Abstract
Proper centrosome positioning is critical for many cellular functions, such as cell migration and maintenance of polarity. During wound healing, fibroblasts orient their centrosomes such that they face the wound edge. The centrosome orientation determines the direction of cells’ migration so that they can close the wound effectively. In this study, we investigated the regulation of centrosome polarization and have identified the phosphatase POPX2 as an important regulator of centrosome orientation. We found that POPX2 inhibits centrosome centration, but not rearward nuclear movement, by regulating multiple proteins that function in centrosome positioning. High POPX2 levels result in reduced motility of the kinesin-2 motor, which, in turn, inhibits the transport of N-cadherin to the cell periphery and cell junctions. Loss of N-cadherin localization to the cell membrane affects the localization of focal adhesions and perturbs CDC42-Par6/PKCζ signaling. In addition, overexpression of POPX2 also results in a loss of Par3 localization to the cell periphery and reduced levels of LIC2 (dynein light intermediate chain 2), leading to defects in microtubule tethering and dynamics at cell-cell contacts. Therefore, POPX2 functions as a regulator of signaling pathways to modulate the positioning of centrosome in fibroblast during wound healing.
Keywords: centrosome, calcium/calmodulin kinase, dynein light intermediate chain 2, Kinesin-2 motor, N-cadherin, Par3, POPX2 phosphatase
Introduction
Control of cell polarity is crucial in processes such as migration, cell division and differentiation. In cells such as fibroblasts and astrocytes, the position of the centrosome is responsible for determining the direction of migration. Migratory polarization is induced when confluent monolayers of fibroblasts are scratch-wounded, resulting in the centrosome, Golgi apparatus and microtubules positioning toward the wound edge.1-3
It is well-established that CDC42 plays an important role in the control of centrosome orientation. CDC42 regulates 2 different pathways which lead to centrosome orientation—one pathway controls nuclear movement, while the other controls centrosome centration.2 The pathway controlling centrosome centration involves Partitioning defective 6 (Par6), protein kinase C zeta (PKCζ)—a serine/threonine kinase belonging to the atypical PKC family, microtubules and dynein.2-5 Cortical dynein has been shown to associate with Par3 through dynein light intermediate chain 2 (LIC2).6 This interaction tethers microtubules to the cell cortex, generating a pulling force on the microtubules that allows the centrosome to be positioned at the cell centroid.6,7 Classical cadherins have also been shown to play a role in centrosome positioning. Cadherin is localized to regions where cell-cell contacts are present, and the centrosome positions itself away from these cell-cell contacts. Changing the geometry of N-cadherin-mediated interactions affects the orientation of the centrosome-nucleus axis.8,9 Meanwhile, focal adhesions localize to the free edge of the cell, where cell-cell contacts are absent. It has been reported that the recruitment of βPIX, a guanine nucleotide exchanger for RAC and CDC42, to the focal adhesion is important in generating active CDC42 at the leading edge of the cells, which in turn mediates a polarity signal involving Par6, atypical protein kinase C (aPKC) and adenomatous polyposis coli (APC).10
POPX2 (partner of PIX 2) is a phosphatase that belongs to the PP2C family. It is found in a complex with βPIX and has been found to dephosphorylate p21-activated kinase (PAK), calcium/calmodulin kinases (CaMKs) and kinesin family member 3A (KIF3A).11-13 Dephosphorylation of KIF3A at serine 690 by POPX2 reduces the motility of the kinesin-2 motor thus inhibiting transport of cargoes such as Par3 and N-cadherin.12 In this study, we found that overexpression of POPX2 in fibroblasts inhibits proper centrosome orientation upon scratch-wounding. Silencing POPX2 can restore the centrosomal-nuclear axis. With the use of a phosphatase-dead mutant, we confirm that the phosphatase activity of POPX2 contributes toward the regulation of centrosome orientation. We also found that POPX2 can regulate the levels of LIC2 thereby affecting the centrosome orientation. Based on our findings, we propose that POPX2 acts as a key regulator of centrosome positioning by controlling LIC2 expression and KIF3A-mediated transport of Par3 and N-cadherin.
Results
POPX2 expression results in loss of directional migration
POPX2 has been previously shown to affect cell migration and invasion, with high expression of the phosphatase leading to an increase in the speed of cell migration and invasiveness.14,15 Tracing and overlaying the paths of control NIH3T3 (Ctrl) and POPX2 stably-expressing (X2) cells reveals that the migratory paths of these cells across scratch wounds (Fig. 1A) also differ, with Ctrl cells migrating in a direction that is perpendicular across a scratch wound, while X2 cells migrate in a more random and less directional manner across the wound (Fig. 1B and C). Cells expressing a phosphatase-dead POPX2 construct (X2m) did not appear to be affected in the direction of cell migration (Fig. 1D) suggesting that the phosphatase activity of POPX2 is involved in the regulation of migratory direction.
Figure 1. POPX2-stable expressing cells do not move perpendicularly across scratch wounds. (A) Representative path taken by a NIH 3T3 fibroblast across the scratch wound. Cells were tracked every 15 min up to 15 h post-wounding using time-lapse microscopy. The wound is oriented such that it runs parallel to the x-axis. (B) Paths taken by Ctrl cells (n = 40). (C) Paths taken by X2 cells (n = 42). (D) Paths taken by X2m cells (n = 46). All paths are oriented such that the start point is normalized to the origin and overlaid on the same graph.
In fibroblasts, the direction of cell migration is determined by the leading edge and the position of the centrosome. Once the cell is polarized and the centrosome moves to a position that lies between the leading edge and the nucleus, the cell will move in the direction in which the centrosome is orientated. As POPX2 overexpressing cells show defects in the directionality of cell migration, we postulate that overexpression of POPX2 interferes with centrosome orientation.
POPX2 stable expressing cells are defective in MTOC orientation
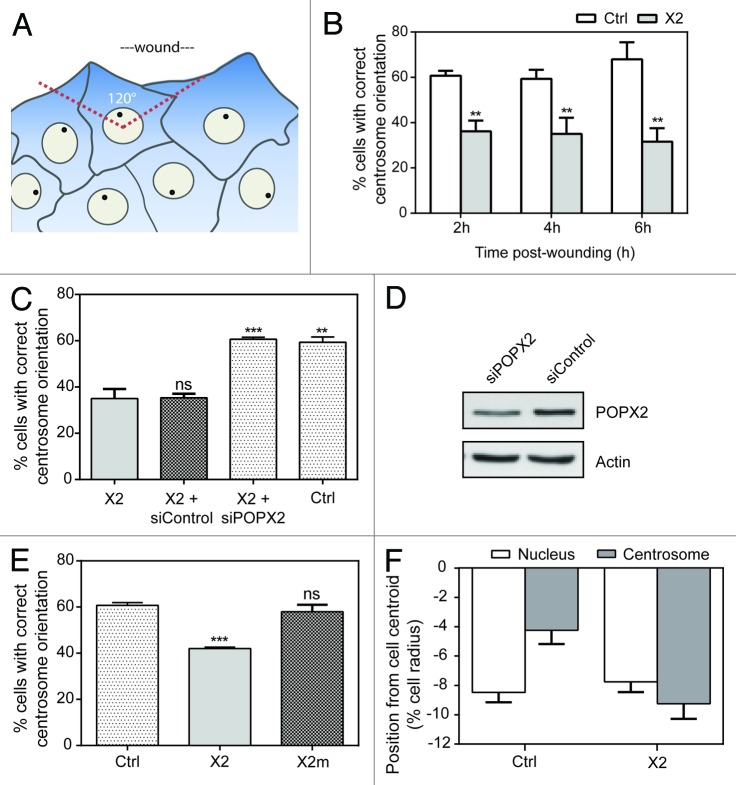
We used a wound-induced centrosome orientation assay to determine the status of centrosome orientation in Ctrl or X2 NIH3T3 fibroblasts. In this assay, confluent monolayers were scratch-wounded, and cells were scored as having correct centrosome orientation if the centrosome was located in a 120° sector facing the wound edge (Fig. 2A). X2 cells consistently showed centrosome mis-orientation at 2, 4, and 6 h post-wounding (Fig. 2B). This phenotype can be reversed by depleting POPX2 in X2 cells using siRNA (Fig. 2C and D), suggesting that overexpression of POPX2 negatively regulates centrosome orientation. Inhibition of centrosome orientation appears to be dependent on the phosphatase activity of POPX2, as overexpression of the phosphatase-dead mutant of POPX2, POPX2m, did not affect centrosome orientation (Fig. 2E; Fig. S1).
Figure 2. POPX2-overexpressing cells are defective in centrosome orientation. (A) Diagram showing classification of cells with correct centrosome orientation. Centrosomes were determined as correctly oriented when they were positioned in the 120° sector defined by the nucleus and leading edge. (B) Histogram showing percentage of Ctrl and X2 fibroblasts with correctly oriented centrosomes at different time points after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (Student t test, n = 100; **P < 0.01). (C) Histogram showing percentage of Ctrl fibroblasts and X2 fibroblasts treated with control or POPX2 siRNA with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (Student t test, n = 100; **P < 0.01, ***P < 0.001). (D) Western blot showing knockdown efficiency of POPX2 in X2 cells treated with POPX2 and control siRNAs. Actin was used as a loading control. (E) Histogram showing percentage of NIH3T3 fibroblasts overexpressing POPX2 or POPX2m with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (Student t test, n = 100; ***P < 0.001). (F) Quantification of the position of the nucleus and centrosome along the axis of the cell perpendicular to the wound in Ctrl and X2 cells. The cell centroid is defined as “0”. Positive values are toward the leading edge and negative values are toward the rear of the cell. Error bars represent SEM (n = 100).
Centrosome reorientation occurs when confluent fibroblast monolayers are scratch-wounded. During this process, the centrosome remains near the cell centroid while the nucleus moves rearward.2 To determine whether POPX2 affects centrosome positioning or rearward nucleus movement, we measured the positions of the nucleus and centrosome relative to the cell centroid in Ctrl and X2 cells. We observed that while there was no significant difference in the position of the nucleus, the centrosome was positioned more toward the rear in X2 cells as compared with Ctrl cells, indicating that POPX2 affects centrosome positioning rather than nuclear movement (Fig. 2F).
N-cadherin is required for centrosome positioning and its localization is affected in X2 cells
Having established that POPX2 affects the polarity of cells at wound edge by controlling centrosome positioning rather than the movement of the nucleus, we next investigated how POPX2 regulates centrosome orientation. Since N-cadherin, Par3 and LIC2 have been implicated in centrosome orientation6,9 we asked if POPX2 modulates N-cadherin- and Par3/LIC2-mediated centrosome positioning.
We have recently reported that POPX2 can negatively regulate N-cadherin transport by inhibiting the kinesin-2 motor.12 N-cadherin, β-catenin and other polarity proteins have been reported to be cargoes of the kinesin-2 motor, which is made up of the KIF3A and 3B motor subunits and a non-motor KAP3 subunit.16 POPX2 regulates the phosphorylation status of serine-690 at the C-terminal tail of KIF3A. The presence of high levels of POPX2 or substitution of serine-690 to alanine resulted in reduced motility of the kinesin-2 motor, suggesting that the phosphorylation status of S690 is important in regulating kinesin motor transport along the microtubules.12 As a result, X2 cells show reduced peripheral N-cadherin localization as compared with Ctrl and POPX2m-overexpressing (X2m) cells.12 As N-cadherin is known to control centrosome positioning by regulating cell-ECM interactions,9 we proceeded to determine if POPX2 negatively regulates centrosome positioning through its effects on kinesin-2-mediated N-cadherin transport. We first confirmed that N-cadherin is required for proper centrosome orientation by treating both Ctrl and X2 cells with 2 different siRNAs targeting N-cadherin. Reduction of N-cadherin expression resulted in a reduced number of Ctrl cells with proper centrosome orientation (Fig. 3A and B). Overexpression of POPX2 does not affect N-cadherin levels in the cells as western blot analysis showed that endogenous N-cadherin levels were similar in both Ctrl and X2 cells (Fig. 3C). Overexpression of N-cadherin failed to rescue the loss of centrosome orientation in X2 cells (Fig. 3D) suggesting that it is not the lack of N-cadherin protein, but rather the defects in kinesin-2 motor transport that is negatively impacting centrosome orientation in X2 cells. We then verified that the microtubule network appears normal in X2 cells. Immunofluorescent staining of the microtubule network with anti-tubulin antibodies did not show any observable differences between Ctrl and X2 cells (Fig. S2). In order to demonstrate that kinesin-2 transport plays a role in centrosome orientation, we overexpressed the KIF3A tail domain in Ctrl cells. The KIF3A tail domain acts in a dominant-negative manner as it is able to compete with the endogenous KIF3A for cargoes but is unable to transport them. Overexpression of the KIF3A tail in Ctrl cells inhibits centrosome orientation, showing the requirement of proper KIF3A motor activity in centrosome positioning (Fig. 3E). We next determined if wild-type (WT) KIF3A, or the phospho-mimic S690D KIF3A mutant could rescue the centrosome orientation defect in X2 cells. Indeed, overexpression of WT KIF3A and KIF3A-S690D in X2 cells partially restores proper centrosome orientation, while overexpression of the mutant KIF3A-S690A has no effect (Fig. 3F). We have earlier reported that calcium/calmodulin kinase II (CaMKII) is a kinase likely responsible for regulating phosphorylation of serine-690 of KIF3A motor.12 Indeed, inhibition of CaMKII activity by small molecule inhibitors, KN-93, and KN-62, resulted in impairment of centrosome orientation (Fig. S3).
Figure 3. N-cadherin is required for centrosome positioning and its localization is effected in POPX2-overexpressing cells. (A) Histogram showing percentage of Ctrl and X2 fibroblasts transfected with control or N-cadherin siRNA with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100, Student t test, *P < 0.05). (B) Western blot showing knockdown efficiency of N-cadherin in NIH3T3 fibroblasts transfected with N-cadherin siRNA. Actin was used as a loading control. (C) Western blot showing levels of endogenous N-cadherin in Ctrl and X2 fibroblasts. Actin was used as a loading control. (D) Histogram showing percentage of Ctrl and X2 fibroblasts overexpressing N-cadherin-GFP with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments. (E) Histogram showing percentage of NIH3T3 fibroblasts overexpressing KIF3A tail with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100; Student t test, **P < 0.01). (F) Histogram showing percentage of NIH3T3 fibroblasts overexpressing KIF3A, KIF3A-S690A or KIF3A-S690D with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100; Student t test, **P < 0.01).
X2 cells show defects in focal adhesion localization and βPIX recruitment to the leading edge
Cell-cell and cell–matrix adhesions are spatially segregated due to negative feedback between both systems, leading to focal adhesions and N-cadherin being localized in a mutually exclusive manner, i.e., focal adhesions are only observed at the free edge of the cell and not at the cell-cell junctions.9,17-19 In scratch-wounded monolayers, N-cadherin localizes to cell-cell contacts while focal adhesions are mostly found at the free edge of the cell facing the wound. The CDC42- and RAC-GEF, βPIX, is recruited to the focal adhesions to activate CDC42 signaling, which in turn plays a role in centrosome positioning through Par6 and PKCζ.3,10 Since X2 cells show reduced peripheral localization of N-cadherin,12 they are also likely to have mislocalized focal adhesions and defects in βPIX recruitment. We confirmed this by immunostaining for paxillin and βPIX in Ctrl, X2, and X2m cells. While paxillin was largely localized to focal adhesions at the free edge in Ctrl and X2m cells, it was not restricted to the leading edge but instead found throughout X2 cells (Fig. 4A). Similarly, staining for βPIX revealed that βPIX accumulated at the free edge in Ctrl and X2m, but not in X2 cells (Fig. 4B). Since βPIX recruitment to the free edge is required to activate CDC42-Par6/PKCζ signaling to control centrosome orientation, it is likely that activation of this pathway is perturbed in X2 cells.

Figure 4. POPX2-overexpressing cells show loss of focal adhesion localization and βPIX recruitment to the leading edge. Immunostaining of (A) paxillin or (B) βPIX in cells infected with retroviral vector encoding GFP, GFP-POPX2, or GFP-POPX2m. Dotted lines in the middle panels represent the wound edge. Scale bar: 20 μm.
Par3 is required for centrosome positioning and its localization is affected in X2 cells
Another signaling pathway implicated in the regulation of centrosome orientation is mediated by Par3 and LIC2. Since POPX2 inhibits KIF3A motor activity, transport of not only N-cadherin but other kinesin-2 cargoes is also expected be affected in X2 cells. Therefore, we investigated if X2 cells show defects in Par3 localization as Par3 is a cargo of kinesin-2 and is also known to regulate centrosome positioning.6,20 We first confirmed the requirement for Par3 for proper centrosome orientation by treating Ctrl and X2 cells with 2 different siRNAs targeting Par3. Knockdown of Par3 resulted in a loss of proper centrosome orientation in Ctrl cells, but had no effect in X2 cells (Fig. 5A and B). Immunostaining for Par3 showed Par3 localization to regions of cell-cell contact in Ctrl and X2m, but not X2 cells (Fig. 5C). Overexpression of Par3 in X2 cells could not rescue the loss of proper centrosome orientation (Fig. 5D). Western blot analysis showed that both Ctrl and X2 cells expressed similar levels of Par3 (Fig. 5E), suggesting that defects in Par3 localization in X2 cells were likely due to impaired Par3 transport and localization. We confirmed this by immunostaining of Par3 in NIH3T3 fibroblasts overexpressing WT KIF3A, KIF3A-S690A or KIF3A-S690D. Par3 localized normally to cell-cell contacts in WT KIF3A- and KIF3A-S690D- but not KIF3A-S690A-overexpressing cells (Fig. 5F), indicating that: (1) the phosphorylation status of KIF3A on S690, which is affected by POPX2, is important for proper Par3 transport and localization; and (2) POPX2 affects centrosome positioning through its effects on kinesin-2-mediated Par3 transport.
Figure 5. Par3 is required for centrosome positioning and its localization is affected in POPX2-overexpressing cells. (A) Histogram showing percentage of Ctrl and X2 fibroblasts transfected with control or Par3 siRNAs with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100; Student t test, **P < 0.01, ***P < 0.001). (B) Western blot showing knockdown efficiency in NIH3T3 fibroblasts transfected with Par3 siRNA. Actin was used as a loading control. (C) Immunostaining of Par3 in NIH3T3 fibroblasts infected with retroviral vector encoding GFP, GFP-POPX2, or GFP-POPX2m. Dotted lines in the middle panels represent the wound edge. Scale bar: 20 μm. (D) Histogram showing percentage of Ctrl and X2 fibroblasts overexpressing Par3 with correctly oriented centrosome 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100). (E) Western blot showing endogenous levels of Par3 in Ctrl and X2 fibroblasts. Actin was used as a loading control. (F) Immunostaining of Par3 in NIH3T3 fibroblasts infected with retroviral vector encoding GFP-KIF3A, GFP-KIF3A-S690A, or GFP-KIF3A-S690D. Dotted lines in the middle panels represent the wound edge. Scale bar: 20 μm.
POPX2 affects LIC2 expression, which controls centrosome position and orientation
It is known that Par3 functions together with LIC2 in the regulation of centrosome positioning. Par3 is an interacting partner of LIC2, and is thought to serve as a cortical factor that controls centrosome positioning by tethering microtubules via LIC2.6 Since we observed loss of proper Par3 localization in X2 cells, we next investigated if POPX2 overexpression might affect the Par3 partner, LIC2. Treatment of Ctrl cells with 2 different siRNAs against LIC2 resulted in a loss of proper centrosome orientation, while the same treatment had no effect in X2 cells (Fig. 6A and B). Western blot analysis revealed lower levels of LIC2, but not dynein light intermediate chain 1 (LIC1), in X2 cells as compared with Ctrl (Fig. 6C). Expression of LIC2 could be increased in X2 cells when treated with a siRNA against POPX2 (Fig. 6D), suggesting that POPX2 negatively regulates the levels of LIC2.
Figure 6. The levels of LIC2, which controls centrosome position and orientation, were found to be lower in POPX2 stable expressing cells. (A) Histogram showing percentage of Ctrl and X2 fibroblasts transfected with control or LIC2 siRNA with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100; Student t test, **P < 0.01, ***P < 0.001). (B) Western blot showing knockdown efficiency of LIC2 in NIH3T3 fibroblasts transfected with LIC2 siRNA. Actin was used as a loading control. (C) Western blot showing endogenous levels of LIC1 and LIC2 in Ctrl and X2 fibroblasts. Actin was used as a loading control. (D) Western blot showing increase in LIC2 levels in X2 fibroblasts treated with POPX2 siRNA. Actin was used as a loading control. (E) Histogram showing percentage of Ctrl and X2 fibroblasts transfected with LIC1 or LIC2 cDNA constructs with correctly oriented centrosomes 4 h after wounding. Results are shown as means +/− standard deviation of 3 independent experiments (n = 100; Student t test, **P < 0.01). (F) Overexpression of LIC2 is able to rescue the loss of centrosome positioning in X2 cells. Quantification of the position of the nucleus and centrosome along the axis of the cell perpendicular to the wound in Ctrl and X2 cells overexpressing LIC1 or LIC2. The cell centroid is defined as “0.” Positive values are toward the leading edge and negative values are toward the rear of the cell. Error bars represent SEM (n = 100).
Since LIC2 is required for proper centrosome orientation, and X2 cells express lower levels of LIC2, then restoring the levels of LIC2 in X2 cells would be expected to restore proper centrosome orientation. Indeed, overexpression of LIC2, but not LIC1, restored proper centrosome orientation in X2 cells (Fig. 6E). By measuring the positions of the centrosome and nucleus relative to the cell centroid, we also show that overexpression of LIC2, but not LIC1, in X2 cells restores centrosome positioning such that it is closer to the cell centroid (Fig. 6F). Thus, POPX2 is able to regulate both components of the Par3-LIC2 complex.
Loss of LIC2 expression and peripheral Par3 localization affects MT pausing at cell-cell contacts
The dynein motor at the cell cortex is important in the regulation of microtubule dynamics and generation of pulling forces which are required for positioning of the mitotic spindle and centrosomes in dividing cells and migrating fibroblasts, respectively.7 It has been reported that Par3 and LIC2 contribute to the regulation of microtubule dynamics at the cell-cell contact since cortical dynein stabilizes microtubule plus ends, and Par3 and LIC2 play a role in microtubule tethering.6,7,21 We next monitored microtubule dynamics at cell-cell contacts in Ctrl and X2 cells by measuring the growth; shrinkage and pausing time of microtubules at cell-cell contacts and determined if POPX2 could influence microtubule dynamics. NIH3T3 cells were transfected with mCherry-β-tubulin, microtubules present at cell-cell contacts were imaged and measured to determine their dynamics (Fig. 7A; Video S1–5). Depletion of LIC2 or Par3 in Ctrl cells decreased the microtubule pausing time and increased microtubule dynamics while treatment with control siRNA had no effect (Fig. 7B and C). Overexpression of POPX2 gave rise to the same phenotype as LIC2 or Par3 depletion; there was a decrease in microtubule pausing and an increase in dynamicity (Fig. 7B and C; Table S1). Our observations suggest that in addition to the regulation of centrosome orientation through its effect on Par3 localization and LIC2 levels, POPX2 also regulates the microtubule dynamics through its modulation of Par3 and LIC2.
Figure 7. Loss of LIC2 expression and peripheral Par3 localization affect microtubule (MT) pausing at cell-cell contacts. (A) Frames of movies of NIH3T3 fibroblasts showing MT dynamics at cell-cell contacts under the indicated conditions. Arrowheads indicate dynamic MT ends. Scale bar: 5 μm. (B) Quantification of % time MTs spent pausing. (C) MT dynamicity at cell-cell contacts in NIH3T3 fibroblasts treated as indicated. Data for each condition are from at least 2 independent experiments and 95–100 MTs. Error bars show SEM (***P < 0.001).
Discussion
Centrosome positioning involves many proteins that function in multiple pathways, and is affected by cell shape and confluence. It is controlled differently in single cells and confluent monolayers.22 In this study, we focus on centrosome positioning in confluent fibroblast monolayers. While many proteins functioning in centrosome orientation have been identified and the pathways that they function are elucidated, it remains unclear whether these pathways are regulated collectively or independently. We show that POPX2 affects centrosome orientation by inhibiting centrosome centration and not nuclear movement. Our findings also indicate that POPX2 acts as a regulator of centrosome orientation by affecting multiple proteins that function in centrosome positioning (Fig. 8).

Figure 8. POPX2 inhibits centrosome orientation by affecting LIC2 expression and kinesin-2 motor transport. POPX2 dephosphorylates KIF3A on S690 either directly or through inhibition of CaMKII activity. This results in a reduction of KIF3A motility, inhibiting the transport of kinesin-2 cargoes such as N-cadherin and Par3. Due to the loss of N-cadherin localization at cell-cell contacts, focal adhesions are no longer restricted to the leading edge, and instead localize throughout the cell periphery.9 This perturbs the activation of the Cdc42-dependent polarity pathway.3,10 In addition, loss of peripheral Par3 localization as well as decreased LIC2 levels result in defects in tethering MTs at cell-cell contacts.6 The combined effects of POPX2 on these different pathways lead to the loss of proper centrosome orientation.
It is currently thought that centrosome positioning is controlled by CDC42, which when activated, recruits and activates the Par6/PKCζ complex.2-5 Activation of CDC42 is localized, and requires integrin activation at the free cell edge.3 This is achieved through crosstalk and spatial segregation between cell-cell and cell-matrix adhesions,9,19 thus implicating N-cadherin transport and expression as well as focal adhesion distribution and signaling in the control of centrosome orientation.
Centrosome positioning also requires the dynein-dynactin complex, which functions downstream of Par6/PKCζ.2-5 It is not known how Par6/PKCζ affects the dynein-dynactin complex. However, dynein at the cell cortex is known to function in centrosome positioning by tethering and stabilizing microtubule plus ends. This requires LIC2 to be present within the dynein complex and Par3 to be present at the cell periphery, which result in the generation of a pulling force on the centrosome to position the centrosome close to the cell center.6,7,21 Thus, loss of peripheral Par3, LIC2, or the dynein–dynectin complex would impair centrosome orientation.
Our findings implicate POPX2 as a negative regulator of centrosome orientation through 2 main effects: (1) impairing transport of Par3 and N-cadherin to the cell periphery; and (2) reducing LIC2 protein expression. POPX2 inhibits transport of Par3 and N-cadherin by keeping KIF3A, a subunit of the kinesin-2 complex, in a dephosphorylated state. This is achieved through either direct dephosphorylation of KIF3A on serine 690, or indirectly through inhibition of CaMKII, which is responsible for phosphorylating KIF3A on this residue (Fig. 8).12 How POPX2 affects LIC2 expression, however, is yet to be determined. POPX2 could either inhibit LIC2 transcription or regulate LIC2 degradation, and this is would be of interest in future studies.
Our findings also highlight the importance of regulating POPX2 levels or activity in the cell. The amount of POPX2 present in the cell may influence cytoplasmic dynein 1 function through its effects on LIC2 expression. The cytoplasmic dynein 1 heavy chain binds to either the LIC1 or LIC2 subunit in a mutually exclusive manner,23,24 and this influences the function of the cytoplasmic dynein 1 complex. LIC1 is thought to function in centrosome assembly, organization and function, as well as at the Golgi,23,25,26 while LIC2 participates in centrosome orientation and at recycling endosomes.6,23 High levels or increased activity of POPX2 phosphatase inhibits LIC2 expression, and could shift the balance of heavy chain binding toward LIC1, while low POPX2 levels could allow more LIC2 binding, thus targeting the cytoplasmic dynein 1 complex toward different functions.
POPX2 levels also have an impact on directional cell migration. Directed cell migration is important in processes such as embryogenesis, wound healing and the immune response. Centrosome orientation dictates the direction of cell migration in a number of cell types. By keeping POPX2 expression under tight control, cells can ensure that they are able to orient their centrosomes correctly, and thus migrate in the right direction. On the contrary, loss of regulation of POPX2 expression results in defects in cell migration, which could lead to developmental defects. Loss of cell polarity is also linked to tumorigenesis and cancer cell invasion. Interestingly, the levels of POPX2 have been found to be higher in invasive breast cancer cells compared with non-invasive ones.15 POPX2 can potentially be a major regulator of cell polarity by integrating both cell-cell (through kinesin-2 and N-cadherin) and cell-matrix (through the regulation of βPIX-CDC42-Par3/LIC2 pathway) dependent signaling pathways as well as controlling the localization of cell polarity proteins such as Par3.
Materials and Methods
Reagents
Anti-N-cadherin was from BD Biosciences, anti-paxillin from Millipore (05-417), anti-LIC1 from GeneTex (GTX120114) and anti-LIC2 from AbCam (ab 118082). Anti- βPIX was from Millipore (07-1450) while anti-α-tubulin (T9026) and anti-γ-tubulin (T6557) were from Sigma. Anti-actin (MAB1501) and anti-Par3 (07-330) were from Millipore. Anti-GFP was from Life Technologies (G10362) and anti-sera against POPX2 were from Koh’s lab. Constructs encoding POPX2 and the phosphatase-dead mutant, POPX2m were previously described.11 N-cadherin-eGFP (Addgene plasmid 18870) was from Addgene and constructs encoding KIF3A and its mutants were previously described.12 Par3 and Dynein LIC1 and 2 constructs were generated from mouse cDNA. mCherry-tubulin was from a gift from Dr Hoi-Yeung Li, Nanyang Technological University. All siRNAs were from Life Technologies (Invitrogen).
Cell culture, transfection and retrovirus infection
NIH3T3 and X2 cells were grown in DMEM high glucose medium supplemented with 10% FBS. Characterization of X2 cells was described in Singh et al. (2011).14 Transfections were performed using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s instructions, or by retroviral infection.
Analysis of cell migration paths
NIH3T3 cells were seeded and allowed to grow to confluence overnight. A scratch was made across the cell monolayer with a 200 μl pipette tip. Imaging was performed for 15 h using a Zeiss Axiovert 200M inverted microscope at 37 °C, and cell nuclei were tracked manually using ImageJ software. Cell paths were traced and aligned such that their start points lie at the origin. Cells were tracked unless they underwent division.
Centrosome orientation assay
NIH3T3 cells were grown to confluence and individual wounds were made using a 200 μl pipette tip. Cells were fixed 4 h post-wounding and stained with γ-tubulin and DAPI. Centrosome orientation was assessed as previously described,4 where the centrosome was considered as correctly oriented if it was in the 120° sector defined by the nucleus and leading edge. At least 100 cells from 3 independent experiments were analyzed for each condition and error bars represent standard deviation, SD.
Analysis of nucleus and centrosome position
Scratch-wounded cells were fixed and stained with γ-tubulin and DAPI 4 h post-wounding. Images were aligned with the wound parallel to the x-axis. The perimeters of the whole cell, nucleus and centrosomes were traced and their centroids were calculated using ImageJ. The distance between the centroids of the nucleus and centrosomes to the cell centroid was calculated, and normalized to cell size to correct for cell size differences. At least 100 cells from 2 independent experiments were analyzed for each condition, and the errors bars represent standard error of the mean, SEM.
Immunofluorescence staining
NIH3T3 cells were fixed with either 4% paraformaldehyde or cold methanol, permeabilized in 0.2% Triton X-100 and blocked with 4% BSA. Incubation with primary antibody was performed at 4 °C overnight and incubation with secondary antibody was performed at room temperature for 1 h. Coverslips were mounted using Vectorshield with DAPI (Vector Labs). Images were obtained on a Carl Zeiss axiovert microscope using Plan-Apochromat 40×/1.25 or Plan-Apochromat 63×/1.4 objectives and recorded on a Roper Scientific CoolSNAP CCD camera.
Time-lapse imaging of MT dynamics
NIH3T3 cells were transfected with mCherry-tubulin and GFP-POPX2 or siRNA against LIC2 or Par3. Cells were imaged with Carl Zeiss axiovert microscope equipped with a humid chamber at 37 °C and 5% CO2. The Zeiss objective Plan Neo-fluar 100X/1.3 oil was used. Images were captured with Axiovision software using a Roper Scientific CoolSNAP CCD camera. MTs at cell-cell contacts were imaged at 5 s/frame for 3 min. Microtubules that could be clearly observed for at least 2 min were tracked using ImageJ. 95–100 MTs from at least 2 independent experiments were used for analysis, and the error bars plotted represent SEM. The play-back rate for all movies is 7 frames/s.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the MOE Tier 2, Ministry of Education Singapore and the National Research Foundation, Singapore for funding support.
REFERENCES
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–63. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–98. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 4.Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–41. doi: 10.1016/S0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 5.Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem. 2003;278:31020–3. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- 6.Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol. 2009;19:1065–74. doi: 10.1016/j.cub.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, López MP, Vale RD, Jülicher F, Reck-Peterson SL, Dogterom M. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 2012;148:502–14. doi: 10.1016/j.cell.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci. 2009;122:905–11. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185:779–86. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–9. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol. 2002;12:317–21. doi: 10.1016/S0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 12.Phang H-Q, Hoon J-L, Lai SK, Zeng Y, Chiam K-H, Li HY, Koh CG. POPX2 phosphatase regulates the KIF3 kinesin motor complex. J Cell Sci. 2014;127:727–39. doi: 10.1242/jcs.126482. [DOI] [PubMed] [Google Scholar]
- 13.Harvey BP, Banga SS, Ozer HL. Regulation of the multifunctional Ca2+/calmodulin-dependent protein kinase II by the PP2C phosphatase PPM1F in fibroblasts. J Biol Chem. 2004;279:24889–98. doi: 10.1074/jbc.M400656200. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, Gan CS, Guo T, Phang HQ, Sze SK, Koh CG. Investigation of POPX2 phosphatase functions by comparative phosphoproteomic analysis. Proteomics. 2011;11:2891–900. doi: 10.1002/pmic.201100044. [DOI] [PubMed] [Google Scholar]
- 15.Susila A, Chan H, Loh AX, Phang HQ, Wong ET, Tergaonkar V, Koh CG. The POPX2 phosphatase regulates cancer cell motility and invasiveness. Cell Cycle. 2010;9:179–87. doi: 10.4161/cc.9.1.10406. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–99. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camand E, Peglion F, Osmani N, Sanson M, Etienne-Manneville S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J Cell Sci. 2012;125:844–57. doi: 10.1242/jcs.087668. [DOI] [PubMed] [Google Scholar]
- 18.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–95. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burute M, Thery M. Spatial segregation between cell-cell and cell-matrix adhesions. Curr Opin Cell Biol. 2012;24:628–36. doi: 10.1016/j.ceb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–34. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks AG, Lazarus JE, Perlson E, Gardner MK, Odde DJ, Goldman YE, Holzbaur EL. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol. 2012;22:632–7. doi: 10.1016/j.cub.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale CM, Chen WC, Khatau SB, Daniels BR, Lee JS, Wirtz D. SMRT analysis of MTOC and nuclear positioning reveals the role of EB1 and LIC1 in single-cell polarization. J Cell Sci. 2011;124:4267–85. doi: 10.1242/jcs.091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer KJ, Hughes H, Stephens DJ. Specificity of cytoplasmic dynein subunits in discrete membrane-trafficking steps. Mol Biol Cell. 2009;20:2885–99. doi: 10.1091/mbc.E08-12-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tynan SH, Purohit A, Doxsey SJ, Vallee RB. Light intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. J Biol Chem. 2000;275:32763–8. doi: 10.1074/jbc.M001536200. [DOI] [PubMed] [Google Scholar]
- 25.Tynan SH, Gee MA, Vallee RB. Distinct but overlapping sites within the cytoplasmic dynein heavy chain for dimerization and for intermediate chain and light intermediate chain binding. J Biol Chem. 2000;275:32769–74. doi: 10.1074/jbc.M001537200. [DOI] [PubMed] [Google Scholar]
- 26.Purohit A, Tynan SH, Vallee R, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol. 1999;147:481–92. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.