in caspase signaling, and caspase inhibition in resistant cells.34,35 In this study, we expressed TRAIL delivered by rNDV to improve the effect of cancer therapy. The results indicated that rNDV-TRAIL was an efficient anti-neoplastic drug both in vitro and in vivo.
IL-2 showed a promise anti-tumor agent and demonstrated potential immunotherapeutic effects in a number of cancers. Previous studies have shown that IL-2 was an ideal anti-tumor drug for cancer therapy.4,25,36-42 Here we further studied that human IL-2 expressed by rLaSota was beneficial with regard to survival rate of the malignant melanoma and hepatic carcinoma models, which keeps consistency with previous results. And previous studies also have reported that the murine IL2 protein expressed by the recombinant mesogenic viruses increased the number of CD4+ and CD8+ T cells and survival rate in the tumor-bearing animals.4,7 Our results confirmed therapeutic effects of IL-2 expressed by mesogenic NDV and further analyzed in HCC and melanoma models.
In this study, we compared anti-tumor effects of rNDV-IL-2 with rNDV-TRAIL. Each of them had its own advantages in cancer therapy. rNDV-IL-2 induced T cell proliferation to enhance antitumor activity through tumor-reactive CD8+ T cells in vivo.6 rNDV-TRAIL could induce apoptosis of tumors cells by expressing TRAIL in tumor cells to enhance oncolytic effects. However, rNDV expressing IL-2 alone has limitations in oncolytic cancer cells, and rNDV expressing TRAIL alone also has imperfection in immunotherapy levels outcome. Therefore, we constructed and investigated the anti-tumor potential of the rNDV-IL-2-TRAIL.
The synergistic partners between IL-2 and TRAIL expressed by rNDV-IL-2-TRAIL opened a new way for cancer therapy. First, results in the HCC and melanoma models indicated that simultaneous expression of IL-2 and TRAIL significantly inhibited tumor growth. TRAIL did not negate tumor suppressor function of IL-2, but worked synergistically with IL-2 in vivo. Second, though systemic and local human IL-2 concentration was enough to enhance immunologic function without side effects. In addition, NDV lentogenic strain LaSota is safe and pathogenic free for man and animals.43 Third, TRAIL expressed by rNDV-IL-2-TRAIL elevated relevant apoptosis genes of Bax, FasL, caspase-8, caspase-9 and caspase-3 expression in tumor cells. And we found that anti-apoptotic protein Bcl-2 mRNA expression was increased after infected by rNDV-TRAIL and rNDV-IL-2-TRAIL. Bcl-2 can interfere or inhibit Fas mediated apoptosis signaling system.44,45 This may be the reason why parts of tumor cells resistance to apoptosis. Other data revealed that rLaSota showed tumor selective replication and had a monocyclic replication cycle in tumor cells.15 Therefore, lentogenic LaSota strain of NDV will be a better alternative for future clinical application.
In summary, NDV LaSota has been shown a safe and environmental-friendly virus, which fitting with a growing theme of safe drug. In this study, by integrating tumor-specific of NDV, immune regulation of IL-2 and inducing apoptosis of TRAIL for tumor cells, we have found that tumors were regressed greatly in 120-d survival of animals in rNDV-IL-2-TRAIL-treated mice. These results indicated that rNDV-IL-2-TRAIL will be a powerful anti-tumor agent in cancer therapy.
Materials and Methods
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Chinese Association For Laboratory Animal Sciences (CALAS), Animal Health Products, Committee on the Ethics of Animal Experiments Defense Research and Development, China, and Animal Experiments of the University of Northeast Agricultural (approval number: SCXK-2012-0002). All surgery and euthanasia were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Cell lines, viruses, and other reagents
rNDV, rNDV-IL2, rNDV-IL15, rNDV-TRAIL, and rNDV-IL-2-TRAIL viruses were grown in embryonated specific-pathogen-free (SPF) eggs. All viruses were propagated in DF-1 chicken fibroblasts cells (ATCC) with DMEM (BioWhittaker, Inc.) containing 10% FBS (Sigma) and 1% chicken allantoic fluid (AF). Mouse malignant melanoma cells (B16-F10 cells) were a kind gift from Dr Jiahuai Han (XiaMen University). H22 was provided by the Technology Center, Harbin Pharmaceutical Group, and primary chicken embryo fibroblasts were prepared from 9- to 11-d-old SPF embryos. BHK-21, A549 (human lung cancer cells), and CTLL2 T cells were purchased from ATCC. H22, B16-F10, and BHK-21 cells were maintained in DMEM supplemented with 10% FBS, 1% nonessential amino acids, and 1% sodium pyruvate. CT-26 (mice colon tumor cells), DF-1, and chicken embryo fibroblast cells were maintained in DMEM with 10% FBS, 1% penicillin/streptomycin. All cell lines were grown at 37 °C under 5% CO2. All cell cultures were regularly tested for mycoplasma contamination.
Construction of the recombinant NDV containing the IL-2, TRAIL, IL-2-TRAIL, or EGFP gene
The NDV LaSota strain was used as a backbone for construction of recombinant NDV cDNA clones. The IL-2 gene flanked by the NDV gene end and the gene star was cloned into pMD18-T vector (NEB) by PCR using the sense primer (5′-CGACGCGTTT AAGAAAAAAT GTACGGGTAG AACCCGCCAC CATGTACAGG ATGCAACTCC tgt-3′) and antisense primer (5′-CGACGCGTTA ATCAAGTCAG TGTTGAGATG ATGCT-3′). The resulting plasmid was named pMD-IL-2. The EGFP, TRAIL, and IL-2-TRAIL genes was cloned in to pMD18-T vector by same the method using the sense primer (5′-AGGCGCGCCT TAAGAAAAAA TACGGGTAGA ACCGCCACCA TGGTGAGCAA GGGCGAGGAG-3′), (5′-GTTAACTTAA GAAAAAATAC GGGTAGAACC GCCACCATGG AGACAGACAC ACTCCTGCT-3′), (5′-CGACGCGTTT AAGAAAAAAT GTACGGGTAG AACCCGCCAC CATGTACAGG ATGCAACTCCT GT-3′) and the antisense primer (5′-AGGCGCGCCT AACTTGTACA GCTCGTCCAT GCCGAG-3′), (5′-GTTAACTAAG CCAACTAAAA AGGCCCCGAA A-3′), (5′-GTTAACTAAG CCAACTAAAA AGGCCCCGAA A-3′), respectively. IL-2 and TRAIL genes were connected by the gene star and the gene end. The resulting plasmids were named pMD-EGFP, pMD-TRAIL, and pMD-IL-2-TRAIL. The EGFP, IL-2, TRAIL and IL-2-TRAIL genes were subcloned into the HN-L gene junction of rNDV at the Pme1 or Asc1 site, to generate the recombinant plasmids pBl-rNDV-EGFP, pBl-rNDV-IL-2, pBl-rNDV-TRAIL, and pBl-rNDV-IL-2-TRAIL, respectively. All plasmid sequences were verified by sequencing.
Rescue of recombinant viruses and virus titration
Rescue of rNDV, rNDV-TRAIL, rNDV-IL-2, rNDV-IL-2-TRAIL, and rNDV-EGFP viruses were performed as previously described.46 In brief, BHK-21 cells in six-well plates were co-transfected using Lipofectamine 2000 with 0.5 μg pBL-N, 0.25 μg pBL-P, 0.1 μg pBL-L, and 1 ug of the recombinant plasmids pBl-rNDV, pBl-rNDV-IL-TRAIL, pBl-rNDV-IL-2, pBl-rNDV-IL-2-TRAIL, or pBl-rNDV-EGFP (DNA: Lipofectamine¼ 2:3). Three days after transfection recombinant viruses were harvested from the supernatant of the transfected cells. Recombinant viruses were grown in 10-d-old embryonated SPF chicken eggs and purified as described previously.47 The rescued viruses were termed rNDV, rNDV-TRAIL, rNDV-IL-2, rNDV-IL-2-TRAIL, and rNDV-EGFP, respectively.
Viral titers were determined by plaque-formation assay using DF1 cells in a 24-well format. After attachment (2 h) of viruses to the cells at different multiplicity of infection (MOI), the inoculum was removed and replaced with 1% agarose-containing medium. Plaques were counted 48 h after infection.
MTT cytotoxicity assays
H22, B16-F10, A549, and CT-26 cells were infected with viruses at 0.1 MOI in 96-well plates for 24, 48, 72, and 96 h in triplicate for each condition, and PBS was added instead of the virus as a control. At each time point, 20 μL of MTT (5 mg/mL) in PBS solution was added to each well, the plate was further incubated for 4 h. Most of the medium was removed and 100 μL of DMSO (dimethylsulfoxide) was added into the wells to solubilize the crystals. Finally, the OD was measured by a BIO-RAD (ELISA) reader at wavelength of 450 nm.
ELISA
H22, B16-F10, A549, and CT-26 cells were infected with rNDV, rNDV-TRAIL, rNDV-IL-2, or rNDV-IL-2-TRAIL at a MOI of 0.1 and the infected supernatants were harvested 48 h later. The supernatants were diluted 1:10, 1:50, 1:250, and analyzed by ELISA for presence of foreign protein using Quantikine M kit (R&D Systems).
4.7 Induction of apoptosis in H22 and B16-F10 cells
H22 and B16-F10 cells were grown at 37 °C with 5% CO2 until 70% to 80% confluence was reached. Apoptosis was assessed by incubation these cells with rNDV-TRAIL or rNDV-IL-2-TRAIL (0.1 MOI) for 48 h. After incubation with the recombinant viruses, as described above, the cells were trypsinized and collected. The cells were then washed in cold PBS, adjusted to 1 × 106 cells/mL with PBS, labeled with annexin V-FITC and PI (Annexin V-FITC Kit, BD), and analyzed with a FACScan flow cytometer (BD). The treatments were performed in triplicate, and the percentage of labeled cells undergoing apoptosis in each group was determined and calculated.
Purification of IL-2 and TRAIL from supernatant of infected cells
DF-1 cells were infected with rNDV-IL-2, rNDV-TRAIL, and rNDV-IL-2-TRAIL at 0.1 MOI for 72 h, respectively. Supernatants were harvested after clarification in membranes (0.45 μm) and concentrated by tangential filtration (Millipore System, 10 kDa cassette). The IL-2 from rNDV-IL-2-infected DF-1 cells was partially purified by hydrophobic interaction chromatography and AcA 54 gel filtration. The IL-2 and TRAIL from rNDV-IL-2-TRAIL-infected DF-1 cells was purified by hydrophobic interaction chromatography, phenyl-Sepharose, DEAE Sephacel, and AcA 54 gel filtration. The TRAIL from rNDV-TRAIL-infected DF-1 cells was partially purified by hydrophobic interaction chromatography, DEAE Sephacel and gel filtration.
IFN-γ release assay
Spleens were removed from the euthanized animals. Splenocytes from the virus-treated and PBS-treated mice in two tumor models were isolated by passing the spleens through 100 μm nylon mesh filters. Cultured B16-F10 or H22 cells (5 × 105) were treated with 50 µg/mL of mitomycin C for 2 h at 37 °C to induce cell cycle arrest. After the treatment, the cells were washed with PBS and incubated with 1 × 107 splenocytes for 3 d in Roswell Park Memorial Institute medium with 10% fetal calf serum to assess for IFN-γ release.
Flow cytometry analysis
Cell suspensions from tumors of treated mice were prepared for flow cytometric analysis (FACS). Cell suspensions were filtered through a 100 μm nylon strainer. Tumor cells were labeled with CD3 and CD45 plus CD4 and CD8 staining to get the frequency of CD4 and CD8 cell subsets in total infiltrated immune cells. The stained cells were analyzed by using Becton Dickinson FACScan flow cytometer.
An apoptosis assay using flow cytometry was performed to detect B16-F10 and H22 cells apoptotic rates. After 24 h of incubation with the NDVs at a MOI of 0.1, as described above, the cells were trypsinized and collected. The cells were then washed in cold PBS, adjusted to 1 × 106 cells/mL with PBS, labeled with annexin V-FITC and PI (Annexin V-FITC Kit, BD), and analyzed with a FACScan flow cytometer (BD). The treatments were performed in triplicate, and the percentage of labeled cells undergoing apoptosis in each group was determined and calculated.
Cytokine-dependent proliferation assay
IL-2-dependent T (CTLL2) cells were used to analyze the biological activity of the integrated cytokine gene products release from the viral infected DF-1 cells. One day prior to the assay, the indicator cells were transferred to cytokine-free medium (BioWhittaker, Inc.) and cultured for 12 h. For the assay, the cells were harvested, washed three times with cytokine-free medium and adjusted to 2 × 105/mL. Two hundred microliters of cell suspension were added to a 96-well plate, which contained 20 ng IL-2 from the supernatants of infected DF-1 cells. The assay was performed in duplicate for each dilution. After 48 h at 37 °C, the proliferation index of CTLL2 was measured by MTT assay.
Preparation of LAK cells and suppression of B16-F10 and H22 cells by LAK cells
LAK cells were induced by culturing splenocytes in the presence of IL-2 as described previously.48 Briefly, spleens from C57BL/6 and Balb/c mice were aseptically excised and crushed into a single cell suspension with the end of a syringe. The cell suspension was filtered through nylon mesh and centrifuged. Erythrocytes were lysed in a buffered ammonium chloride solution. After washing twice with cytokine-free medium, the cells were transferred to 24-well plates at a concentration of 5 × 106 cells/mL in cytokine-free medium and stimulated with 20 ng of the human IL-2 from the supernatants of rNDV-IL-2-infected tumor cells. The 24-well plates were incubated at 37 °C in 5% CO2 for 3 d. The cells were then harvested, washed in PBS and used as LAK cells. A total of 2 × 104/mL H22 and B16-F10 cells were co-cultured with the LAK cells at ratios of 5:1, 10:1, 20:1, and 40:1 in 96-well plates containing 20 μL AF for 72 h in triplicate for each condition and lymphocytes and PBS were used instead of the virus as control. The antitumor effects of the LAK cells were determined by LDH release assays.
LDH release assays
The media were removed and the cells were washed with 1 mL of PBS. Cells were subsequently incubated with 1% Triton X-100 at 37 °C for 30 min. LDH activity in the lysates was determined using the Promega CytoTox 96 assay kit, according to the manufacturer's instructions. Briefly, 50 μL of cell lysates were incubated with 50 μL of assay reagent and the absorbance was recorded at 490 nm.
Analysis of apoptosis using reverse transcription and quantitative real-time PCR
Total RNA from H22 and B16-F10 cells (infected with rNDV-TRAIL or rNDV at MOI of 0.1) was extracted using the TRIZOL (Invitrogen) reagent. Chloroform was then added (200 μL for each 1 mL TRIZOL) and the samples were centrifuged at high speed for 15 min at 4 °C. The aqueous layer was then transferred into a new tube and RNA was precipitated with iso-propanol followed by one wash using 70% ethanol. The RNA precipitate was then dissolved in 10–15 μL of RNase free water and analyzed for quantity and quality using a spectrophotometer. Total RNA was reverse transcribed using the GeneAmp kit (Applied Biosystems; ABI). Twenty nanograms of the resulting cDNA was then used in the real-time PCR step. Six genes were tested by real-time PCR including. All real-time PCR assays were performed in triplicate in a 96-well plate using the 7900 Sequence Detector System (ABI) according to the manufacturer's protocol. Data analysis was performed using the Sequence Detector System (SDS) software (ABI) and the results were expressed as fold-change in relative mRNA expression level, calculated using the ΔCt method with β-actin (ACTB) as the reference gene and the non-treated cells as baseline.
Animal experiments
Six- to eight-week-old female C57BL/6 and Balb/c mice were purchased from the West China Experimental Animal Center of Sichuan University. Mice were permitted 1 wk to acclimate to their environment before manipulation. All surgical procedures were completed in accordance with the guidelines on the care and use of laboratory animals for research purposes by the West China Hospital Cancer Center's Animal Care and Use Committee. Mice of C57BL/6 were inoculated with 5 × 107 B16-F10 cells in the right footpad and Balb/c mice were inoculated with 5 × 106 hepatic carcinoma cells in the right inguinal. Primary tumors usually became palpable on day 5–6 and with an average diameter of 6–8 mm. On day 7, the tumor-bearing mice in two modes were randomly and respectively assigned into 6 groups and each group contained 16 mice. Each mouse in virus-treated group received 5 × 107 pfu recombinant Newcastle disease viruses (rNDVs) injection on days 7, 9, 11, 13, and 15 for a total of 5 times.7 The mice in the control groups received normal saline, serving as injection control. The details of the treatment were described previously.49 Tumor dimensions were measured with calipers every 2 d for a total of 10 times. The tumor volumes were calculated according to the following formula: length × width2 × 0.52. On day 25 after innoculation, tumors, brain, liver, heart, and spleen in mice in two tumor models were used to HA assay.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by National Natural Science Foundation of China (NSFC, 31200121), Heilongjiang Funds for Young Scientists (QC2012C099), and Northeast Agriculture University Funds for PhD (2012RCB43).
References
- 1.Sinkovics JG, Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16:1–15. doi: 10.1016/S1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 2.Song KY, Wong J, Gonzalez L, Sheng G, Zamarin D, Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J Mol Med (Berl) 2010;88:589–96. doi: 10.1007/s00109-010-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sui H, Bai Y, Wang K, Li X, Song C, Fu F, Zhang Y, Li L. The anti-tumor effect of Newcastle disease virus HN protein is influenced by differential subcellular targeting. Cancer Immunol Immunother. 2010;59:989–99. doi: 10.1007/s00262-010-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, Martínez-Sobrido L, Woo SL, García-Sastre A. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–92. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 5.Wei D, Sun N, Nan G, Wang Y, Liu HQ, Peeters B, Chen ZN, Bian H. Construction of recombinant Newcastle disease virus Italien strain for oncolytic virotherapy of tumors. Hum Gene Ther. 2012;23:700–10. doi: 10.1089/hum.2011.207. [DOI] [PubMed] [Google Scholar]
- 6.Zamarin D, Martínez-Sobrido L, Kelly K, Mansour M, Sheng G, Vigil A, García-Sastre A, Palese P, Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamarin D, Vigil A, Kelly K, García-Sastre A, Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Janke M, Fournier P, Schirrmacher V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 2008;136:75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Peeters BP. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J Gen Virol. 2003;84:781–8. doi: 10.1099/vir.0.18884-0. [DOI] [PubMed] [Google Scholar]
- 10.Altomonte J, Marozin S, Schmid RM, Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol Ther. 2010;18:275–84. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A, Villar E, García-Sastre A, Palese P. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001;75:11868–73. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigil A, Martinez O, Chua MA, García-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16:1883–90. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morán IS, Cuadrado-Castano S, Barroso IM, Kostetsky EY, Zhadan G, Gómez J, Shnyrov VL, Villar E. Thermal stability of matrix protein from Newcastle disease virus. Int J Biol Macromol. 2013;61:390–5. doi: 10.1016/j.ijbiomac.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Fábián Z, Csatary CM, Szeberényi J, Csatary LK. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J Virol. 2007;81:2817–30. doi: 10.1128/JVI.02490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy S, Takimoto T, Scroggs RA, Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80:5145–55. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter RJ, Attar BM, Rafiq A, Delimata M, Tejaswi S. Two avirulent, lentogenic strains of Newcastle disease virus are cytotoxic for some human pancreatic tumor lines in vitro. JOP. 2012;13:502–13. doi: 10.6092/1590-8577/977. [DOI] [PubMed] [Google Scholar]
- 17.Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schütz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–10. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XD, Franco AV, Nguyen T, Gray CP, Hersey P. Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol. 2000;164:3961–70. doi: 10.4049/jimmunol.164.8.3961. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–61. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–53. [PubMed] [Google Scholar]
- 21.Qiu B, Sun X, Zhang D, Wang Y, Tao J, Ou S. TRAIL and Paclitaxel Synergize to Kill U87 Cells and U87-Derived Stem-Like Cells in Vitro. Int J Mol Sci. 2012;13:9142–56. doi: 10.3390/ijms13079142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 23.Boytim ML, Lilly P, Drouvalakis K, Lyu SC, Jung R, Krensky AM, Clayberger C. A human class II MHC-derived peptide antagonizes phosphatidylinositol 3-kinase to block IL-2 signaling. J Clin Invest. 2000;105:1447–53. doi: 10.1172/JCI8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. doi: 10.1089/cbr.2010.0902. [DOI] [PubMed] [Google Scholar]
- 25.Janke M, Peeters B, Zhao H, de Leeuw O, Moorman R, Arnold A, Ziouta Y, Fournier P, Schirrmacher V. Activation of human T cells by a tumor vaccine infected with recombinant Newcastle disease virus producing IL-2. Int J Oncol. 2008;33:823–32. [PubMed] [Google Scholar]
- 26.Bai F, Niu Z, Tian H, Li S, Lv Z, Zhang T, Ren G, Li D. Genetically engineered Newcastle disease virus expressing interleukin 2 is a potential drug candidate for cancer immunotherapy. Immunol Lett. 2014;159:36–46. doi: 10.1016/j.imlet.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava RK. Intracellular mechanisms of TRAIL and its role in cancer therapy. Mol Cell Biol Res Commun. 2000;4:67–75. doi: 10.1006/mcbr.2001.0265. [DOI] [PubMed] [Google Scholar]
- 28.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–33. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 29.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 30.Griffith TS, Fialkov JM, Scott DL, Azuhata T, Williams RD, Wall NR, Altieri DC, Sandler AD. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–9. [PubMed] [Google Scholar]
- 31.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 32.Kuijlen JM, Bremer E, Mooij JJ, den Dunnen WF, Helfrich W. Review: on TRAIL for malignant glioma therapy? Neuropathol Appl Neurobiol. 2010;36:168–82. doi: 10.1111/j.1365-2990.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 33.Mora R, Abschuetz A, Kees T, Dokic I, Joschko N, Kleber S, Geibig R, Mosconi E, Zentgraf H, Martin-Villalba A, et al. TNF-alpha- and TRAIL-resistant glioma cells undergo autophagy-dependent cell death induced by activated microglia. Glia. 2009;57:561–81. doi: 10.1002/glia.20785. [DOI] [PubMed] [Google Scholar]
- 34.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–56. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 36.Dreicer R, Stadler WM, Ahmann FR, Whiteside T, Bizouarne N, Acres B, Limacher JM, Squiban P, Pantuck A. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs. 2009;27:379–86. doi: 10.1007/s10637-008-9187-3. [DOI] [PubMed] [Google Scholar]
- 37.Acres B. Cancer immunotherapy: phase II clinical studies with TG4010 (MVA-MUC1-IL2) J BUON. 2007;12(Suppl 1):S71–5. [PubMed] [Google Scholar]
- 38.Ko YJ, Bubley GJ, Weber R, Redfern C, Gold DP, Finke L, Kovar A, Dahl T, Gillies SD. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27:232–9. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E, Krzakowski M, Hess D, Tartour E, Chenard MP, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 40.Schwager K, Hemmerle T, Aebischer D, Neri D. The Immunocytokine L19-IL2 Eradicates Cancer When Used in Combination with CTLA-4 Blockade or with L19-TNF. J Invest Dermatol. 2013;133:751–8. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 41.Coventry BJ, Ashdown ML. The 20th anniversary of interleukin-2 therapy: bimodal role explaining longstanding random induction of complete clinical responses. Cancer Manag Res. 2012;4:215–21. doi: 10.2147/CMAR.S33979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oguariri RM, Dai L, Adelsberger JW, Rupert A, Stevens R, Yang J, Huang D, Lempicki RA, Zhou M, Baseler MW, et al. Interleukin-2 (IL-2) Inhibits HIV-1 Replication in Some HTLV-1-Infected Cell Lines via the Induction and Incorporation of APOBEC3G into the Virion. J Biol Chem. 2013;288:17812–22. doi: 10.1074/jbc.M113.468975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9:495–8. doi: 10.3748/wjg.v9.i3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–7. [PubMed] [Google Scholar]
- 45.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 46.Krishnamurthy S, Huang Z, Samal SK. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology. 2000;278:168–82. doi: 10.1006/viro.2000.0618. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Krishnamurthy S, Panda A, Samal SK. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J Virol. 2003;77:8676–85. doi: 10.1128/JVI.77.16.8676-8685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunji Y, Vujanovic NL, Hiserodt JC, Herberman RB, Gorelik E. Generation and characterization of purified adherent lymphokine-activated killer cells in mice. J Immunol. 1989;142:1748–54. [PubMed] [Google Scholar]
- 49.He QM, Wei YQ, Tian L, Zhao X, Su JM, Yang L, Lu Y, Kan B, Lou YY, Huang MJ, et al. Inhibition of tumor growth with a vaccine based on xenogeneic homologous fibroblast growth factor receptor-1 in mice. J Biol Chem. 2003;278:21831–6. doi: 10.1074/jbc.M300880200. [DOI] [PubMed] [Google Scholar]