Cilia are cell protrusions containing an axoneme of microtubules. There are two types of cilia: non-motile cilia which mediate signaling perception, and motile cilia which are required for cell motility and extracellular fluid movement. Mucociliary epithelia contain multiciliated cells (MCCs) that project hundreds of motile cilia from their apical surface. These cilia beat in a coordinate fashion, generating a directional transport of fluids and mucus produced by neighboring secretory cells. In the airway, a mucociliary epithelium lines the trachea, bronchi, and nasal cavities, constituting a first-line defense against infections by removing inhaled particles and pathogens from the respiratory tract.
Several recent studies have revealed a role of miR-34/449 microRNAs (miRNAs) in motile ciliogenesis of vertebrate MCCs.1-3 miRNAs are small, non-coding RNAs that regulate diverse cellular processes through post-transcriptional repression of many target mRNAs. In some cell types, homologous miRNAs with conserved seed sequences can collectively constitute a significant portion of expressed miRNAs, and act redundantly to confer functional robustness. The evolutionarily conserved miR-34/449 miRNAs represent such a miRNA family with extensive genomic redundancy and predominant expression pattern in MCCs. The miR-34/449 miRNA family consists of six homologous miRNAs from three genomic loci, which collectively constitute the most highly enriched miRNAs in mammalian airway MCCs, as well as in the Xenopus embryonic epidermis.1,2 Complete miR-34/449 deficiency in mice results in defective motile ciliogenesis in MCCs, causing airway infections as well as infertility, thereby resembling a subset of symptoms seen in human primary ciliary dyskinesia (PCD) patients.1 Interestingly, the effect of miR-34/449 on motile cilia is tissue specific, as left-right axis asymmetry is normal and hydrocephaly does not occur in miR-34/449 deficient mice and frogs.1,2
Impaired ciliogenesis caused by miR-34/449 deficiency is largely due to aberrant maturation and docking of basal bodies, which normally attach to the apical membrane of MCCs and act as microtubule-organizing centers to nucleate axonemes.1 A key target of miR-34/449 miRNAs is Cp110, a centriolar protein that needs to be removed from centrioles to facilitate centriole-to-basal body transition, and which was previously described to inhibit cilia formation in monociliated cells.4 Upregulation of Cp110 levels in MCCs, due to either miR-34/449 deficiency or cp110 overexpression, also inhibits ciliogenesis by impairing basal body maturation and docking to the apical membrane of MCCs.1 Furthermore, cp110 knockdown in miR-34/449-deficient MCCs rescues basal body maturation and docking, and subsequently restores ciliogenesis.1
The clinical relevance of miR-34/449 extends beyond ciliogenesis in vertebrate model organisms. Among other miRNAs, a reduction in miR-34/449 expression was correlated with exposure of airway cells to cigarette smoke, which is the most common cause of chronic obstructive pulmonary disease. Furthermore, downregulation of all miR-34/449 miRNAs occurs in airway epithelial cells of asthma patients, when compared with healthy specimens.5 This miR-34/449 reduction could contribute to the impaired motile cilia function in both conditions. Interestingly, the asthma phenotype also includes mucus metaplasia, i.e., a decrease in MCCs accompanied by an increase in secretory cells.5 An important signal that regulates the cell-type composition in mucociliary epithelia is the Notch pathway, which contains multiple components regulated by miR-34/449 miRNAs in a cell type- and context-dependent manner.2,5 Although no obvious cell fate specification defects were observed in miR-34/449 deficient mucociliary epithelia during normal development,1,2 it would still be interesting to determine if miR-34/449 downregulation induced by inflammation in asthma patient could act on airway epithelial cell specification.
Importantly, the connection between miR-34/449 miRNAs and motile cilia in MCCs is evolutionary conserved: ciliation defects caused by miR-34/449 deficiency occur in MCCs of the murine airway and fallopian tube, the embryonic epidermis of Xenopus,, and the multiciliated pronephros and nasal pits of zebrafish embryos.1-3 In most cases, miR-34/449 miRNAs were shown to be required for basal body docking to the apical membrane, demonstrating an evolutionary conserved cellular function throughout the vertebrates. Furthermore, the connection between ciliogenesis in MCCs and miR-34/449 miRNAs likely extends beyond vertebrates, as miR-34 expression is also found in MCCs of sea urchin and worm larvae.6
Similar to miR-34/449 miRNAs in MCCs, miR-129 negatively regulates Cp110 abundance and ciliogenesis in motile monociliated cells of the zebrafish Kupffer's vesicle that are important for left-right axis laterality,4 indicating cell-type specific miRNA regulation of cp110 during ciliogenesis. In addition, the miR-183 family is expressed in another type of ciliated cells, namely ciliated neurosensory cells, which contain non-motile sensory cilia in vertebrates and are found in the inner ear, the retina, and the lateral line organ.7 Expression of miR-183 family members is also found in ciliated neurosensory cells of non-vertebrate deuterostomes (sea urchins and acorn worms) and protostomes (Drosophila and C. elegans), suggesting a conserved function for this miRNA family in another class of ciliated cells.7
Taken together, miRNAs constitute important components of highly conserved pathways that regulate different types of specialized ciliated cells in animals. miR-34/449 and miR-129 regulate ciliogenesis of motile cilia in MCCs and monociliated cells, respectively; and miR-183 being associated with ciliated neurosensory cells containing non-motile primary cilia. Future studies will reveal additional mechanistic details of miRNA function in ciliated tissues and further contribute to our understanding of post-transcriptional control mechanisms in cilia-dependent animal development and human disease. (Fig. 1)
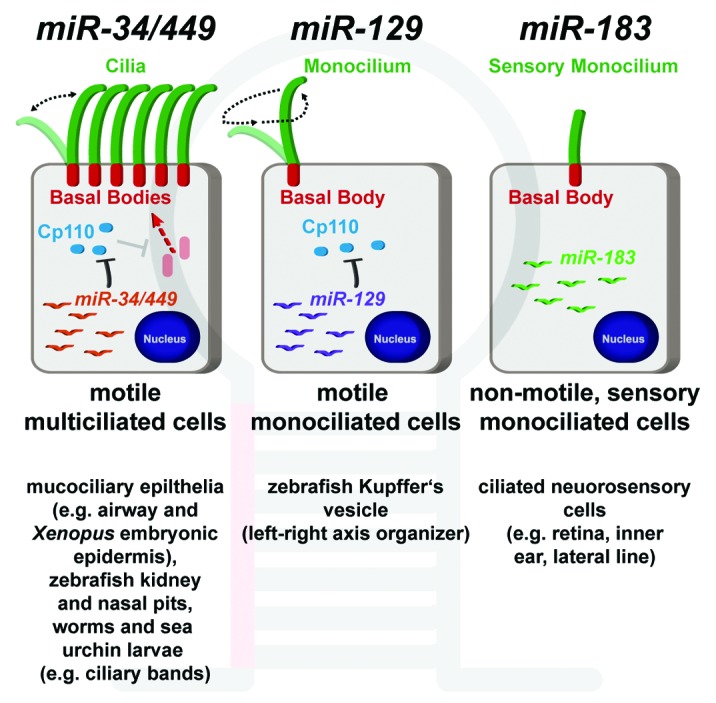
Figure 1. Specific expression of microRNAs in ciliated cell types. The miR-34/449 family is expressed in mucociliary multiciliated cells, where it inhibits cp110 to promote ciliogenesis of motile cilia (left). miR-129 is required for inhibition of cp110 and ciliogenesis of motile mono-cilia (middle). The miR-183 family is expressed in non-motile ciliated neurosensory cells, but their precise role is not elucidated yet (right).
Song R, et al. Nature. 2014;510:115–20. doi: 10.1038/nature13413.
References
- 1.Song R, et al. Nature. 2014;510:115–20. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcet B, et al. Nat Cell Biol. 2011;13:694–701. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, et al. Development. 2013;140:2755–64. doi: 10.1242/dev.092825. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, et al. Nat Cell Biol. 2012;14:697–706. doi: 10.1038/ncb2512. [DOI] [PubMed] [Google Scholar]
- 5.Solberg OD, et al. Am J Respir Crit Care Med. 2012;186:965–74. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christodoulou F, et al. Nature. 2010;463:1084–8. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce ML, et al. Evol Dev. 2008;10:106–13. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


