Abstract
Background and Purpose
The IMS III study tested the effect of IV t-PA alone as compared to IV t-PA followed by endovascular therapy and collected cost data to assess the economic implications of the two therapies. This report describes the factors affecting the costs of the initial hospitalization for acute stroke subjects from the US.
Methods
Prospective cost analysis of US subjects treated with IV t-PA alone or IV t-PA followed by endovascular therapy in the IMS III trial. Results compared to expected Medicare payments.
Results
The adjusted cost of a stroke admission in the study was $35,130 for subjects treated with endovascular therapy following IV t-PA treatment and $25,630 for subjects treated with IV t-PA alone (p<0.0001). Significant factors related to costs included treatment group, baseline NIH Stroke Scale, time from stroke onset to IV t-PA, age, stroke location, and comorbid diabetes. The mean cost for subjects who had routine use of general anesthesia as part of endovascular therapy was $46,444 as compared to $30,350 for those who did not have general anesthesia. The costs of embolectomy for IMS III subjects and patients from the NIS cohort exceeded the Medicare DRG payment in more than 75% of patients.
Conclusion
Minimizing the time to start of IV t-PA and decreasing the use of routine general anesthesia, may improve the cost-effectiveness of medical and endovascular therapy for acute stroke.
Keywords: stroke, cost, hospital, acute, ischemic, reperfusion, arterial, IMS III
INTRODUCTION
Costs for the initial hospital admission for acute ischemic stroke (AIS) patients are affected by stroke severity, presence of pre-stroke comorbid conditions, type of reperfusion treatment selected, response to therapy including adverse events, hospital care processes, and hospital charging patterns related to use of specific services.1,2 Understanding the relative contributions of these complex factors to the overall cost of stroke care is critical so that the processes of stroke care can be designed to be cost-effective.
Recent studies have reported the costs associated with the use of intravenous (IV) t-PA alone. Brinjikji and colleagues used 2001–2008 HCUP National Inpatient Sample data to identify median charges for AIS patients treated with IV-t-PA, as well as patient and hospital characteristics associated with variations in charges.1 Most previous United States (US) studies of t-PA have used a decision analysis modeling approach.3,4 Fagan and colleagues demonstrated that patients treated with IV t-PA, as compared to placebo-treated patients, had a shorter length of stay (LOS) in the hospital (10.9 versus 12.4 days, p=0.02), long-term health outcomes of 564 (3 to 850) quality-adjusted life-years saved over 30 years of the model per 1,000 patients, and a greater likelihood of discharge to home than to inpatient rehabilitation or a nursing home (48% versus 36%; p = 0.002).5 However, LOS at the time of the NINDS t-PA Trial (1991–1994) was much longer than LOS today. A pooled analysis of the ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators revealed a strong relationship between the speed of initiation of IV t-PA after stroke onset and improved functional outcome at 90 days, but did not examine the economic impact.6 Luengo-Fernandez and colleagues identified an inverted U-shaped relationship between initial National Institute of Health Stroke Scale (NIHSS) score and cost of care in the year subsequent to the initial stroke, with more severe patients expending more, but with costs attenuated for the sickest patients because of early death.7 A recent study compared mechanical thrombectomy as an adjunct to IV t-PA using a Markov model and Monte Carlo simulation based on a hypothetical 68-year-old patient with large vessel ischemic stroke.8 This simulation study reported an interventional strategy has the potential for being cost effective, but the assumptions of the model have been questioned.9
The present economic study was planned as part of the IMS III Trial - an international, multicenter, randomized, open-label clinical trial with a blinded outcome assessment at 3 months - that tested the approach of IV t-PA followed by protocol-approved endovascular treatment as compared with standard IV t-PA.10 The IMS III trial began enrollment in August 2006. In April 2012, after 656 of a planned 900 participants had undergone randomization, the Data and Safety Monitoring Board (DSMB) recommended to the sponsor (the National Institute of Neurological Disorders and Stroke) that enrollment be terminated owing to the crossing of the pre-specified boundary for futility. The clinical trial analysis found no overall difference in outcomes for the two study arms. A pre-specified subgroup analysis showed a statistical trend that patients with more severe stroke may do better with IV+IA, but this relationship did not achieve statistical significance (p=0.06). The current analysis compares initial hospital charges and costs estimated from the recorded charges for IMS III subjects treated in the United States with IV t-PA alone and those treated with IV t-PA followed by endovascular therapy (heretofore referred to as the endovascular treated group) for whom UB04 billing data were prospectively collected as part of the clinical trial.
METHODS
Study Population
The IMS III Trial planned to enroll a maximum of 900 subjects, aged 18 to 82 years, at 58 centers in the United States, Canada, Australia, and Europe. Eligibility criteria included receipt of IV t-PA within 3 hours after symptom onset and a moderate-to-severe neurologic deficit (defined as an NIHSS score ≥10 or, after approval of amendment 3, an NIHSS score of 8 to 9 with CT angiographic (CTA) evidence of an occlusion of the first segment of the middle cerebral artery [M1], internal carotid artery, or basilar artery at institutions where CTA imaging at baseline was the standard of care for patients with acute stroke). Informed consent was obtained from the eligible patient or a legal representative before randomization. Detailed inclusion and exclusion criteria have been published.10 The original economic study of the IMS III Trial was designed with 80% power to detect a 10% reduction in cost over one year post stroke.11 The present analysis has a shorter time horizon for the accumulation of cost difference and contains only US subjects’ UB04 charge data because UB04 data are not collected in Australia, Canada or Europe. The results of this analysis of hospital cost differences should be interpreted in view of these limitations.
Determination of Cost
Cost of the initial hospital admission was estimated based on the actual hospital charges documented on UB04 billing forms provided by the treating hospitals. Total charges were summed if more than one UB04 form was present. Thus, study charges included cases where more than one bill was submitted from a hospital, as well as multiple individual bills submitted from referring and receiving hospitals. Subjects were not charged for the intra-arterial (IA) t-PA (study drug provided by Genentech); thus the cost of one 50mg vial of t-PA was added to the cost estimate for all subjects who received this therapy. Free devices, or hospital-requested device replacements, were provided by some vendors over parts or all of the trial period (EKOS, Concentric, and Johnson and Johnson). This variation was corrected by adding the cost of the primary device to the estimated cost for the affected bills. Charges were converted to costs using the treating hospital’s Medicare cost-to-charge ratio filed with the CMS. All cost estimates were converted to 2012 US dollars (USD) using the Medical Care Services Consumer Price Index (CPI). Since hospital costs vary greatly across geographic regions in the US based on differences in the cost of labor, we also estimated an adjusted cost value, using the CMS 70% labor adjustment factor for each of the hospitals in the study; however, this cost estimate modification did not significantly affect the results, so these data are not reported. Thus, three separate measures of the amount of hospital resources used for the initial ischemic stroke event were estimated in the cost analyses. These were charges as submitted in the year of the event, costs in currency of the year of the event, and Present Value Costs (PVC) inflated to represent costs in 2012. All analyses were performed using SAS, version 9.3 (Cary, NC).
Intent-to-treat analysis included data for all subjects randomized in the study who had economic data collected for the initial stroke hospitalization. Basic descriptive statistics were used to compare costs of subjects randomized to endovascular treatment to subjects receiving IV t-PA alone. The effects of differences in subject characteristics at baseline on observed charges, costs, and 2012 PVC were examined using generalized linear multivariable models with a gamma-distributed log link function. All cost models included as covariates any measures listed in the Online Supplemental Material that were significant at p<0.05. The distribution of hospital charges for the admissions were examined using the standard CMS charge groupings with the exception of the operating or procedure room charges and anesthesia charges, which we combined in the “OR” category, and blood charges, which we included in the “Other” category. Finally, the cost profiles of the IMS treatment groups were compared to the cost profiles of a sample of US patients who were treated under usual care conditions using all payer data from the Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS) for 2010. NIS patients were selected if they had a principal diagnosis ICD-9 code of acute ischemic stroke similar to the principal ICD-9 diagnosis codes observed for the IMS subjects, while also having a procedure code indicating receipt of t-PA. Within this group we then identified the subgroup of admissions with an ICD-9 procedure code of endovascular embolectomy (39.74). While the NIS comparison group was selected to be as similar to the IMS stroke cohort as possible, it is likely that those treated with IV t-PA had substantially lower stroke severity than the subjects in the IMS III Trial, because the trial excluded patients with an NIHSS of < 10 except for patients with NIHSS of 8–9 with a larger artery occlusion on imaging. The charges for the NIS cohort were adjusted to costs using the cost-to-charge ratios for the discharging hospital. For consistency with the IMS III Trial cost analysis, the NIS data were then inflated to 2012 PVC using the Medical Care Services CPI.
RESULTS
A total of 430 (66% of subjects randomized in the study), out of the 454 US subjects in the trial, had usable economic data collected for the initial stroke hospitalization. The 202 non US subjects were not considered in this analysis (Appendix).
The mean observed charges and costs for the subjects with moderate to severe stroke randomized to endovascular treatment and to IV t-PA alone are provided in Table 1. The mean hospital charges per admission recorded on the UB04 form was $113,185, this amount is equal to $35,130 when adjusted to reflect the 2012 costs of a stroke admission (controlling for effects of age, stroke location, NIHSS, diabetes, and time to IV t-PA administration). The $35,130 cost incurred for subjects treated with endovascular therapy and is compared to a cost of $25,630 for subjects treated with IV t-PA alone, a difference of $9,500 in 2012 currency (p<0.0001). The cost of the endovascular devices used to administer IA t-PA or to perform thrombectomy in this population can range from $1,250 to $11,000.12 Thus, the higher cost in the endovascular therapy arm may be largely explained by the cost of the device. As expected, treatment group (IV plus endovascular treatment) and baseline NIHSS (higher severity with higher costs) did affect costs, as did time to t-PA (lower costs with earlier treatment), age (higher costs with older age), stroke location (higher cost with right hemispheric location) and comorbid diabetes (higher costs with diabetes).
Table 1.
Hospital Charges as Incurred, Costs as Incurred, and Costs in 2012 US Currency for the Initial Treatment of US Patients with Moderate or Severe Stroke Enrolled in the Study†
| Treatment Group |
Charges (Year of Stroke*) |
Costs (Year of Stroke*) |
Present Value Costs (2012 Value) |
Estimated Mean 2012 Cost** |
|---|---|---|---|---|
| Endovascular Therapy: | ||||
| Mean (SD) | $113,185 (82,797) | $31,074 (18,756) | $35,175 (20,702) | $35,130 |
| Median (Range) | $86,481 (23,350–552,279) | $26,935 (6,602–179,491) | $29,848 (7,856–205,609) | |
| IV t-PA Alone: | ||||
| Mean (SD) | $86,880 (91,057) | $23,833 (21,799) | $26,266 (24,042) | $25,630 |
| Median (Range) | $58,247 (13,701–830,652) | $18,134 (6,602–179,491) | $19,768 (5,992–209,299) | |
No physician charges are included in the estimates.
IV = intravenous; t-PA = tissue plasminogen activator;
Costs in year of stroke indicates the actual costs at the time of patient enrollment. These costs are not adjusted for inflation.
Multivariable model controlling for age, NIHSS, time to IV t-PA administration, stroke location and diabetes.
The multivariable analysis assessing the effects of differences in subject characteristics at baseline on observed charges, costs, and PVC revealed a significant effect of the “study year” of the trial on charges (p<0.0001) and costs (p=0.0002). However, the inflation-adjusted costs do not show a time effect over the course of the study. Gender, race, history of hypertension, coronary artery disease, congestive heart failure, hyperlipidemia, statin or antiplatelet use at baseline, atrial fibrillation, baseline SBP, baseline INR, withdrawal of care, or pre-existing disability did not significantly affect the estimated cost of a hospital admission for stroke in subjects treated within the Trial.
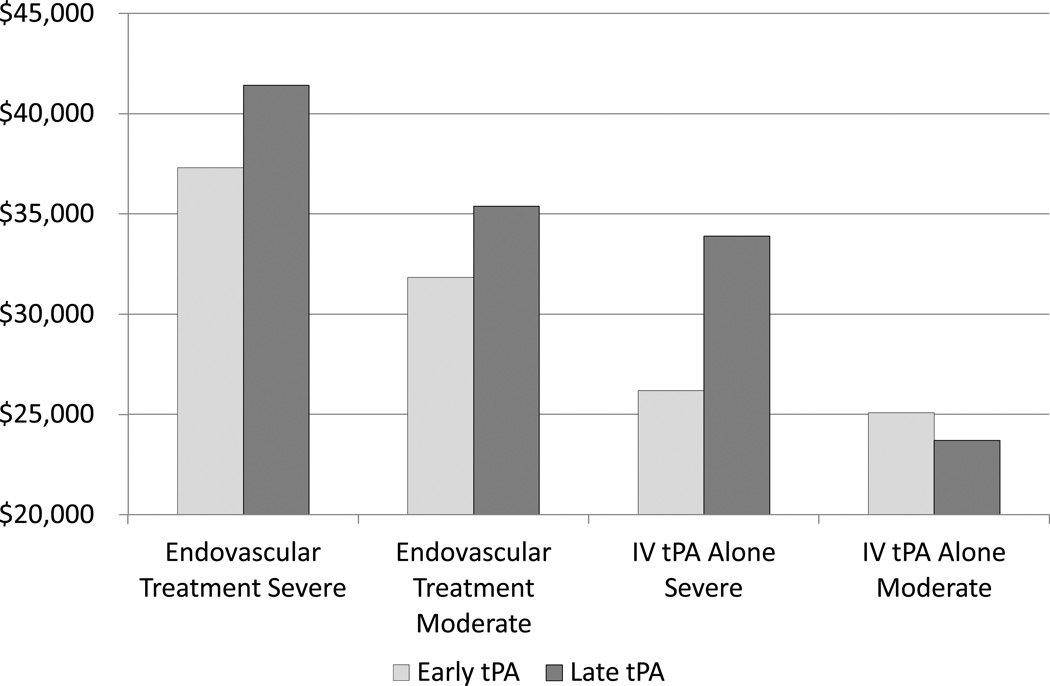
The effects of treatment group, stroke severity and time to t-PA administration on mean PVC of hospital care for stroke are shown in Figure 1. The adjusted mean cost of the initial hospital admission for stroke subjects randomized to endovascular therapy following IV t-PA is, as expected, consistently higher than the mean cost of care for subjects treated with IV t-PA alone, regardless of the timing of t-PA administration. However, the mean cost of care for subjects randomized to endovascular therapy following IV t-PA differed based on whether or not patients actually received an endovascular therapy. Multivariable model estimates (controlling for age, stroke severity and early t-PA administration) of mean cost for subjects randomized to endovascular therapy following IV t-PA who did not receive the intra-arterial intervention was $30,313 compared to $38,424 for subjects who were treated with intra-arterial t-PA or an embolectomy device. For comparison, mean cost for the IV t-PA alone group was $25,630.
Figure 1. Adjusted* Present Value Costs by Treatment Group, Time of IV t-PA Administration and Stroke Severity.
*adjusted for intubation
Subjects with a severe stroke (NIHSS >= 20) have approximately $7,000 greater hospital costs than subjects with a moderate stroke (Figure 1). However, in the group randomized to endovascular therapy, administration of t-PA within two hours of stroke onset reduced the cost of a stroke by an estimated $3,248 (p=0.0442) regardless of stroke severity. A different cost pattern was observed for subjects treated with IV t-PA alone. Early administration of t-PA made no significant difference (-$1,385) in observed cost for those with moderate stroke (p=0.1267) but reduced the mean cost by $7,706 for those with severe stroke. Thus, the greatest cost savings for subjects treated with IV t-PA alone may be expected from improvement in the time to t-PA administration for subjects with severe stroke.
The pattern of adjusted mean length of hospital stay reflects those observed for the costs for subgroups of subjects defined by timing of t-PA and stroke severity. Length of hospital stay was 2.4 days shorter for subjects who received t-PA within 2 hours of stroke onset (p<0.0001); 1.5 days shorter for subjects with baseline NIHSS less than 20 (p=0.0190); and length of hospital stay was 1.3 days longer for subjects with a right hemispheric stroke (p=0.0361). We observed a trend toward slightly shorter mean hospital length of stay (1.0 day) for subjects who received endovascular therapy (p=0.0848) than the mean length of hospital stay observed for subjects who received IV t-PA alone, after controlling for the effects of timing of t-PA and baseline covariates. There was no statistical difference in at 7 days post stroke (p=0.57) for the two study arms, so cost differences are unlikely to be due to survival. Thus, differences in length of hospital stay explain some, if not much, of the cost difference observed in the study.
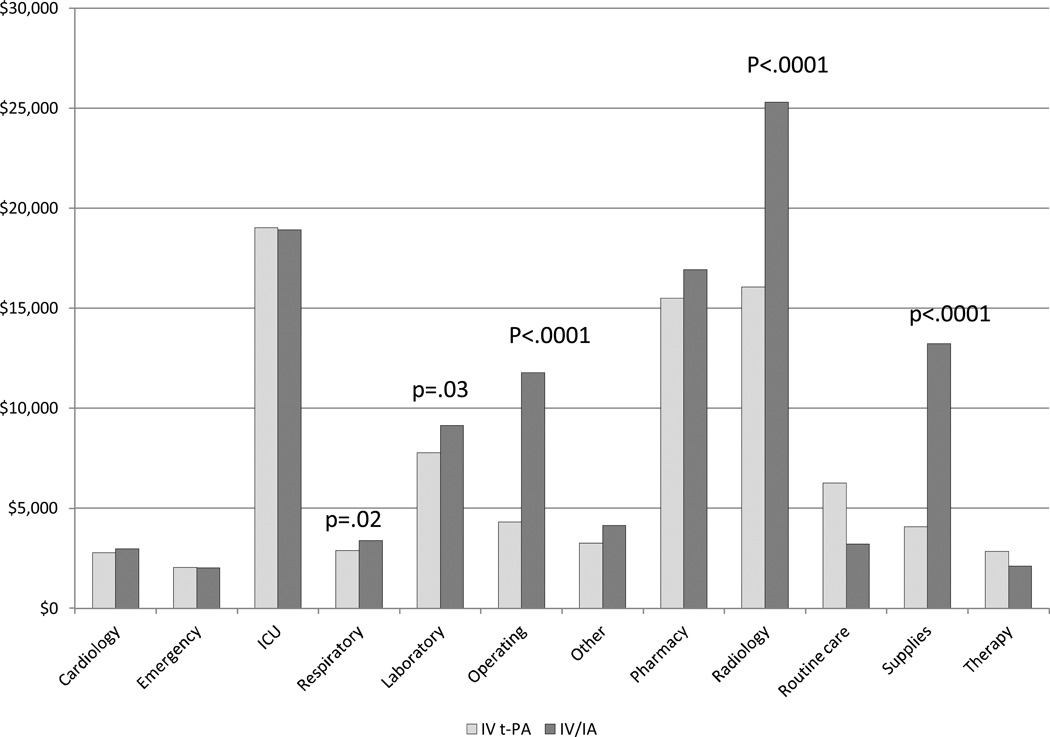
When we examined the distribution of mean charges by charge category (Figure 2), we observed significantly higher charges billed for respiratory services (p=0.02), laboratory (p=0.03), operating or procedure room use (including anesthesia) (p<0.0001), radiology services (p<0.0001) and supplies (p<0.0001) for endovascular therapy subjects when compared to the mean charges for the IV t-PA alone group.
Figure 2. Mean Charges by Category.
Note: Anesthesia is grouped with Operating costs, and blood products are grouped with Other costs.
As expected, we observed some variations in mean cost across the treatment groups depending on the endovascular device types used and the choices made for the use of anesthesia. Some subjects who were randomized to receive endovascular therapy were not candidates for thrombectomy, and three subjects randomized to IV t-PA alone received endovascular therapy. The mean cost observed for subjects by use of intubation for general anesthesia, whether routinely used for the endovascular procedure or as medically indicated for control of airway and respiration, are presented in Table 2.
Table 2.
Adjusted Cost for IV t-PA Alone and Anesthesia as Indicated by Intubation in First Seven Hours After Stroke.
| Treatment Type and Anesthesia Status |
N=430 | Mean Cost per Admission (SD) |
Estimated Mean Cost per Admission * |
|---|---|---|---|
| IV t-PA alone, no intubation ** | 135 | $22,982 ($14,745) | $23,027 |
| IV t-PA alone, with medically indicated intubation | 9 | $59,784 ($61,035) | $57,145 |
| IV t-PA alone with procedural intubation | 2 | $97,137 ($57,114) | $95,829 |
| Endovascular therapy, no intubation | 186 | $30,216 ($15,825) | $30,350 |
| Endovascular therapy, with medically indicated intubation | 45 | $42,760 ($20,196) | $41,690 |
| Endovascular therapy with procedural intubation | 53 | $46,139 (28,912) | $46,444 |
Multivariable model adjusted for effects of NIHSS and early t-PA administration
'no intubation' groups include subjects missing intubation information
IV = intravenous
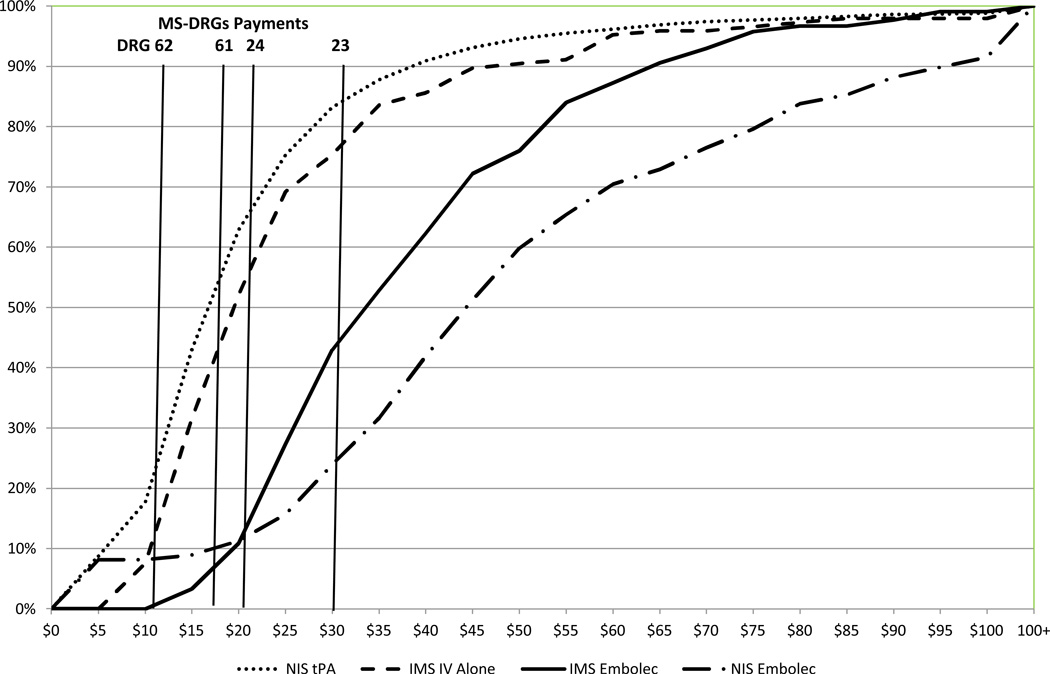
We examined the cost distributions by therapy type and compared the cumulative cost curves for the IMS III subjects to the cost curves for patients from the 2010 NIS inflating their costs to 2012 values to be comparable to the IMS III PVC presented here. The cumulative cost curves for the IMS subjects and the NIS patients are provided in Figure 3.
Figure 3. Subject Treated with t-PA Alone and Those Treated with t-PA followed by Endovascular Therapy Using Embolectomy Devices in IMS III and NIS Cohorts.
Cumulative cost curves for AIS patients receiving IV t-PA alone (red curves) included data from IMS III subjects randomized to t-PA Alone (IMS IV Alone) and HCUP National Inpatient Sample (NIS) patients who received IV t-PA but did not have an endovascular procedure code or a DRG classification indicating an endovascular intervention (NIS t-PA).
Cumulative cost curves for IMS III subjects who were randomized to endovascular treatment with embolectomy devices (black solid line) and NIS patients who had both a t-PA procedure code (indicating received t-PA) and an endovascular procedure code or DRG classification indicating embolectomy (black interrupted line) are provided.
Medicare 2012 payment levels (without any hospital-specific payment for teaching or regional factors) for the DRGs most relevant to IMS III are indicated by the vertical lines. DRG 61 is national payment for AIS with use of thrombolytic agent with major comorbid condition (MCC), DRG 62 is payment with comorbid condition (CC), and DRG 63 is payment without MCC or CC. To best reflect IMS III in this figure DRG 23 is payment for AIS with embolectomy (Craniotomy with major device implant or acute complex CNS PDX w MCC) and a t-PA procedure code, and DRG 24 is payment for AIS with embolectomy as defied above without MCC and a t-PA procedure code. Payment and cost of providing care is equal (on average) if the payment line crosses the cumulative cost cure at the 50% mark. Payments are inadequate for covering cost of care (on average) if the payment line crosses the cumulative cost curve at a point lower than 50%, and payment exceeds cost of care (on average) if the payment line crosses the cumulative cost curve above the 50% mark.
The observed cost curves for IMS III subjects treated with IV t-PA alone appear slightly higher than the cost curve for the NIS stroke patients with an ICD-9 code of t-PA, reflecting the exclusion of mild strokes from the trial. Further, the expected Medicare payments for MS-DRGs 61, 62 and 63 fall below the 50th percentile on the cumulative cost curves, indicating that payment for these stroke patients may be slightly less than the cost of care provided.
A different pattern is observed for subjects who had embolectomy following t-PA. Here the cumulative cost curve for IMS III subjects fall to the left of the curve for NIS patients. This indicates that the IMS subjects have lower median cost than the costs estimated for the NIS patients. Furthermore, the expected payment for both groups fall far left of the median of the cost curves. This means that the typical Medicare DRG payment will be lower than what it costs a hospital to treat such a patient in a majority of cases. For the IMS III subjects, we may expect the care of 75 percent of subjects to cost more than the Medicare DRG payment will reimburse, while the expected DRG payment will not cover the cost of care for 85 percent of patients in the NIS cohort.
DISCUSSION
The financial burden of stroke is large from all viewpoints: person, society and payer. While the cumulative costs of stroke care in the long term far exceed the costs of acute care, costs associated with acute care have not been well characterized using detailed billing information.13,14 As expected, emergency endovascular intervention using embolectomy devices in the IMS III Trial resulted in greater cost per subject as compared to subjects treated with IV t-PA alone. This cost difference is explained primarily by the use of the embolectomy devices, intra-arterial t-PA, and the angiographic procedure. However, the IMS III Trial also provides insights into three important drivers of acute care costs in subjects who undergo reperfusion therapy: baseline stroke severity, time from stroke onset to start of reperfusion therapy, and routine use of general anesthesia during endovascular stroke therapy.
Stroke prognosis and care needs are very much governed by stroke severity, and costs and outcomes are closely linked in our findings. Severe stroke was much more expensive in the acute hospital care than moderate stroke in the IMS III Trial, irrespective of treatment assignment. This is an intuitive result for clinicians because patients with severe stroke are less likely to be discharged quickly and to go home. It also complements the recent report by Fonarow and colleagues using the Get With The Guidelines (GWTG) database in which baseline stroke severity is the most important driver of subject outcome and mortality.2
Evidence of a relationship between time to IV tPA and clinical outcome was found in only one of the four (US) subgroups examined: endovascular subjects with NIHSS ≥20. Interestingly, in the pre-specified subgroup analyses of all IMS III subjects comparing IV t-PA with IV t-PA followed by endovascular therapy, there was a trend (non-significant) toward better overall outcomes among participants in the endovascular group in those patients treated with IV t-PA within 2 hours after stroke onset.
Though we do not have evidence to show association of time to IV t-PA and outcome in the subgroups with significantly different costs, this may be due to lack of power. In addition, the pattern of adjusted mean length of hospital stay reflects those observed for the costs for subgroups of subjects defined by timing of t-PA and stroke severity. Length of hospital stay was 2.4 days shorter for subjects who received t-PA within 2 hours of stroke onset (p<0.0001); 1.5 days shorter for subjects with baseline NIHSS less than 20 (p=0.0190 – already in results). It is not clear why decreased costs are not seen in the subgroup of patients treated with IV t-PA with NIHSS 8–19; imbalances in other variables related to both outcome and costs between patients treated early and late could be a factor and small numbers can lead to differences simply related to chance as the confidence intervals include both positive and negative relationships.
This finding provides strong evidence to motivate physicians and hospitals to put into place systems of care that allow for and demand very rapid treatment times with special focus on assuring that these systems are effective for both patients with moderate and severe stroke. While we recognize the importance of a 60-minute door-to-needle time yet uncommonly meet that benchmark, the Helsinki group routinely manages treatment under 30 minutes.15 Thus, decreasing the time from stroke onset to start of reperfusion therapy currently saves money as it saves ischemic brain for severe stroke patients. More work may be needed to assure that patients with moderate stroke have equal benefits.
Costs for endovascular therapy were substantially increased by the routine use of general anesthesia with intubation as part of an endovascular procedure. Increases in respiratory therapy, radiography and anesthesia services were major drivers of the increased costs. Given the association of anesthesia with worse clinical outcomes in several prior reports,16,17 and the substantial increased costs with use of general anesthesia and intubation, this practice needs great scrutiny going forward.
Importantly from the overall societal costs perspective, the charges for subjects in the IV t-PA treatment group within the Trial were close to charges for a similar cohort from the NIS. This suggests that these economic results from the IMS III Trial are generalizable to typical care in the United States. The costs for endovascular therapy with devices in IMS III were less than the NIS sample of patients treated with embolectomy, likely because embolectomy devices were used in a minority of IMS III treated patients who received endovascular therapy as compared to those in the recent NIS sample. The costs of care for endovascularly treated patients far exceed typical remuneration patterns in the NIS, implying that these procedures are under-supported financially.
The current study has several limitations. First, we analyzed only hospital costs and did not include any physician costs in our analyses. It would be expected that the use of endovascular treatment and routine anesthesia would add a larger amount of separately billed physician costs to the overall total cost difference between the two approaches. Second, this analysis examines only the initial hospitalization and does not examine the costs over subsequent years that are highly correlated with disability following stroke. A subsequent paper will address the one-year costs and projected future costs in the IMS III cohort. Finally, we cannot generalize our cost estimates to other countries. However, we expect that the increase in costs associated with stroke severity, endovascular treatment, later treatment with IV t-PA, and routine use of intubation will be mirrored in other countries as well.
Supplementary Material
Acknowledgments
FUNDING SOURCES
Supported by grants from the National Institutes of Health and the National Institute of Neurological Disorders and Stroke (UC U01NS052220, MUSC U01NS054630, and U01NS077304) and by Genentech ®, EKOS® Corporation, Concentric Medical Inc., Cordis ® Neurovascular Corporation, and Boehringer-Ingelheim Pharmaceuticals, Inc.
Joseph P Broderick, MD
Research Grant: NIH IMS III grant; Significant. Genentech PRISMS Steering Committee; Significant. Other Research Support; Significant; Genentech and Schering-Plough; -study medication for NINDS-funded IMS III and CLEARER trials, Genentech -PRISMS Trial consultant on steering committee, Penumbra-THERAPY Trial co-inv (paid to Dept). EKOS and Concentric study devices for first years of IMS III trial.
Lydia D. Foster, MS- Research Grant; Significant; NIH-IMS III Trial
Michael Hill, MD
Research grant: Hoffmann-La Roche Canada Ltd. Stock Ownership: Calgary Scientific Inc. No compensation.
Edward C. Jauch, MD - Research Grant; Significant; NIH-IMS III Trial.
Pooja Khatri, MD
Other Research Support; Significant; Genentech-PRISMS Trial PI (paid to Dept), Penumbra-THERAPY Trial PI (paid to Dept).
Yuko Palesch, PhD
Research Grant: NIH The work presented in the manuscript was supported by NINDS grant funds for IMS III; Significant.
Patrick D. Mauldin, PhD - Research Grant: NIH-IMS III Trial; Modest.
Judith Spilker, RN BSN
Research Grant; -Significant; NIH-IMS III Trial
Sharon Yeatts, PhD
Research Grant: NIH IMS III Co-investigator: Significant.
Footnotes
Author Disclosures
Dawn Kleindorfer, MD – None.
Renee Martin, PhD – None.
Kit Simpson, DrPH: None.
Annie Simpson, PhD: None.
REFERENCES
- 1.Brinjikji W, Rabinstein AA, Cloft HJ. Hospitalization costs for acute ischemic stroke patients treated with intravenous thrombolysis in the United States are substantially higher tha Medicare payments. Stroke. 2012;43:1131–1133. doi: 10.1161/STROKEAHA.111.636142. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, Broderick JP, et al. Comparison of 30-Day Mortality Models for Profiling Hospital Performance in Acute Ischemic Stroke With vs Without Adjustment for Stroke Severity. JAMA. 2012;308:257–264. doi: 10.1001/jama.2012.7870. [DOI] [PubMed] [Google Scholar]
- 3.Jackson D, Earnshaw SR, Farkouh R, Schwamm L. Cost-Effectiveness of CT Perfusion for Selecting Patients for Intravenous Thrombolysis: A US Hospital Perspective. Am J Neuroradiol. 2010;31:1669–1674. doi: 10.3174/ajnr.A2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzauskas GF, Boudreau DM, Villa KF, Levine SR, Veenstra DL. The Cost-Effectiveness of Primary Stroke Centers for Acute Stroke Care. Stroke. 2012;43:1617–1623. doi: 10.1161/STROKEAHA.111.648238. [DOI] [PubMed] [Google Scholar]
- 5.Fagan SC, Morgenstern LB, Petitta A, Ward RE, Tilley BC, Marler JR, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 6.ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 7.Luengo-Fernandez R, Silver LE, Gutnikov SA, Gray A, Rothwell P. Hospitalization resource use and cost before and afte TIA and stroke: Results from a population-based cohort study (OXVASC) Value Health. 2013;16:280–287. doi: 10.1016/j.jval.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Kim AS, Nguyen-Huynh M, Johnston SC. A cost-utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke. 2011;42:2013–2018. doi: 10.1161/STROKEAHA.110.606889. [DOI] [PubMed] [Google Scholar]
- 9.Hill MD, Khatri P, Tomsick TA, Simpson KN, Broderick JP. Letter by Hill et al regarding article, "A cost-utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke". Stroke. 2011;42:e641–e642. doi: 10.1161/STROKEAHA.111.637603. [DOI] [PubMed] [Google Scholar]
- 10.Broderick J, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular Therapy after Intravenous t-PA versus t-PA Alone for Stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauldin PD, Simpson KN, Palesch YY, Spilker JS, Hill MD, Broderick JP the IMS III Investigators. Design of the Economic Evaluation for the Interventional Management of Stroke Trial. Int. J. Stroke. 2008;3:138–144. doi: 10.1111/j.1747-4949.2008.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turk AS, III, Campbell JM, Spiotta A, Vargas J, Turner RD, Chaudry MI, et al. An investigation of the cost and benefit of mechanical thrombectomy for endovascular treatment of acute ischemic stroke. J NeuroIntervent Surg. 2014;6:77–80. doi: 10.1136/neurintsurg-2012-010616. [DOI] [PubMed] [Google Scholar]
- 13.Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Coté R, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Canadian Journal of Neurological Sciences. 2012;39:793–800. doi: 10.1017/s0317167100015638. [DOI] [PubMed] [Google Scholar]
- 14.Krueger H, Lindsay P, Cote R, Kapral MK, Kaczorowski J, Hill MD. Cost Avoidance Associated With Optimal Stroke Care in Canada. Stroke. 2012;43:2198–2206. doi: 10.1161/STROKEAHA.111.646091. [DOI] [PubMed] [Google Scholar]
- 15.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41:1175–1179. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 17.Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, et al. Calgary Stroke Program. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;2:396–405. doi: 10.1097/ALN.0b013e318242a5d2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.