Abstract
Background
Meaningful delays occurred in the IMS III trial. Analysis of the workflow will identify factors contributing to the in-hospital delays.
Methods and Results
In the endovascular arm of the IMS III trial, these time intervals were calculated: stroke onset to ED arrival; ED to CT; CT to IV tPA start; IV tPA start to randomization; randomization to groin puncture; groin puncture to thrombus identification; thrombus identification to start of endovascular therapy; start of endovascular therapy to reperfusion. The effects of enrollment time, CTA use, inter-hospital transfers, and intubation on workflow were evaluated. Delays notably occurred in the time intervals from IV tPA initiation to groin puncture (median 84 minutes) and start of endovascular therapy to reperfusion (median 85 minutes). The CT to groin puncture time was significantly shorter during working hours than after. Times from ED to reperfusion and groin puncture to reperfusion decreased over the trial period. Patients with CTA had shorter ED to reperfusion and onset to reperfusion times. Transfer of patients resulted in a longer onset to reperfusion time compared to those treated in the same center. Age, sex, NIHSS, and intubation did not impact delays.
Conclusions
Important delays were identified prior to reperfusion in the IMS III trial. Delays decreased as the trial progressed. Use of CTA and endovascular treatment in the same center were associated with time savings. These data may help in optimizing workflow in current and future endovascular trials.
Clinical Trial Registration Information
http://clinicaltrials.gov. Identifier: NCT00359424.
Keywords: Stroke, Infarction, Interventional neuroradiology
BACKGROUND
The outcome of acute ischemic stroke therapies is time-dependent.1, 2 Early restoration of blood flow to ischemic brain tissue increases the potential of mitigating the ischemic insult and restoring the normal function of the affected brain.3, 4 In a meta-analysis of randomized trials of intravenous (IV) tissue plasminogen activator (tPA) within six hours from onset, patients treated with IV tPA within 3 hours achieved the greatest treatment benefit.5 Evidence from intra-arterial (IA) cohorts has shown similar results with evidence of improved recovery and lower mortality in patients who achieved short onset to reperfusion times.6
The recent introduction of stentrievers resulted in higher rates of successful reperfusion with procedure times reduced by more than half when compared to prior techniques.7 For reperfusion therapies, attention to minimizing time to reperfusion via workflow improvement, targeting the various steps from Emergency Department (ED) arrival to microcatheter delivery at the thrombus interface is paramount.8 While some studies demonstrate the feasibility of shortening ED to IV tPA needle time to as low as 20 minutes,9, 10 studies on workflow in stroke patients treated with endovascular therapies are scarce.
Multiple factors may contribute to considerable delays prior to achieving endovascular reperfusion. Coordinating endovascular therapy is more complex given the resource requirement prior to treatment, variability in vascular access, and intensive nature of the procedures.11 With the multiple issues that require attention in the acute stroke setting, delays often go unrecognized by stroke team members, and potential strategies to reduce time to reperfusion may be overlooked.
To improve the various processes and to appropriately allocate resources, an understanding of the flow of patients through the hospital system from arrival to the ED, the time loss associated with acquiring additional imaging, and the time of various components within the angiography suite until final reperfusion will be useful. In this study, we examine the patients in the endovascular arm of the Interventional Management of Stroke (IMS) III trial to not only evaluate the systems processes, but also to identify the factors which ultimately contribute to delays in achieving reperfusion.
METHODS
The IMS III trial was an NIH-funded, phase III, randomized, multi-center, open-label clinical trial designed to determine whether a combined approach with endovascular therapy after IV tPA is superior to standard IV therapy alone when initiated within 3 hours of acute stroke onset in moderate to severe strokes, as determined by a modified Rankin Scale (mRS) score of 0-2 at 90 days. Patients were randomized in a 2:1 ratio with more patients assigned to the endovascular arm.
The IMS III Trial enrolled 656 patients before enrollment was halted for futility in May of 2012, based on a recommendation by the Data and Safety Monitoring Board. The details of the study methods and results have been published.12, 13
Of the 434 patients randomized to the endovascular arm, 16 patients were inpatients at the time of their strokes and were excluded from the workflow analysis. In the remaining 418 patients, the following time intervals were calculated: (1) Stroke onset to ED arrival; (2) ED arrival to CT start; (3) CT to start of IV tPA bolus; (4) IV tPA bolus to randomization; (5) Randomization to groin puncture; (6) Groin puncture to thrombus identification; (7) Thrombus identification to start of endovascular therapy; (8) Start of endovascular therapy to reperfusion. Among those imaged with CT angiography (CTA), the CT to CTA time and CTA to start of IV tPA bolus times were also assessed.
The time of thrombus identification was defined as the time of the angiographic run that shows the intracranial occlusion. The time of start of endovascular therapy is the time of start of IA tPA bolus (via Micro-Sonic SV infusion system [EKOS] or a standard microcatheter), or the start time of balloon occlusion (if Merci retriever [Concentric Medical] was the primary IA device), or the start time of thrombus aspiration (if Penumbra system [Penumbra] was the primary IA device), or the time of first deployment of the device (if Solitaire FR [Covidien] was the primary IA device). The reperfusion time is the time of the last angiographic image.
The impact of patient transfer, baseline CTA performance (yes vs. no), intubation within 7 hours of onset (yes vs. no), as well as time of randomization (working hours “Mon-Fri; 0800-1700” vs. after-hours) on the overall workflow time were evaluated. Patient transfer type was classified into those who received IV tPA and then transferred to another facility for endovascular therapy (drip and ship), those who were transferred to the endovascular facility prior to IV tPA initiation (ship and drip), and those who presented and treated within the same center (mother-ship). In addition, change in times from ED to reperfusion and from groin puncture to reperfusion over the entire study period were assessed.
All times are reported as medians (with IQR). The Kruskal-Wallis test was used to compare the median times, with adjustment for multiple comparisons by the Bonferroni method where applicable. To investigate factors associated with delays, univariable and multivariable linear regression models were constructed with both ED to reperfusion time and onset to reperfusion time individually as outcomes. Potential predictors include age, baseline National Institute of Health Stroke Scale (NIHSS) stratum (≤19 vs ≥20), sex, warfarin use, quartiles of enrollment, randomization during working hours transfer type, baseline use of CTA, and intubation status. The model for ED to reperfusion time was fit only within patients treated in the mother-ship paradigm and thus excluded transfer type as a predictor. Similarly, the impact of CTA conduct, patient transport, and the enrolment time on favorable outcome (mRS ≤2) was assessed in univariable and multivariable logistic regression models. Models assumptions and goodness of fit were assessed and found to be valid. Analyses were performed using SAS® 9.3 Software. A two-tailed significance level of 0.05 was used.
Informed consents were obtained from all participants (or their legal representatives) prior to enrollment. The study was approved by the institutional review committee of the participating centers.
RESULTS
The clinical characteristics and outcomes of these patients were previously published.12 The specified time intervals are shown in Table 1. The median time from onset to ED arrival was 50 minutes (IQR 34). Prior to the start of the endovascular procedure, the longest time interval was from IV tPA initiation to groin puncture (85 minutes, IQR 41). During the endovascular procedure, the longest time interval was from start of endovascular therapy until the last angiographic image was acquired (85 minutes; IQR 74). There was a weak negative correlation between the time from onset to ED arrival with the puncture to reperfusion time; and between the time interval from ED arrival to baseline CT with the CT to puncture time.
Table 1.
The various time intervals in the endovascular arm of the IMS-III trial. (IQR: interquartile range)
| Time Interval (Minutes) | Median | IQR |
|---|---|---|
| Stroke Onset to ED Arrival (N= 418) | 50 | 34 |
| ED Arrival to baseline CT (N= 413) | 19 | 15 |
| Baseline CT to IV bolus (N=412) | 42 | 30 |
| Baseline CT to IV (patients with CTA, N=206) | 39 | 27 |
| Baseline CT to CTA (N= 206) | 6 | 7 |
| Baseline CTA to IV bolus (N= 207) | 31 | 24 |
| Baseline CT to IV bolus (patients without CTA, N= 206) | 47 | 28 |
| IV tPA Bolus to groin Puncture (N=407) | 85 | 41 |
| IV tPA Bolus to randomization (N= 417) | 24 | 25 |
| Randomization to groin Puncture (N= 408) | 62 | 38 |
| Groin puncture to thrombus identification (N= 327) | 15 | 15 |
| Thrombus identification to Endovascular therapy initiation (N= 310) | 20.5 | 24 |
| Endovascular therapy initiation to reperfusion (N= 312) | 85 | 74 |
| IV tPA bolus to Endovascular therapy initiation (N= 314) | 125.5 | 46 |
Delays Due to Inter-hospital Patient Transfer (Figure 1, Table 2)
Figure 1.
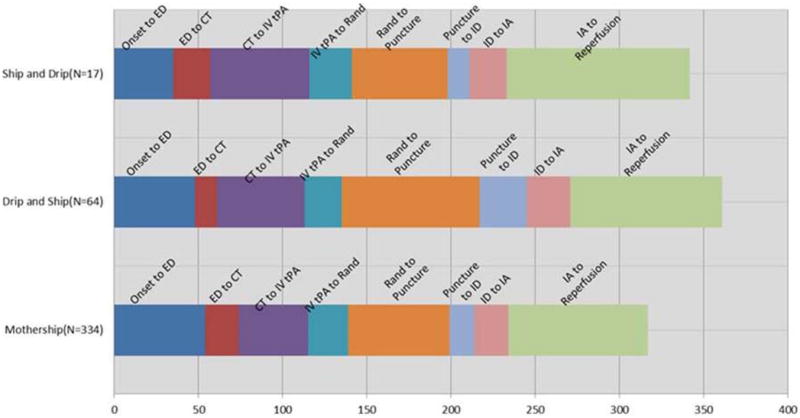
Various time intervals in patients treated within the same institution (mother-ship) vs. those who were transferred from another before (ship and drip) or after (drip and ship) IV tPA therapy. (CTA: CT angiogram, ED: emergency department, IA: start of endovascular therapy, ID: thrombus identification, IV tPA: intravenous tPA, Puncture: groin puncture; Rand: randomization).
Table 2.
Time intervals according to treatment location.
| Time interval (Minutes) | Mother-shi (N = 334) |
Drip and ship (N = 64) |
Ship and drip (N = 17) |
|||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| IV tPA bolus to randomization | 24 | 25 | 22 | 32 | 25 | 27 |
| Randomization to groin puncture* | 60 | 35 | 82† | 34 | 57 | 52 |
| IV tPA bolus to groin puncture* | 83 | 37 | 105† | 47 | 65 | 57 |
P-value for Kruskal-Wallis test: <0.0001
Comparing to Mother-ship only, P-value < 0.0001
Time from IV tPA bolus to groin puncture was affected by transfer status. This difference appears to be driven largely by the time from randomization to puncture, as the time from IV bolus to randomization is not different according to transfer type. Patients who were treated in the drip and ship paradigm had a significantly longer time from IV tPA to puncture than the patients randomized and treated in the same facility (p-value <0.0001 for both the IV tPA bolus to puncture and randomization to puncture time intervals). There was no difference in the time from IV to puncture in patients who were treated in the ship and drip paradigm compared to those who were randomized and treated in the same facility (p-value >0.2 for both time intervals).
The odds of a good clinical outcome (mRS≤2) for subjects treated under the drip and ship paradigm are less than the odds for subjects treated under the mother-ship paradigm (OR 0.56, 95% CI 0.31, 0.99; p = 0.045). However, this association was not significant after adjustment for baseline CTA, age, baseline NIHSS, baseline ASPECTS, and reperfusion status. Given the small number of patients in the ship and drip model, a comparative outcome analysis of this group was not performed.
Use of CT Angiography (Figures 2 & 3)
Figure 2.
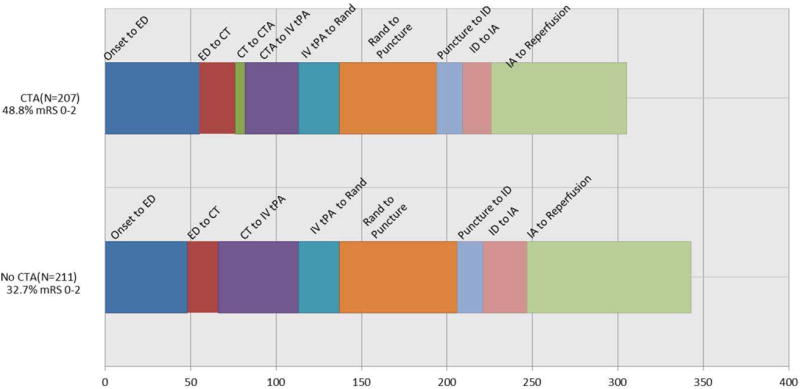
Time intervals in patients investigated by CT and CTA vs. CT alone. (CTA: CT angiogram, ED: emergency department, IA: start of endovascular therapy, ID: thrombus identification, IV tPA: intravenous tPA, Puncture: groin puncture; Rand: randomization).
Figure 3.
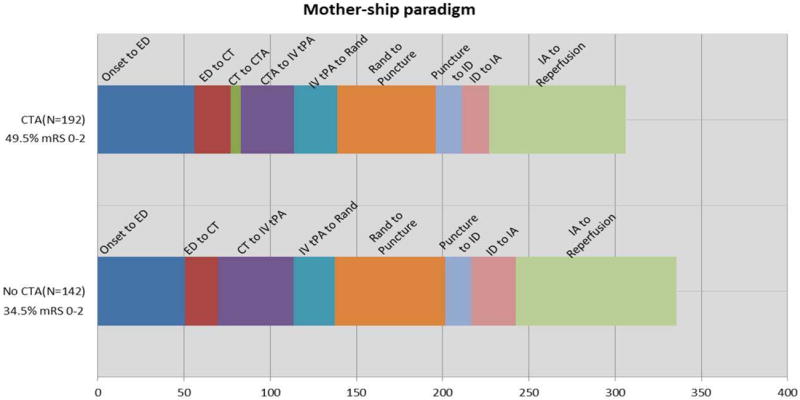
Time intervals in patients investigated by CT and CTA vs. CT alone in the mother-ship paradigm only. (CTA: CT angiogram, ED: emergency department, IA: start of endovascular therapy, ID: thrombus identification, IV tPA: intravenous tPA, Puncture: groin puncture; Rand: randomization).
The use of CTA prior to randomization was not mandatory. However, a total of 207 patients (49.5%) in the endovascular arm had baseline CTA performed. The median time from baseline CT to CTA was 6 minutes (IQR 7). The use of CT angiography did not cause delays in IV tPA bolus initiation. The median times from CT to IV tPA bolus in those who underwent CTA (39 minutes) was significantly shorter than those who did not undergo CTA (47 minutes; Figure 2). Patients who underwent CTA or MRA had a slightly higher proportion of proximal occlusions compared to those who underwent CT alone: ICA or M1 occlusions 66.9% in CTA/MRA vs 61.0% in the CT alone group (p-value >0.05).
Transfer patients were less likely to have a baseline CTA and experienced a longer time from randomization to puncture. To minimize the impact of this potential confounding, the effect of baseline CTA use on favorable outcome was analyzed only under the mother-ship paradigm (Figure 3). The odds of favorable outcome among subjects with a baseline CTA were 2.1 times the odds for subjects with CT alone (95% CI 1.1 to 3.8), after adjustment for age, baseline NIHSS stratum, baseline Alberta Stroke Program Early CT score (ASPECTS), site of occlusion, and successful reperfusion (defined as TICI 2b-3).
Intubation (Supplemental Table 1)
The overall workflow time did not vary significantly according to intubation utilization. The median time from randomization to groin puncture was 60 minutes for those who did not require intubation (n=251 patients) compared to 66 minutes in those who were intubated as per the routine practice of that institution for endovascular therapy (n=73 patients) and 68 minutes in those who required intubation for medical reasons (n=67 patients).
Delays Due to Procedural Timing (Figure 4)
Figure 4.
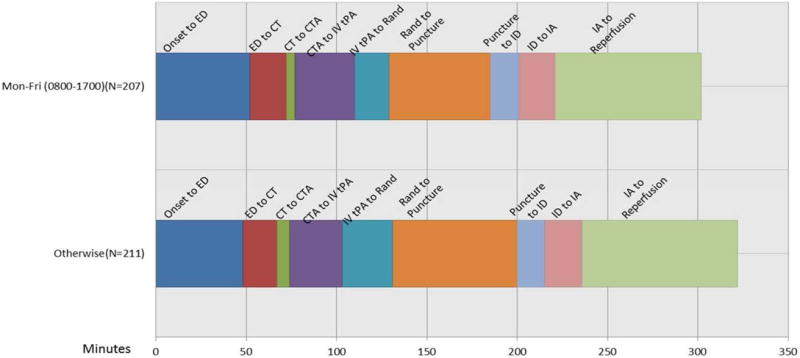
Time intervals in patients who underwent endovascular procedures during working hours (Mon-Fri; 0800-1700) compared to patients treated outside these hours. (CTA: CT angiogram, ED: emergency department, IA: start of endovascular therapy, ID: thrombus identification, IV tPA: intravenous tPA, Puncture: groin puncture; Rand: randomization).
Randomization occurred during the working hours (Mon-Fri; 0800-1700) in 207 patients vs. 211 patients randomized outside these hours. The ED to imaging time during working hours was 20 minutes (IQR 15minutes) compared to 19 minutes in those treated outside these hours (IQR 15 minutes, p = 0.20). The time from CT to groin puncture during working hours (119 minutes, IQR 49 minutes) was shorter than the 141 minutes for those presenting after these hours (IQR 54 minutes, p <0.0001). In those who were randomized during day time (0800-2100), the time from CT to groin puncture was 127 minutes (n=341; IQR 51 minutes) compared to 142 minutes during night time (n=63; IQR 60 minutes, p=0.0012).
Workflow Changes during the Enrollment Period (Figure 5)
Figure 5.
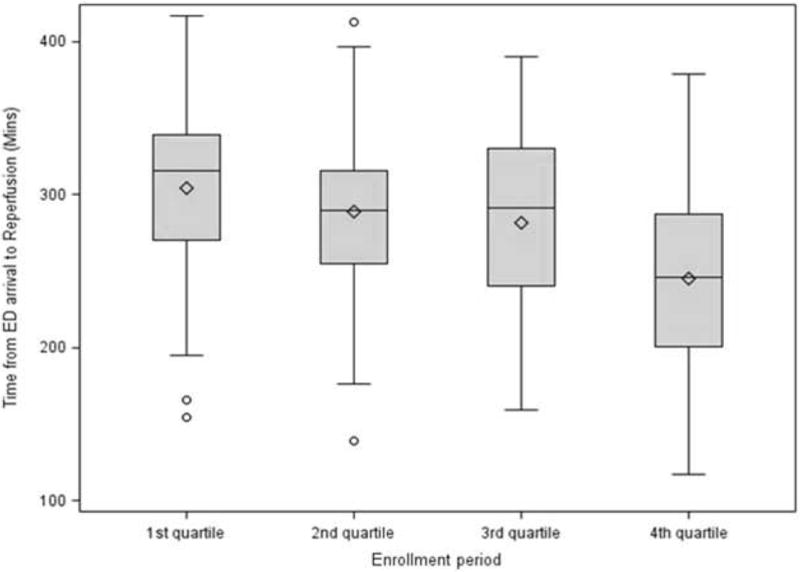
The change in the time from ED arrival until reperfusion by enrollment quartile. (Mins: minutes)
There was significant improvement in the time from ED arrival to reperfusion as well as the time from groin puncture to reperfusion during the course of the trial. During the first quartile of enrollment, the median time from ED arrival to reperfusion was 316 minutes compared to 246 minutes in the last quartile (p-value <0.0001). Similarly, the time from groin puncture to reperfusion decreased from 145 minutes during the first quartile of enrollment to 120 minutes in the last quartile (p-value = 0.0005).
Predictors of Delays in ED Arrival to Reperfusion and Onset to Reperfusion Times
A multivariable linear regression model was fitted to identify predictors of time from ED arrival to reperfusion in 261 patients without missing data who were treated in the same facility (mother-ship). Significant predictors were the use of CTA, procedural timing, and timing of enrolment during the course of the trial. Patients who underwent CTA had about 20 minutes shorter ED to reperfusion times compared to those who did not have CTA (p-value = 0.008). Similarly, patients who were randomized during working hours had 15 minutes shorter ED to reperfusion time compared to those who were randomized after hours (p-value = 0.03). Finally, patients who were enrolled during the last quartile of the study had 46 minutes shorter ED to reperfusion time compared to patients who were enrolled during the first quartile of the study (p-value <0.0001). The ED to reperfusion times for patients enrolled in the second and third quartiles were not significantly different from those enrolled during the first quartile.
We also investigated factors that impacted the onset to reperfusion times. Subjects with baseline CTA had 17 minutes shorter onset to reperfusion times compared to patients who did not have baseline CTA (p-value = 0.016). Compared to patients who were enrolled during the first quartile of the study, patients who were enrolled during the last quartile had 42 minutes shorter onset to reperfusion time (p-value <0.0001). However, there was not a significant difference in the onset-to-reperfusion time between patients enrolled during the second or third quartiles and those enrolled in the first. Finally, patients treated in the drip and ship paradigm had 21 minutes longer onset to reperfusion time compared to those treated in the same center (p-value =0.039). There was insufficient evidence to demonstrate a longer onset to reperfusion time (mean 22 minutes) in those treated in the ship and drip paradigm compared to those treated in the same center (p-value = 0.16); however, there are only 17 patients in this ship and drip category, resulting in limited power for this test. In these two models, age, sex, baseline NIHSS, and intubation status were not shown to have significant impact on time delays.
DISCUSSION
In acute ischemic stroke therapy with IV tPA, ED to needle time within 60 minutes has become an important benchmark in evaluating the efficiency of the workflow for delivering IV tPA and measuring the quality of stroke centers. Despite the evidence behind IV tPA and the resources allocated to meeting this target, only a quarter of IV tPA-treated patients met this time metric in the Get With The Guidelines” (GWTG).14 There are multiple proposed target times for endovascular therapy including the picture-to-puncture,15 puncture-to-reperfusion, etc. Certain time metrics capture delays at specific stages of the treatment continuum, e.g. delays in patients’ transport are captured by the picture to puncture time. However, there is no consensus on a single time metric that would capture the elements unique to those treated with the combined approach of IV tPA plus endovascular therapy. Such time metric is increasingly needed to better understand these delays and to provide a target that all centers should aspire to achieve.16
The importance of a fast onset to treatment time in acute ischemic stroke cannot be overemphasized. Considerable delays were encountered in starting endovascular therapy in the IMS III trial. Although 63.4% of the patients presented to the ED within 60 minutes, delays occurred from the start of IV tPA infusion to randomization followed by further delays during the endovascular procedures until reperfusion was achieved. With the known strong correlation of time and stroke outcome, such delays are expected to produce lower than anticipated outcomes with endovascular therapy.17 While the time from ED arrival to endovascular reperfusion improved during the course of the trial, significant variability in the times within each enrollment quartile may have prevented these shorter times from reflecting on the overall trial results.
Some of the delays encountered during the endovascular procedures could be device-related. The current first choice endovascular devices stentrievers were used in only few patients in the endovascular arm which may have affected both the success and speed of reperfusion. With the increasing use of stentrievers and the progressively short puncture to reperfusion times reported, endovascular procedures are already shorter and yield higher rates of successful reperfusion.7, 18, 19
Our data did not show a longer time to IV tPA administration when baseline CTA imaging was performed. When added to the other valuable information gained from CTA regarding the exact occlusion site and the vascular bed anatomy, CTA becomes an instrumental tool that will result in a net save of the time from onset to reperfusion.20 In addition to outlining the anatomical and pathological aspects of the aortic arch and carotid system to help plan the endovascular procedure, CTA also serves to localize the exact site of occlusion. It is not uncommon for a thalamic stroke due to posterior cerebral artery occlusion to mimic MCA occlusions, or for a clinical right ACA occlusion to require endovascular access from the left carotid system when both ACAs originate from the left side (azygous variant). Moreover, CTA helps in planning the use of appropriate catheters and other tools, and obviates the need for a complete angiogram and hence may serve to save procedural time. However, the association of CTA use with shorter times to reperfusion have other potential explanations. Routine CTA use is expected in large-volume centers where protocols for IV tPA use are practiced routinely with subsequent efficient and timely execution. In addition, the interpretation of CTA is anticipated to be faster in centers using this imaging modality routinely. We did not measure the time required for CTA interpretation due to numerous practical and perceived difficulties. However, CTA has the advantage of being readily-available upon acquisition with no need for post-processing. Moreover, interpretation of CTA images could take place in parallel with other treatment steps without delaying the overall workflow. Our data did not show a significantly longer time to groin puncture in patients who were intubated for the endovascular procedure. However, the time required for intubation may become a factor in patients treated using stentrievers where short imaging to reperfusion times are achievable.4 In addition, we did not investigate the effects of general anesthesia on stroke outcome in these patients.21
Significant delays were noted from IV tPA administration to groin puncture in the IMS III study. There are many potential reasons for delays in this time interval. One component might be delays encountered during patient transport after IV tPA is initiated. Patients who received IV tPA in the drip and ship paradigm had longer times from tPA to groin puncture compared to those transported without receiving IV tPA. While IV tPA therapy should be initiated as soon as possible in all eligible patients, this finding highlights the need for protocols to guide the care of patients planned for transport for endovascular stroke therapy to minimize any delays introduced by tPA administration before transportation. Pre-hospital assessment and triage of the most severe stroke patients directly to comprehensive stroke centers that are experienced in endovascular therapy is another potential mechanism to decrease delays. To measure delays encountered during patient transfer, the American Heart Association defined a time metric (door-in / door-out) for acute coronary syndrome patients to capture the time interval from admission to the outside hospital to ambulance departure toward the treatment center.22 When this metric was achieved in 30 minutes or less, faster treatment times and lower mortality were described. To account for delays encountered in the endovascular drip and ship approach, investigators devised the picture-to-puncture time metric to capture delays occurring from the time of baseline CT scan until groin puncture is done.15 Patients with a picture-to-puncture time over 90 minutes had a significantly lower likelihood of independent functional recovery at 90 days. This stresses the importance of coordinated, protocol-driven steps for the expedited transport and treatment of such patients especially when imaging needs to be repeated prior to the endovascular procedure.
Delays from IV tPA administration to randomization were encountered in patients treated with endovascular therapy in the same center. Some of these delays can be attributed to the time spent screening the patient for the trial, obtaining the informed consent, and the randomization process. Such delays are well-documented and occur despite best efforts.23 Deferral of consent, surrogate consent or shortened consent forms have been proposed to shorten this time interval. Further, the time required for the assembly of the interventional team, for intubation (in centers routinely performing these procedures under GA), and for preparing the endovascular devices can result in significant delays. Alerting the endovascular team as soon as possible, having an angiography tray ready for use, and using the same catheters/ devices setup for all endovascular stroke cases are measures that can be considered to decrease these delays. Comprehensive stroke centers with high patient volume might be more accustomed to these practices compared to centers with relatively low patient volume.
Patients treated after hours and on weekends had longer CT to groin puncture times compared to those treated in the working or during daytime hours. Worse in-hospital outcomes have been reported in stroke patients admitted during weekends compared to regular working hours.24, 25 However, some studies suggest that comprehensive stroke centers seem to avoid this effect.26 Although our analysis did not account for outcomes, such delays likely impacted outcomes. One of the proposed solutions for weekend delays is to cross-train X-ray or CT technologists to assist in the angiography suite coverage during these times.27 This requires that the other members of the interventional team are also readily available. This could be addressed by establishing a group alert paging system which links members of the stroke and interventional teams to provide enough time to travel to the hospital as IV tPA is being administered.28
This study has limitations. An inherent bias exists that people tend to function better when they are being watched or recorded. This may cause the times recorded in the setting of a trial to look better than real life times. However, any time saved due to this bias is likely counteracted by times lost in obtaining the trial consent. Our data and analysis of factors affecting workflow are restricted to the variables available in the study. Other variables that may influence time delay are not available for analysis, such as individual centers’ case volume and catchment area. We performed exploratory analyses to assess the impact of some of the factors we studied on outcomes. These post-hoc analyses do not account for many important baseline differences which could explain any outcome difference. Therefore, the results of the outcome analyses should be viewed in the context of these important limitations and we hope they will serve to stimulate further research in this subject.
Conclusions
In the endovascular arm of the IMS III trial there were significant delays from start of IV tPA to groin puncture. Improvement in workflow times were noted as the trial advanced. The use of CTA correlated with an overall shorter time to reperfusion and was associated with better clinical outcomes compared to patients’ who underwent CT alone. Use of intubation did not result in additional delays. Endovascular treatment outside of working hours did result in additional delays. These data may help in designing, optimizing and documenting workflow in current and future endovascular trials.
Supplementary Material
Acknowledgments
Funding Sources: This study was funded by the US National Institutes of Health and National Institute of Neurological Disorders and Stroke (grant numbers UC U01NS052220, MUSC U01NS054630, and U01NS077304). Genentech supplied the study drug used for intra-arterial thrombolysis in the endovascular group. EKOS Corporation, Concentric, and Cordis Neurovascular supplied study catheters during amendments 1–3. In Europe, IMS III investigator meeting support was partly provided by Boehringer Ingelheim.
Footnotes
Conflict of Interest Disclosures: M. Goyal received a modest research Grant from Covidien. He is a paid consultant for Covidien. He received lecture fees from Covidien and owns Stock in Calgary Scientific and NoNO. M.A. Almekhlafi has nothing to disclose. L. Fan has nothing to disclose. B. Menon received research Support from Heart and Stroke Foundation of Canada Research Scholarship. A. Demchuk received Grant support and lecture fees Covidien. S.D. Yeatts has nothing to disclose. M. Hill received research grant from Hoffman-La Roche Canada, and Heart and Stroke Foundation of Alberta. . He is a paid consultant for Vernalis Group. He received lecture fees from Hoffman-La Roche Canada and owns Stock in Calgary Scientific,. T. Tomsick received Research Grant; from Covidien. P. Khatri serves as an expert Witness in Medico-Legal Consulting. She is a paid consultant for Penumbra, Genentech, and Janssen Pharmaceuticals. She received travel support from Genentech. O. Zaidat has nothing to disclose. E. Jauch has nothing to disclose. Muneer Eesa has nothing to disclose. T.G. Jovin is a paid consultant for Silk Road Medical and owns Stock in Silk Road Medical. J.P. Broderick received Lecture fees from Oakstone Publishing and consulting fees from PhotoThera.
References
- 1.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Ecass AN, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G Group Er-PS. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 2.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, Ims I, Investigators II. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 4.Almekhlafi MA, Eesa M, Menon BK, Demchuk AM, Goyal M. Ultrashort imaging to reperfusion time interval arrests core expansion in endovascular therapy for acute ischemic stroke. J Neurointerv Surg. 2013;5(Suppl 1):i58–61. doi: 10.1136/neurintsurg-2012-010486. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM Penumbra Pivotal Stroke Trial Investigators CSP, the Seaman MRRC. Effect of baseline ct scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42:93–97. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 7.Almekhlafi MA, Menon BK, Freiheit EA, Demchuk AM, Goyal M. A meta-analysis of observational intra-arterial stroke therapy studies using the merci device, penumbra system, and retrievable stents. AJNR Am J Neuroradiol. 2013;34:140–145. doi: 10.3174/ajnr.A3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford AL, Williams JA, Spencer M, McCammon C, Khoury N, Sampson TR, Panagos P, Lee JM. Reducing door-to-needle times using toyota’s lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–3398. doi: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meretoja A, Weir L, Ugalde M, Yassi N, Yan B, Hand P, Truesdale M, Davis SM, Campbell BC. Helsinki model cut stroke thrombolysis delays to 25 minutes in melbourne in only 4 months. Neurology. 2013;81:1071–1076. doi: 10.1212/WNL.0b013e3182a4a4d2. [DOI] [PubMed] [Google Scholar]
- 10.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Shamy M, Menon BK, Saver JL, Diener HC, Mocco J, Pereira VM, Jovin TG, Zaidat O, Levy EI, Davalos A, Demchuk A, Hill MD. Endovascular stroke trials: Why we must enroll all eligible patients. Stroke. 2013;44:3591–5. doi: 10.1161/STROKEAHA.113.002522. [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer R, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TA. Interventional Management of Stroke IIII. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, Demchuk AM, Martin R, Mauldin P, Dillon C, Ryckborst KJ, Janis S, Tomsick TA, Broderick JP. Interventional Management of Stroke IIII. Methodology of the interventional management of stroke iii trial. Int J Stroke. 2008;3:130–137. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: Patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 15.Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, Camp D, Frankel MR, Belagaje SR, Anderson AM, Isakov AP, Gupta R. “Picture to puncture”: A novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–1148. doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 16.Zaidat OO, Lazzaro MA, Gupta R, Rasmussen PA, Frei DF, Goyal M. ‘Time’ for success. J Neurointerv Surg. 2013;5:391–392. doi: 10.1136/neurintsurg-2013-010868. [DOI] [PubMed] [Google Scholar]
- 17.Goyal M. Acute stroke intervention results: The “denominator” fallacy. AJNR Am J Neuroradiol. 2014;6:616–618. doi: 10.3174/ajnr.A3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS, Trialists T. Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (trevo 2): A randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira R, Clark W, Budzik R, Zaidat OO. the ST. Solitaire with the intention for thrombectomy (swift) trial: Design of a randomized, controlled, multicenter study comparing the solitaire flow restoration device and the merci retriever in acute ischaemic stroke. Int J Stroke. 2012:1747–4949. doi: 10.1111/j.1747-4949.2012.00856.x. [DOI] [PubMed] [Google Scholar]
- 20.Goyal M, Almekhlafi MA. Dramatically reducing imaging-to-recanalization time in acute ischemic stroke: Making choices. AJNR Am J Neuroradiol. 2012;33:1201–1203. doi: 10.3174/ajnr.A3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, Archer DP, Calgary Stroke P. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396–405. doi: 10.1097/ALN.0b013e318242a5d2. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK American College of Cardiology/American Heart Association Task Force on Performance M, American Academy of Family P, American College of Emergency P, American Association of C, Pulmonary R, Society for Cardiovascular A, Interventions, Society of Hospital M. Acc/aha 2008 performance measures for adults with st-elevation and non-st-elevation myocardial infarction: A report of the american college of cardiology/american heart association task force on performance measures (writing committee to develop performance measures for st-elevation and non-st-elevation myocardial infarction): Developed in collaboration with the american academy of family physicians and the american college of emergency physicians: Endorsed by the american association of cardiovascular and pulmonary rehabilitation, society for cardiovascular angiography and interventions, and society of hospital medicine. Circulation. 2008;118:2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 23.Goyal M. Acute stroke trials and consent. J Neurointerv Surg. 2014;6:254–255. doi: 10.1136/neurintsurg-2012-010595. [DOI] [PubMed] [Google Scholar]
- 24.Saposnik G, Baibergenova A, Bayer N, Hachinski V. Weekends: A dangerous time for having a stroke? Stroke. 2007;38:1211–1215. doi: 10.1161/01.STR.0000259622.78616.ea. [DOI] [PubMed] [Google Scholar]
- 25.Reeves MJ, Smith E, Fonarow G, Hernandez A, Pan W, Schwamm LH Committee GW-SS, Investigators. Off-hour admission and in-hospital stroke case fatality in the get with the guidelines-stroke program. Stroke. 2009;40:569–576. doi: 10.1161/STROKEAHA.108.519355. [DOI] [PubMed] [Google Scholar]
- 26.McKinney JS, Deng Y, Kasner SE, Kostis JB Myocardial Infarction Data Acquisition System Study G. Comprehensive stroke centers overcome the weekend versus weekday gap in stroke treatment and mortality. Stroke. 2011;42:2403–2409. doi: 10.1161/STROKEAHA.110.612317. [DOI] [PubMed] [Google Scholar]
- 27.Magid DJ, Wang Y, Herrin J, McNamara RL, Bradley EH, Curtis JP, Pollack CV, Jr, French WJ, Blaney ME, Krumholz HM. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute st-segment elevation myocardial infarction. JAMA. 2005;294:803–812. doi: 10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 28.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med. 2006;355:2308–2320. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


