Abstract
Downregulation or loss of α-catenin occurs in multiple human cancer types. The traditional view of α-catenin is that it is one of the core components of the E-cadherin-catenin complex and is required for maintaining the integrity of the intercellular adherens junction, a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. Therefore, loss of α-catenin can result in loss of cell-cell adhesion, a common characteristic of cancer cells. There is an emerging recognition; however, that α-catenin also regulates multiple signaling pathways independent of adherens junctions. For instance, α-catenin functions as a tumor suppressor in E-cadherin-negative basal like breast cancer cells by inhibiting NF-κB signaling. In this perspective, we discuss the role and mechanisms of α-catenin in regulating several signaling pathways in cancer.
Keywords: Hedgehog, NF-kB, YAP, cancer, tumor suppressor, α-catenin, β-catenin
Introduction
The cadherin-catenin protein complex forms the adherens junction between neighboring cells, which is critical for maintaining cell-cell adhesion, cellular polarity and tissue organization.1 E-cadherin is a transmembrane protein that forms the core of the adherens junction, and its cytoplasmic tail interacts with p120 catenin and β-catenin. β-catenin in turn binds to α-catenin which connects adherens junctions to actin filaments (Fig. 1).
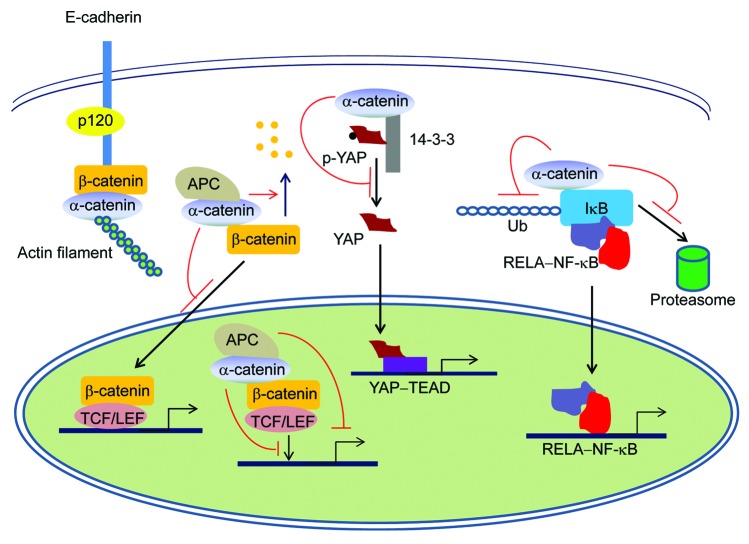
Figure 1. α-catenin signaling in cancer. In addition to maintaining the integrity of the cadherin-catenin complex and linking adherens junctions to the actin cytoskeleton, α-catenin can: (1) inhibit the Wnt/β-catenin pathway by preventing β-catenin nuclear translocation or formation of the β-catenin-TCF-DNA complex, by promoting β-catenin degradation, or by recruiting APC to the β-catenin-TCF complex; (2) regulate the Hippo-YAP pathway by blocking YAP dephosphorylation and nuclear localization; and (3) suppress the NF-κB pathway by inhibiting IκB ubiquitination and its association with the proteasome, in a tissue type-specific manner. The Hedgehog pathway is not shown, as it is unknown how this pathway is regulated by α-catenin.
While adherens junctions are abundant in normal epithelia, disruption or loss of the cadherin-catenin protein complex occurs commonly in human cancers and plays important roles in tumorigenesis and malignant progression. For instance, mutations in CDH1 (encoding E-cadherin) are found in hereditary gastric cancer,2 and loss of E-cadherin induces epithelial-mesenchymal transition and promotes breast tumor invasion and metastasis.3,4 A common gene alteration in colon cancer is mutation of the APC tumor suppressor,5 which results in stabilization and nuclear translocation of β-catenin. β-catenin, which links adherens junctions to the Wnt signaling pathway, promotes tumorigenesis and metastasis in many cancers.6,7 Moreover, p120 catenin has been shown to stimulate lung cancer cell proliferation.8
Owing to the important roles of E-cadherin and β-catenin in cancer, many studies have been focused on these two components of the adherens junction. However, α-catenin, the bridge between adherens junctions and actin filaments, received less attention in the field of cancer. Here we summarize recent progress on the role and mechanisms of α-catenin in tumorigenesis and discuss future directions.
α-catenin is a Tumor Suppressor in Human Cancer
Although α-catenin is less studied in cancer compared with E-cadherin and β-catenin, it has been recognized that α-catenin expression is downregulated in multiple cancers. In the early 1990s, loss of α-catenin in tumor cells was first reported in a human lung cancer cell line, PC-9. Despite abundant expression of E-cadherin, this cell line exhibits impaired cell-cell adhesion, which is likely to be a result of homozygous deletion of the α-catenin gene, CTNNA1.9 Subsequently, downregulation or loss of α-catenin was found in many other cancer cell lines or primary tumor tissues,10 including myeloid leukemia,11,12 breast cancer,13-15 colon cancer,16 lung cancer,17 and prostate cancer,18 which correlated with high tumor grade, metastasis and poor survival.16,17,19 Loss of α-catenin expression in these tumors results from multiple reasons, including chromosomal deletion,11,13 DNA methylation,12 gene mutation,15 and modulation of expression by ischemic microenvironment.20
In addition to these expression analyses, a growing body of evidence has demonstrated a functional role for α-catenin in cancer. For instance, overexpression of α-catenin in glioma cells inhibited cell proliferation, migration and invasion.21 Restoration of α-catenin in HL-60 cells, a myeloid leukemia cell line lacking α-catenin expression, resulted in reduced proliferation and apoptotic cell death.22 Through loss-of-function and gain-of-function analyses, our laboratory identified α-catenin as a tumor suppressor in basal-like breast cancer.23 Moreover, mice with tissue-specific deletion of α-catenin in the skin developed squamous cell carcinoma.24,25 These findings suggest that α-catenin has a tumor-suppressing role.
α-catenin signaling in cancer
It is widely accepted that loss of α-catenin leads to loss or reduction of cell-cell adhesion, a common characteristic of cancer cells. Thus, α-catenin may inhibit tumor formation and progression by maintaining the integrity of cadherin-catenin protein complexes. However, recent studies by us and others have revealed other mechanisms by which α-catenin suppresses tumorigenesis.
α-catenin suppresses Wnt/β-catenin signaling
β-catenin is a key molecule that links adherens junctions to the Wnt/Wingless signaling pathway.26 In the presence of Wnt ligands, the phosphorylation of β-catenin by glycogen synthase kinase (GSK)-3 is inhibited, leading to reduced ubiquitination-dependent degradation of β-catenin. Subsequently, stabilized β-catenin enters the nucleus, binds to the TCF/LEF-1 transcription factor and activates the transcription of target genes involved in cell proliferation,27 migration and invasion. The Wnt/β-catenin signaling pathway has been shown not only to promote tumor formation and progression in multiple cancers,28,29 including colorectal cancer,7,30,31 lung cancer32 and endometrial cancer,33,34 but also to regulate drug resistance.35
Since α-catenin directly binds to β-catenin, it is tempting to hypothesize that α-catenin modulates β-catenin signaling. Indeed, ectopic expression of α-catenin in EGFR-overexpressing U87 glioma cells promoted cytoplasmic retention of β-catenin, while shRNA-mediated depletion of α-catenin induced β-catenin nuclear translocation even without EGF treatment.21 In addition, Giannini et al. found that α-catenin is present in the nuclei of SW480 and DLD-1 colon cancer cells.36 An α-catenin-deficient clonal variant of DLD-1 cells exhibited increased β-catenin-TCF transcriptional activity, which could be repressed by ectopic expression of α-catenin fused to a nuclear localization signal.36 Interestingly, this inhibitory effect of nuclear α-catenin on β-catenin signaling is not due to interruption of the β-catenin-TCF protein complex, but is caused by disruption of the interaction between the β-catenin-TCF protein complex and DNA.36 Recently, Choi et al. reported that α-catenin directly interacts with the catenin inhibitory domain (CID) of the adenomatous polyposis coli (APC) tumor suppressor and promotes β-catenin ubiquitination and degradation by stabilizing its binding to APC.37 On the other hand, the interaction of α-catenin with APC leads to nuclear translocation of APC and formation of a protein complex consisting of APC:α-catenin:β-catenin and the CtBP:CoREST:LSD1 histone H3K4 demethylase, which inhibits the transcription of Wnt target genes.37 Interestingly, tyrosine phosphorylation of α-catenin at Y177 disrupts its interaction with APC but not β-catenin and releases the transcriptional repression of Wnt target genes.37 These experiments demonstrate that α-catenin can suppress Wnt signaling by promoting β-catenin degradation, by hindering nuclear translocation of β-catenin, by preventing formation of the β-catenin-TCF-DNA complex, or by recruiting APC to the β-catenin-TCF complex (Fig. 1). Whether inhibition of β-catenin signaling mediates the tumor-suppressing effect of α-catenin in vivo warrants further investigation.
α-catenin inhibits NF-κB signaling
The nuclear factor κB (NF-κB) signaling pathway plays critical roles in multiple processes, including cell proliferation, survival, apoptosis, angiogenesis, migration, invasion and immune response, and hyperactivation of NF-κB signaling has been observed in many cancers, including both hematological malignancies and solid tumors.38 NF-κB is a family of transcription factors consisting of five subunits: NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA (p65), cRel, and RelB, which form homodimers or heterodimers. Under non-stimulated conditions, NF-κB dimmers stay predominantly in the cytoplasm due to their association with the inhibitor of NF-κB (IκB) and are transcriptionally inactive. A variety of cytokines, growth factors and kinases can activate NF-κB signaling through either the canonical pathway or the non-canonical pathway. Usually, pro-inflammatory cytokines and viral infections activate the canonical NF-κB pathway whereby the activated IκB kinase (IKK) complex phosphorylates IκB, leading to its ubiquitination and degradation. Subsequently, NF-κB dimmers, including RelA-p50 and cRel-p50, enter the nucleus and regulate gene transcription.39 The non-canonical pathway is mainly activated by members of the tumor necrosis factor (TNF) family. Upon activation, p100, the main inhibitor of RelB, is phosphorylated by IKK and subsequently processed into the mature form, p52. The RelB-p50 and RelB-p50 dimmers are then translocated into the nucleus and activate the transcription of specific genes.38
Conditional deletion of α-catenin in the mouse skin (K14-Cre/α-cateninfl/fl) led to dramatic defects in the skin and limbs and early death.40 Grafting E18.5 α-catenin-knockout skins onto the back of nude mice resulted in squamous cell carcinomas within 40–70 d,24 suggesting a tumor-suppressing role of α-catenin in the skin. Microarray profiling of the epidermis from E18.5 α-catenin-knockout mice revealed upregulation of genes involved in cell survival, growth, migration and invasion, and interestingly, a number of NF-κB target genes were found to be upregulated early after α-catenin deletion.24 However, how depletion of α-catenin activates NF-κB signaling in the skin and whether the NF-κB pathway plays a causal role in skin tumorigenesis were not determined.
Constitutive activation of the NF-κB pathway is frequently observed in triple-negative basal-like breast cancer cells,41 but the cause of this activation was elusive. Recently, our laboratory found that α-catenin acts as a tumor suppressor in E-cadherin-negative basal-like breast cancer by interacting with and stabilizing IκB, which provided a molecular mechanism by which α-catenin regulates NF-κB signaling. In order to determine E-cadherin-independent functions of α-catenin, we performed both loss-of-function and gain-of-function analyses of α-catenin in multiple breast cancer cell lines.23 We found that ectopic expression of α-catenin inhibited breast cancer cell proliferation, while knockdown of α-catenin promoted cell proliferation, migration and anchorage-independent growth. Interestingly, α-catenin suppresses NF-κB signaling specifically in E-cadherin-negative basal-like breast cancer cells, but not in luminal cells or E-cadherin-positive basal-like breast cancer cells.23
Furthermore, biochemical analyses demonstrated that α-catenin physically interacts with IκB protein and stabilizes IκB by blocking its lysine 48-linked poly-ubiquitination and its association with the proteasome, leading to cytoplasmic retention of RelA and p50 and upregulation of multiple NF-κB target genes, without affecting the activity of IKK23 (Fig. 1). Co-immunoprecipitation assays showed that α-catenin binds to IκBα in E-cadherin-negative cell lines, but not in E-cadherin-positive cell lines. In addition, although β-catenin could be detected in α-catenin immunoprecipitates from basal-like breast cancer cells, overexpression or knockdown of α-catenin did not affect β-catenin protein level, localization or activity.23 Therefore, α-catenin regulates different signaling pathways in different cell types.
Does NF-κB signaling mediate the effect of α-catenin on tumorigenesis? We found that knockdown of α-catenin in two basal-like breast cancer cells lines, MDA-MB-231 and BT549, induced anchorage-independent growth in vitro and tumor growth in vivo, which could be reversed by knockdown of RelA. Immunohistochemical staining revealed upregulation of TNFα and downregulation of IκB in tumors formed by α-catenin-depleted MDA-MB-231 cells, confirming that loss of α-catenin can lead to activation of NF-κB signaling in vivo. On the other hand, restoring α-catenin in an α-catenin-null basal-like breast cancer cell line, MDA-MB-157, inhibited tumorigenesis in xenografted mice, which could be reversed by knockdown of IκB.23 Taken together, these data suggest that inhibition of NF-κB signaling mediates the tumor-suppressing effect of α-catenin in basal-like breast cancer cells; this is highly relevant in human tumors, as α-catenin is specifically downregulated in human basal-like breast cancer due to DNA hypermethylation, correlates with relapse-free survival and is negatively associated with the activity of the NF-κB pathway.23
α-catenin regulates the Hippo-YAP pathway
The Hippo-YAP pathway is involved in organ size regulation, and deregulation of this pathway plays critical roles in tumor formation and progression. For example, genetic deletion of Mst1/242-45 or transgenic overexpression of Yap46,47 in mice has been shown to increase liver size and ultimately induce hepatocellular carcinoma, demonstrating a pivotal role of Hippo signaling in organ growth and tumorigenesis. In addition, YAP overexpression promotes colon cancer cell proliferation, probably by synergizing with Wnt/β-catenin signaling.48 Moreover, recent studies by us and others have demonstrated that the downstream effector of mammalian Hippo signaling, YAP, functions as a promoter of breast tumorigenesis and metastasis.49-52
A conserved Hippo kinase cascade is established in Drosophila and mammals: the mammalian Hippo homolog MST1/2 phosphorylates and activates LATS1/2, and the LATS kinases in turn phosphorylate YAP and TAZ, leading to cytoplasmic retention and functional inactivation of these 2 transcriptional co-activators.53 Deregulation of Hippo signaling is associated with a wide range of human cancers, including liver, lung, and colorectal cancer.54
Genetic deletion of α-catenin in the hair follicle stem and progenitor cells (GFAP-Cre/α-cateninfl/fl) led to development of skin squamous cell carcinoma in mice.25 Specifically, GFAP-Cre/α-cateninfl/fl mice exhibited skin inflammation and tumors with squamous cell differentiation, a phenotype similar to that of the mice with transgenic overexpression of Yap in the skin,55 and concurrent deletion of the tumor suppressor p53 in GFAP-Cre/α-cateninfl/fl mice accelerated skin tumor formation.25 Interestingly, an siRNA screen suggested that YAP may be a downstream effector of α-catenin.25 In support of this notion, the proliferation of α-catenin-deficient cells was suppressed by knockdown of YAP.25 Consistently, α-catenin promotes cytoplasmic sequestration of YAP and inhibits its transcriptional activity. The regulation of YAP subcellular localization by α-catenin is not due to modulation of the canonical Hippo kinase cascade, but is mediated by the interaction between α-catenin and YAP.25,55 Moreover, the association of α-catenin with YAP is mediated by 14-3-3, and this physical association prevents YAP dephosphorylation and activation55 (Fig. 1). These findings suggest that loss of α-catenin may lead to skin tumorigenesis by promoting nuclear localization and transcriptional activity of YAP. Although this effect was not found in basal-like breast cancer cells,23 it remains to be determined whether α-catenin regulates YAP localization and activity in other epithelial cancers, such as liver, lung and colon cancer.
α-catenin inhibits Hedgehog signaling
The Hedgehog (Hh) signaling pathway plays an essential role in segmental patterning during development by regulating cell proliferation, migration and differentiation, and deregulation of this pathway has been implicated in multiple human cancers, including lung, breast, brain, colon and pancreatic cancer.56,57 The core components of the Hedgehog pathway are a 12-pass transmembrane receptor, Patched (Ptch1), and a seven-pass transmembrane receptor, Smoothened (Smo). In the absence of ligands, Ptch1 constitutively inhibits Smo. Upon binding of the Hh ligands, including Sonic (SHh), Desert (DHh) and Indian Hedgehog (IHh), to Ptch1, the inhibition of Smo is released and three Gli zinc-finger transcription factors, the transcriptional activators Gli1 and Gli2 and repressor Gli3, are activated, leading to activation or repression of their target genes.58
Neural progenitors express αE-catenin while differentiated neurons expression αN-catenin.59 Conditional deletion of α-catenin in the mouse brain (Nestin-Cre/α-cateninfl/fl) resulted in early death between 2–3 wk-of-age, and the mutant displayed enlarged head with massive hyperplasia in the brain.59 Microarray analysis showed that Fgf15 and Gli1, which are well-known targets of the hedgehog pathway, are the 2 most upregulated genes in α-catenin-deficient brains.59 This indicates that loss of α-catenin in the central nervous system leads to activation of hedgehog signaling; however, the underlying mechanism still remains unclear. Nevertheless, since Hh signaling is involved in tumorigenesis and malignant progression of a variety of human cancers, this provides yet another mechanism by which loss of α-catenin contributes to cancer development.
Conclusion and Future Perspectives
In summary, biochemical and genetic studies and clinical correlation analyses have demonstrated that α-catenin acts as a tumor suppressor in multiple human cancers. The regulation of Wnt/β-catenin, Hippo-YAP, NF-κB, and Hedgehog pathways by α-catenin may provide molecular mechanisms by which loss of α-catenin contributes to tumorigenesis. Given that α-catenin regulates distinct signaling pathways depending on the tissues type and the surrounding microenvironment, future studies should determine the specific α-catenin signaling pathways in different human cancer types and subtypes, and should evaluate its implication in cancer diagnosis, prognosis and treatment. In addition, studies thus far are focused on the role of α-catenin in tumorigenesis. Although some expression analyses showed underexpression of α-catenin in metastatic or invasive tumors,16,60,61 whether α-catenin plays a functional role in tumor progression and metastasis remains to be determined.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors’ research is supported by the US National Institutes of Health grants R00CA138572 and R01CA166051 (to L.M.) and a Cancer Prevention and Research Institute of Texas Scholar Award R1004 (to L.M.). L.M. is an R Lee Clark Fellow (supported by the Jeanne F Shelby Scholarship Fund) of The University of Texas MD Anderson Cancer Center.
Glossary
Abbreviations:
- APC
adenomatous polyposis coli
- GSK
glycogen synthase kinase
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- LATS
large tumor suppressor kinase
- LEF
lymphoid enhancer-binding factor
- MST
mammalian Ste20-like kinase
- NF-κB
nuclear factor κB
- TCF
T-cell factor
- TNF
tumor necrosis factor
- YAP
YES-associated protein
References
- 1.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 2.Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–17. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 5.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–9. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Scholer-Dahirel A, McLaughlin ME. Determinants of Wnt/β-catenin pathway dependency in colorectal cancer. Cell Cycle. 2012;11:9–10. doi: 10.4161/cc.11.1.18728. [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Nam HS, Lee EH, Han SH, Cho HJ, Chung HJ, Lee NS, Choi SJ, Kim H, Ryu JS, et al. Overexpression of a novel regulator of p120 catenin, NLBP, promotes lung adenocarcinoma proliferation. Cell Cycle. 2013;12:2443–53. doi: 10.4161/cc.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992;52:5770–4. [PubMed] [Google Scholar]
- 10.Benjamin JM, Nelson WJ. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horrigan SK, Arbieva ZH, Xie HY, Kravarusic J, Fulton NC, Naik H, Le TT, Westbrook CA. Delineation of a minimal interval and identification of 9 candidates for a tumor suppressor gene in malignant myeloid disorders on 5q31. Blood. 2000;95:2372–7. [PubMed] [Google Scholar]
- 12.Ye Y, McDevitt MA, Guo M, Zhang W, Galm O, Gore SD, Karp JE, Maciejewski JP, Kowalski J, Tsai HL, et al. Progressive chromatin repression and promoter methylation of CTNNA1 associated with advanced myeloid malignancies. Cancer Res. 2009;69:8482–90. doi: 10.1158/0008-5472.CAN-09-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig DW, O’Shaughnessy JA, Kiefer JA, Aldrich J, Sinari S, Moses TM, Wong S, Dinh J, Christoforides A, Blum JL, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013;12:104–16. doi: 10.1158/1535-7163.MCT-12-0781. [DOI] [PubMed] [Google Scholar]
- 15.Hollestelle A, Elstrodt F, Timmermans M, Sieuwerts AM, Klijn JG, Foekens JA, den Bakker MA, Schutte M. Four human breast cancer cell lines with biallelic inactivating alpha-catenin gene mutations. Breast Cancer Res Treat. 2010;122:125–33. doi: 10.1007/s10549-009-0545-4. [DOI] [PubMed] [Google Scholar]
- 16.Raftopoulos I, Davaris P, Karatzas G, Karayannacos P, Kouraklis G. Level of alpha-catenin expression in colorectal cancer correlates with invasiveness, metastatic potential, and survival. J Surg Oncol. 1998;68:92–9. doi: 10.1002/(SICI)1096-9098(199806)68:2<92::AID-JSO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Kimura K, Endo Y, Yonemura Y, Heizmann CW, Schafer BW, Watanabe Y, Sasaki T. Clinical significance of S100A4 and E-cadherin-related adhesion molecules in non-small cell lung cancer. Int J Oncol. 2000;16:1125–31. doi: 10.3892/ijo.16.6.1125. [DOI] [PubMed] [Google Scholar]
- 18.Aaltomaa S, Kärjä V, Lipponen P, Isotalo T, Kankkunen JP, Talja M, Mokka R. Reduced alpha- and beta-catenin expression predicts shortened survival in local prostate cancer. Anticancer Res. 2005;25(6C):4707–12. [PubMed] [Google Scholar]
- 19.Nakopoulou L, Gakiopoulou-Givalou H, Karayiannakis AJ, Giannopoulou I, Keramopoulos A, Davaris P, Pignatelli M. Abnormal alpha-catenin expression in invasive breast cancer correlates with poor patient survival. Histopathology. 2002;40:536–46. doi: 10.1046/j.1365-2559.2002.01392.x. [DOI] [PubMed] [Google Scholar]
- 20.Plumb CL, Adamcic U, Shahrzad S, Minhas K, Adham SA, Coomber BL. Modulation of the tumor suppressor protein alpha-catenin by ischemic microenvironment. Am J Pathol. 2009;175:1662–74. doi: 10.2353/ajpath.2009.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H, Wang J, Fang B, Fang X, Lu Z. α-Catenin inhibits glioma cell migration, invasion, and proliferation by suppression of β-catenin transactivation. J Neurooncol. 2011;103:445–51. doi: 10.1007/s11060-010-0413-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu TX, Becker MW, Jelinek J, Wu WS, Deng M, Mikhalkevich N, Hsu K, Bloomfield CD, Stone RM, DeAngelo DJ, et al. Chromosome 5q deletion and epigenetic suppression of the gene encoding alpha-catenin (CTNNA1) in myeloid cell transformation. Nat Med. 2007;13:78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- 23.Piao HL, Yuan Y, Wang M, Sun Y, Liang H, Ma L. α-catenin acts as a tumour suppressor in E-cadherin-negative basal-like breast cancer by inhibiting NF-κB signalling. Nat Cell Biol. 2014;16:245–54. doi: 10.1038/ncb2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci U S A. 2006;103:2322–7. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–25. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Meng X, Sun X, Liu M, Gao S, Zhao J, Pei F, Yu H. Wnt/beta-catenin signaling pathway may regulate cell cycle and expression of cyclin A and cyclin E protein in hepatocellular carcinoma cells. Cell Cycle. 2009;8:1567–70. doi: 10.4161/cc.8.10.8489. [DOI] [PubMed] [Google Scholar]
- 28.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 29.Cha YH, Kim NH, Park C, Lee I, Kim HS, Yook JI. MiRNA-34 intrinsically links p53 tumor suppressor and Wnt signaling. Cell Cycle. 2012;11:1273–81. doi: 10.4161/cc.19618. [DOI] [PubMed] [Google Scholar]
- 30.Jaitner S, Reiche JA, Schäffauer AJ, Hiendlmeyer E, Herbst H, Brabletz T, Kirchner T, Jung A. Human telomerase reverse transcriptase (hTERT) is a target gene of β-catenin in human colorectal tumors. Cell Cycle. 2012;11:3331–8. doi: 10.4161/cc.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JE, El-Deiry WS. Calcein-effluxing human colon cancer cells are enriched for self-renewal capacity and depend on β-catenin. Oncotarget. 2013;4:184–91. doi: 10.18632/oncotarget.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacheco-Pinedo EC, Morrisey EE. Wnt and Kras signaling-dark siblings in lung cancer. Oncotarget. 2011;2:569–74. doi: 10.18632/oncotarget.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–56. doi: 10.18632/oncotarget.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, van der Zee M, Fodde R, Blok LJ. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010;1:674–84. doi: 10.18632/oncotarget.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad WH, Swift RD, Biechele TL, Kulikauskas RM, Moon RT, Chien AJ. Regulating the response to targeted MEK inhibition in melanoma: enhancing apoptosis in NRAS- and BRAF-mutant melanoma cells with Wnt/β-catenin activation. Cell Cycle. 2012;11:3724–30. doi: 10.4161/cc.21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannini AL, Vivanco Md, Kypta RM. alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J Biol Chem. 2000;275:21883–8. doi: 10.1074/jbc.M001929200. [DOI] [PubMed] [Google Scholar]
- 37.Choi SH, Estarás C, Moresco JJ, Yates JR, 3rd, Jones KA. α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013;27:2473–88. doi: 10.1101/gad.229062.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 40.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–17. doi: 10.1016/S0092-8674(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi N, Ito T, Azuma S, Ito E, Honma R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Watanabe S, et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci. 2009;100:1668–74. doi: 10.1111/j.1349-7006.2009.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–6. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 48.Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11:1090–6. doi: 10.4161/cc.11.6.19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–7. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–7. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–50. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piccolo S. LIF-ting Hippo averts metastasis. Nat Med. 2012;18:1463–5. doi: 10.1038/nm.2955. [DOI] [PubMed] [Google Scholar]
- 53.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–74. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 55.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruat M, Hoch L, Faure H, Rognan D. Targeting of Smoothened for therapeutic gain. Trends Pharmacol Sci. 2014;35:237–46. doi: 10.1016/j.tips.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalderon D. Transducing the hedgehog signal. Cell. 2000;103:371–4. doi: 10.1016/S0092-8674(00)00129-X. [DOI] [PubMed] [Google Scholar]
- 59.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–12. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zschiesche W, Schönborn I, Behrens J, Herrenknecht K, Hartveit F, Lilleng P, Birchmeier W. Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res. 1997;17(1B):561–7. [PubMed] [Google Scholar]
- 61.Gofuku J, Shiozaki H, Tsujinaka T, Inoue M, Tamura S, Doki Y, Matsui S, Tsukita S, Kikkawa N, Monden M. Expression of E-cadherin and alpha-catenin in patients with colorectal carcinoma. Correlation with cancer invasion and metastasis. Am J Clin Pathol. 1999;111:29–37. doi: 10.1093/ajcp/111.1.29. [DOI] [PubMed] [Google Scholar]