Abstract
Objective
Few decision aids emphasize active surveillance (AS) for localized prostate cancer. Concept mapping was used to produce a conceptual framework incorporating AS and treatment.
Methods
Fifty‐four statements about what men need to make a decision for localized prostate cancer were derived from focus groups with African American, Latino and white men previously screened for prostate cancer and partners (n = 80). In the second phase, 89 participants sorted and rated the importance of statements.
Results
An eight cluster map was produced for the overall sample. Clusters were labelled Doctor–patient exchange, Big picture comparisons, Weighing the options, Seeking and using information, Spirituality and inner strength, Related to active treatment, Side‐effects and Family concerns. A major division was between medical and home‐based clusters. Ethnic groups and genders had similar sorting, but some variation in importance. Latinos rated Big picture comparisons as less important. African Americans saw Spirituality and inner strength most important, followed by Latinos, then whites. Ethnic‐ and gender‐specific concept maps were not analysed because of high similarity in their sorting patterns.
Conclusions
We identified a conceptual framework for management of early‐stage prostate cancer that included coverage of AS. Eliciting the conceptual framework is an important step in constructing decision aids which will address gaps related to AS.
Keywords: cancer, concept mapping, decision making, prostate neoplasms, qualitative research
Introduction
More than 80% of prostate cancer diagnoses in the United States are for localized disease, confined to the prostate and about 80–90% of men diagnosed with low‐risk prostate cancer receive some form of treatment.1, 2 There is currently uncertainty about the management of localized prostate cancer. Surgery and radiation, the most common treatments, have largely equivalent survival rates, and each results in significant risk of urinary, bowel and sexual problems.3, 4 Comparisons of active surveillance (AS) and surgery for low‐risk disease have shown small advantages associated with surgery or no differences in survival.5, 6 There is concern that the growth in early detection and immediate treatment has led to treatment for cancers not likely to be clinically significant.
The 2011 U.S. National Institutes of Health (NIH) Consensus and State‐of‐the‐Science Conference concluded that AS should be offered to patients with low‐risk prostate cancer.3 Active surveillance is defined as a management strategy that delays treatment until it is warranted based on indicators of disease progression. It is differentiated from watchful waiting, in which treatment is introduced to relieve symptomatic disease progression. Low‐risk prostate cancer is usually determined by such characteristics as follows: (i) tumour stage of being not detectable clinically or with imaging or a small tumour able to be felt but confined to the prostate, (ii) PSA value of less than 10 μg/l and (iii) histologic grade or Gleason score of less than or equal to 6.
A number of decision aids have been developed for early‐stage localized prostate cancer.7 However, these decision aids frequently give little attention to AS and may not clearly distinguish it from watchful waiting.8
Decision aids tend to be developed on more highly educated men.9 Moving beyond affluent patient populations will require learning whether less advantaged groups, potentially of different ethnicities, have different views of the treatment options. It will be important for decision aids for prostate cancer treatment decisions to address the information needs and incorporate the conceptual frameworks of these men.
Research on treatment decisions is useful in understanding how patients think about their options. Survival and getting rid of cancer are commonly cited factors in the treatment decision.10 Persons choosing some form of radiation therapy often say they want to avoid surgery.11 Patients express concern about the treatment side‐effects of incontinence, impotence and bowel problems, but do not report them as pivotal to the decision.10, 12
Few studies have focused on patients' reasons for selecting AS. Only a minority of low‐risk prostate cancer patients enrol in an AS protocol and approximately 10–50% of those men elect secondary treatment, despite an absence of clinical disease progression.13, 14 Men selecting AS emphasized the importance of avoiding side‐effects. They also noted the importance of physician support for AS.15 Qualitative research done as part of the PROTECT trial comparing AS, surgery and radiation therapy has shown that information about the options can be presented such that men accept AS as an option in relation to the major treatments.16
Studies in the USA examining variation in treatments by race or ethnicity or socio‐economic position have found that African Americans and Latinos were less likely to be treated with surgery or radiation therapy and were monitored less frequently than whites during the 5‐year period.17, 18 These studies show ethnic variations in treatment selection and experience and the importance of high‐quality observant management strategies. However, it is important to note that these studies combined watchful waiting and unknown treatment categories which reinforces the importance of clear definitions of AS and watchful waiting.
The views of the spouse may also influence decisions. Men say that they want their partners involved, and physicians commonly involve men's partners in discussions of management options.10 However, husbands and wives may value outcomes of treatment differently, for example, in one study wives placed higher value on survival and a lower one on avoiding side‐effects than husbands.19 Single or separated men had higher rates of watchful waiting, while married men were more likely to be treated by radical prostatectomy.20, 21
The primary aim of this study was to learn more about the conceptual framework and information needs of men and partners in relation to considering AS if they were facing a decision for early‐stage, localized prostate cancer. A secondary aim was to examine variation in the conceptual framework by ethnicity and gender. The results will be used to inform the development of patient education and decision support tools to support treatment decisions for localized prostate cancer.
Methods
Overview
Concept mapping is a participatory mixed qualitative–quantitative method that results in a graphical view of a group's ideas about a topic and the relations among them.22, 23, 24 The general sequence of activities is (i) to generate a set of statements about a particular topic, (ii) sorting and rating of the statements, (iii) statistical analysis and (iv) interpretation of the concept map. The term concept map has also been used to show important attributes related to a concept, sometimes based on content analysis of the scientific literature.25
Sometimes the sequence of steps involves a single group of participants. We modified that procedure such that overlapping groups of people took part in the different steps. This was done to reduce the burden of participation.26
Participants
We chose convenience samples of African American, Latino and White, non‐Hispanic men in Houston and El Paso, TX, USA. Eligible men were 50–70 years of age or 40–70 years if African American had a PSA test within the previous 2 years and had never been diagnosed with or tested positive for prostate cancer. The recruited participants represent men interested in prostate cancer screening but who have not faced the treatment decision or its side‐effects. In recruitment, men were asked if they wished to bring a partner, that is, a person he would involve in health decisions. Partner sessions were conducted separately. Sessions took place between May 2010 and February 2011.
In Houston, African American men were recruited by an outreach programme of a comprehensive cancer centre. Non‐Hispanic white men were recruited using flyers and local newspaper advertisements. In El Paso, a community cancer education organization recruited Latino and non‐Hispanic white men. Participants received $75 for their time and parking ($50 in El Paso).
Statement generation
Statements about prostate cancer treatment/management were drawn from focus groups and from research reports.
Focus groups were homogeneous with respect to gender, ethnicity and language (English, Spanish). Participants gave written consent and completed a background questionnaire. The focus groups began with an overview of active treatments for early‐stage prostate cancer (i.e. radical prostatectomy, beam radiation and brachytherapy) and their potential side‐effects so that the discussion had a base of information. This segment featured a 3½ min video on prostate cancer and treatment options27 and a 2½ min video extract about treatments and AS.28
The discussion was about how they would make treatment decisions if they (or their partners) were diagnosed with early‐stage, localized prostate cancer. It covered what information is needed, how they would think about or evaluate information, and who they would involve in the decision. The discussion moderator asked the participants specific questions about the treatment options described in the videos.
Sessions were audio‐taped, transcribed and translated, if in Spanish. The translation was reviewed by bilingual coders to ensure that there was good correspondence with the original transcripts.
Coding statements
Pairs of reviewers independently extracted excerpts from the transcripts that related to the focal prompt: ‘When deciding what to do for early‐stage, localized prostate cancer, a man should…’. The result was short statements summarizing ideas from the focus group discussion. Disagreements about coded statements were resolved in team meetings.
We added a few statements from studies of decision making for early‐stage, localized prostate cancer. Articles were restricted to studies of men in the midst of a treatment decision or a hypothetical decision. Studies of past decisions were excluded. The decision alternatives included AS or watchful waiting. Of 12 articles that met our eligibility criteria, ten were found by two independent reviewers to have relevant content. Following the editing, six statements were added.
Statements were edited to eliminate redundancy, correct double‐barrelled statements and simplified such that the reading levels did not exceed 6th grade Flesch‐Kincaid. The final list had 54 statements. Statements were translated into Spanish and reviewed by native Spanish speakers. (The Spanish translations can be obtained from the corresponding author.) Statements were randomly ordered and printed on card stock. Cards for Spanish‐speaking participants had the English statement on one side and Spanish on the other.
Sorting and rating
Participants independently sorted cards within small groups homogeneous in ethnicity, gender and language. The same video clips and handouts from the focus groups were shown to inform participants about treatment alternatives and AS.
Each participant was asked to sort the cards according to their similarity, so that those seen as most similar in meaning were in the same pile, making as many piles as he/she wanted. The focus prompt for sorting was ‘When deciding what to do for early‐stage, localized prostate cancer, a man should…’. Participants were encouraged to have at least three piles, no ‘miscellaneous’ pile and no single statement piles.
After sorting, participants rated the statements for their importance (compared to the rest) in making a decision. Response alternatives were 1 = relatively unimportant, 2 = somewhat important, 3 = moderately important, 4 = very important and 5 = extremely important.
Analysis and identification of clusters based on sorting
We used The Concept System software29 for analysis. We produced a binary symmetric 54 by 54 matrix for each sorter where cell entries of ‘1’ indicate that two statements were placed together in a pile and ‘0’ otherwise. The individual binary matrices were summed across sorters to produce an aggregate matrix where cell entries were the number of participants placing each pair of statements in the same pile. A map in two dimensions, convenient for displaying the relationships among the statements, was produced with multidimensional scaling (MDS).23, 30 The distance between points (statements) represents the estimates from MDS of how similar they are judged to be. The position of each point on the map (e.g. top, bottom, right, left) is not important – only the distance.
The MDS spatial coordinates were analysed using hierarchical cluster analysis with Ward's algorithm31 to produce non‐overlapping clusters of more similar statements. Cluster boundaries enclose the statements grouped in each cluster. The size and shape of a cluster generally corresponds with whether it is a broader or narrower conceptual area.23
We examined a range of cluster solutions (5–10) to determine the appropriateness of merging or splitting of statement groups and selected the eight cluster solution. The central decision described here is the number of clusters. The content of clusters or the cluster‐tree structure is statistically determined, but the ‘best’ number of clusters depends on the level of specificity desired and context, factors judged substantively.23
Interpretation of concept map
Researchers and a subset of participants interpreted the concept map. The research team selected the number of clusters and proposed preliminary labels shaped by the statements included in the clusters, their importance ratings and the bridging values of clusters. Clusters of statements with low bridging values are more cohesive.
Six groups of community participants discussed the maps in gender‐specific discussion sessions. They looked at the statements in each cluster and discussed what the cluster means to them. At the conclusion, men and their partners reassembled to discuss their joint reactions. The paired interpretations of participants and research group ensured that the researchers' interpretations did not diverge too far from the participants.
The study was approved by the Committee for the Protection of Human Subjects, The University of Texas Health Sciences Center and the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
Results
A total of 80 individuals (45 men and 35 women) participated in statement generation sessions, 89 participants (52 men and 36 women) took part in sorting and rating, and 33 individuals (19 men and 14 women) participated in the interpretation step. The totals include 43 persons, 21 in Houston and 22 in El Paso, from the statement generating focus groups who also participated in the sorting and rating phase. All 18 persons in the El Paso interpretation groups were prior participants, as were nine of 15 in Houston.
To simplify the presentation, Table 1 describes participants in the sorting/rating phase. There were equal numbers of participants from Houston and El Paso, and an approximately even ethnic distribution. There were more men than women, because some men did not invite a partner and some brought a male friend or family member. There was a fair amount of variation in education level. Six sorting and rating participants (three African American, two Latino and one non‐Hispanic white) were excluded from the analysis because their sorts had three or fewer piles.
Table 1.
Description of participants in the sorting phase
| N | % | |
|---|---|---|
| Site | ||
| Houston | 45 | 51 |
| El Paso | 44 | 49 |
| Male | 57 | 64 |
| Age | ||
| Less than 50 years | 14 | 16 |
| 50–59 | 27 | 31 |
| 60 or more years | 47 | 53 |
| Ethnicity | ||
| African American | 31 | 35 |
| Hispanic | 30 | 34 |
| White Non‐Hispanic | 28 | 31 |
| Education | ||
| Less than high school | 21 | 24 |
| High school graduate | 26 | 30 |
| More than high school | 41 | 46 |
| Total | 89 | |
One analytic decision was whether to present separate analyses based on the sorts of a single race/ethnicity or gender. We selected the combined map (all ethnic groups, both genders) based on the high correlation of the sorts made by groups. The correlation of statement similarities between ethnic groups or gender ranged from 0.93 to 0.95 when corrected with the Spearman‐Brown prophecy formula.
Figure 1 shows the 8‐cluster map for the combined sample. The text for each numbered statement can be seen in Table 2. The locations of individual statements (points) are indicated with numbers. The clusters are labelled with brief descriptions. The overall fit or stress value was 0.21, an acceptable value (lower stress values indicate better fit).30
Figure 1.
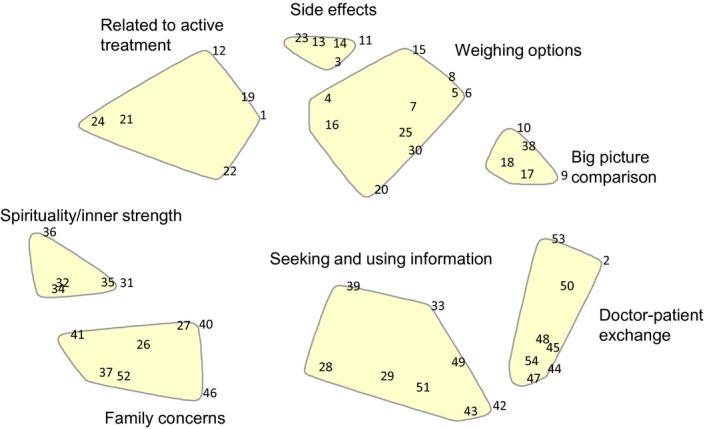
Point map showing cluster labels.
Table 2.
Clusters, ordered by mean importance, with text of statements, statement numbers and mean importance ratings of statements
| Cluster label | Statement (statement number) | Mean | SD |
|---|---|---|---|
| Doctor–patient exchange | Find a doctor he is comfortable with (54) | 4.50 | 0.86 |
| Find out about all of the possible treatments (50) | 4.32 | 0.90 | |
| Know what care would be needed after treatment (53) | 4.28 | 0.93 | |
| Tell his doctor if there's something he doesn't understand (47) | 4.23 | 0.94 | |
| Ask for the information he wants to know (48) | 4.16 | 0.95 | |
| Ask for the most up‐to‐date information (45) | 4.10 | 1.20 | |
| Ask for a second opinion from another doctor (44) | 4.06 | 1.06 | |
| Ask if it is helpful to use natural or alternative medicine (e.g. herbs or acupuncture) to treat his cancer (2) | 3.30 | 1.40 | |
| Big picture comparisons | Find out the survival rates of active treatment and active surveillance. (This means how likely a man is to still be alive 5 years from diagnosis) (18). | 4.07 | 1.16 |
| Ask about the risk that the cancer will spread during treatment (9) | 4.02 | 1.14 | |
| Find out exactly what would be done during active treatment or active surveillance (17) | 3.99 | 1.02 | |
| Find out if anything can be done about treatment side‐effects if they happen (10) | 3.90 | 1.09 | |
| Know that active surveillance is also an option (38) | 3.78 | 0.95 | |
| Weighing options | Compare the risks and benefits of active treatment (5) | 4.18 | 0.89 |
| Consider what effects the active treatment might have on his bowel or urinary function (15) | 4.05 | 1.08 | |
| Consider the risk that the cancer may come back after active treatment (8) | 4.06 | 1.02 | |
| Compare the risks and benefits of active surveillance (6) | 4.06 | 0.99 | |
| Know that he will probably live just as long, whether he chooses active treatment or active surveillance (25) | 4.01 | 1.17 | |
| Understand that if he chooses active surveillance, he may feel worried about the cancer spreading (7) | 3.98 | 1.18 | |
| Think about how willing he is to return for frequent examinations if he chooses active surveillance (30) | 3.76 | 1.02 | |
| Know that if he chooses active surveillance, he can still change his mind later (20) | 3.72 | 1.24 | |
| Ask whether he's likely to have a bad health‐care experience like one he may have had in the past (e.g. slow recovery from surgery) (4) | 3.59 | 1.10 | |
| Consider how often and for how long he would need to go to the clinic or hospital (16). | 3.49 | 1.13 | |
| Seeking and using information | Decide how much input he wants from his doctor in the final decision (29) | 4.17 | 0.89 |
| Make sure to get his doctor's opinion (42) | 4.05 | 0.91 | |
| Realize that he has a choice (39) | 4.05 | 1.00 | |
| Do things to help him remember the information that doctors give him (e.g. taking notes, having someone else go with him) (51) | 4.02 | 1.01 | |
| Be willing to talk about which treatment he'd rather have (28) | 3.92 | 1.22 | |
| Get information from other places such as the Internet (49) | 3.85 | 1.04 | |
| Find out about other people's experiences with early‐stage prostate cancer, both with active surveillance and active treatment (33) | 3.70 | 1.15 | |
| Talk to another health‐care worker, such as a nurse or promoter/a (43) | 3.28 | 1.20 | |
| Spirituality and inner strength | Think about how his positive frame of mind will help him cope with whatever happens (36) | 4.17 | 1.03 |
| Look to his faith for strength and guidance when making the decision (35) | 4.10 | 1.19 | |
| Consider how much help he will need from family and friends during active treatment and recovery (34) | 3.83 | 1.05 | |
| Consider how family members would feel if he had side‐effects from active treatment (32) | 3.42 | 1.29 | |
| Consider how family members would feel if he chose active surveillance (31) | 3.37 | 1.19 | |
| Related to active treatment | Be aware that the older he is, the slower the cancer may grow or spread (1) | 3.94 | 1.03 |
| Consider how strongly he feels about doing something right away to get rid of the cancer (22) | 3.92 | 1.29 | |
| Consider how long it will take to recover (19) | 3.91 | 0.99 | |
| Consider that he may be given fewer treatment options, depending on where he lives, his income or his race/ethnicity (24) | 3.82 | 1.22 | |
| Evaluate how much of the cost of treatment or of active surveillance will have to be paid out of his pocket (e.g. travel costs or co‐payments) (21) | 3.70 | 1.36 | |
| Consider the effect that active treatment might have on his ability to have children (12) | 2.98 | 1.66 | |
| Side‐effects | Consider that active treatment may be harder on him than active surveillance if he is in poor health, has other health conditions or is older (3) | 4.17 | 1.01 |
| Consider the effects of active treatment on his usual activities (11) | 3.86 | 1.02 | |
| Consider how strongly he feels about avoiding side‐effects of active treatments (23) | 3.80 | 1.10 | |
| Consider the effect that active treatment might have on his sex life (13) | 3.48 | 1.41 | |
| Consider the effect that active treatment might have on his sense of manhood (14) | 3.23 | 1.48 | |
| Family concerns | Consider what is important both to him and to his loved ones (37) | 4.23 | 1.02 |
| Say what's important to him in choosing what to do (27) | 4.00 | 1.06 | |
| Make sure loved ones get the information they want (52) | 3.94 | 1.11 | |
| Take as much time as he needs to make the best decision for him (40) | 3.88 | 1.08 | |
| Include family members in making the decision (41) | 3.71 | 1.17 | |
| Resist pressure from others about what to do (26) | 3.52 | 1.41 | |
| Ask friends for advice (46) | 2.66 | 1.24 |
Taken together, the four clusters located towards the right side of the map refer to seeking medical information (Fig. 1) and compare AS and active treatments, that is, surgery and radiation therapy. This set of clusters had the highest importance ratings.
The cluster Doctor–patient exchange has the highest average importance rating. Its statements include ‘finding a doctor he is comfortable with’, ‘asking for information’ and ‘obtaining a second opinion’. The statements also include ‘seeking clarification when he does not understand the doctor’. In the interpretation, participants emphasized the importance of communicating with the doctor. One comment was, ‘Here, it seems like you are doing more communicating – kind of reaching out a little bit. Ask for the information that you need… and this is communicating with the doctor’ (African American men).
Seeking and using information is not so strictly focused on the doctor as the information source. It includes statements about how a man should make the decision, such as being willing to talk about what he wants, realizing that there is a choice to be made, and opening himself up to information from such sources as cancer survivors, other types of health personnel and the Internet. It characterizes the man as a person who actively participates in the treatment decision, reaching out for information. He is seen as deciding about the level of input he wants from the doctor. The cluster emphasizes participating in making treatment decisions and reaching out to the man's network.
The cluster Big picture comparison has statements fundamentally comparing active treatments and AS. Statements include ‘knowing that active surveillance is an option’, ‘finding out what would be done in treatment and active surveillance’ and the survival rates under the two strategies. Pointing to the central theme of comparison, one participant summarized, ‘You may decide you want active surveillance. You may decide you want active treatment. That's about as far apart as they get’ (non‐Hispanic white men).
The cluster Weighing the options has more specific statements about active treatments and AS than the Big picture comparison. Statements related to AS include ‘having a similar length of life to treated men’, ‘a man may worry about the spread of cancer if it is not treated’, the ‘need to return for frequent examinations with active surveillance’ and ‘a man selecting active surveillance can choose to be treated later’. Statements focused on active treatment include ‘men should weigh the overall risks and benefits of treatments', ‘active treatment is associated with a similar length of life to active surveillance’, and ‘there is risk of recurrence following treatment’.
We have grouped together two clusters on the left side of the map as related to faith and family. They are the farthest from the medical clusters.
‘Spirituality and inner strength’ has statements about ‘looking to faith for guidance’, ‘thinking how family members would feel if the man chose active surveillance’ or alternatively ‘if he chose active treatment and suffered side‐effects’. Participants at the interpretation sessions proposed the label ‘Hold on to your faith and stay positive’ (non‐Hispanic white partners). They also suggested ‘Different ways of coping’, because they noted, ‘Some will use faith and some will use family and friends’.
The cluster, Family concerns, has statements related to balancing men's views with those of his family. These include making sure family members get information and weighing or balancing what is valued by the man and his family. There is an interesting confluence of statements about taking the time needed to make the decision and both including and resisting the influence of others. Participants labelled it ‘Consideration of self and loved ones in the decision‐making process’ (African American men). They described the cluster as being about getting input from friends and family in the decision.
The final two clusters bridge the medical and family/spirituality large groups of clusters. Related to active treatment is about general features of the treatment decision. One statement is about the slow growing nature of much prostate cancer such that older men may die with, rather than from, prostate cancer. Another statement is the nearly opposite view of thinking about how important it is to rid the body of cancer. Two statements in the cluster had high bridging values indicating that they were not sorted consistently. These were statements about access to treatment depending on where the man lives, his income or race/ethnicity and about how the financial costs of treatment will be borne.
Side‐effects, a tightly focused cluster, has statements about the effects of treatment on male identity and sex life and the importance of thinking about how strongly the man wishes to avoid side‐effects. Participants labelled the cluster as having to do with the side‐effects of treatment or more generally about quality of life.
Variation in cluster importance
For most clusters, there was no variation by ethnicity in the importance of clusters (see Table 3). There was ethnic variation in the average importance of three of eight clusters. Latino/as rated most clusters as less important than the other ethnic groups, although few differences were statistically significant. One exception was Big picture comparisons. Spirituality and inner strength also varied by ethnicity with African Americans having highest importance ratings, followed by Latinos, then by non‐Hispanic whites. African Americans also rated the Related to treatment cluster as most important.
Table 3.
Cluster importance ratings by ethnicity, mean (SD)
| African American | Latino | Non‐Hispanic white | P‐value | |
|---|---|---|---|---|
| Doctor–patient exchange | 4.09 (0.80) | 3.91 (0.72) | 4.28 (0.66) | 0.18 |
| Big picture comparisons | 4.17 (0.67) | 3.61 (0.98) | 4.07 (0.77) | 0.03 |
| Weighing options | 4.07 (0.63) | 3.71 (0.92) | 3.89 (0.53) | 0.17 |
| Seeking and using information | 3.97 (0.58) | 3.74 (0.84) | 3.92 (0.53) | 0.39 |
| Spirituality and inner strength | 4.03 (0.62) | 3.80 (0.90) | 3.51 (0.77) | 0.05 |
| Related to active treatment | 4.00 (0.70) | 3.58 (0.80) | 3.56 (0.56) | 0.03 |
| Side‐effects | 3.88 (0.73) | 3.54 (0.97) | 3.70 (0.73) | 0.29 |
| Family concerns | 3.80 (0.81) | 3.66 (0.90) | 3.62 (0.72) | 0.70 |
Men and women rated the importance of the clusters similarly for the most part (see Table 4). The two exceptions were that women rated Weighing the options and Seeking and using information as more important than men. Women seemed to value searching out information and actively evaluating options. One group of female participants described the importance of going on a ‘fact‐finding mission’, to assist the man in his decision about prostate cancer management. It is interesting that the importance ratings for clusters Side‐effects of active treatment and Family considerations were similar for men and women.
Table 4.
Cluster importance ratings by gender, mean (SD)
| Men | Women | P‐value | |
|---|---|---|---|
| Doctor–patient exchange | 4.00 (0.74) | 4.23 (0.72) | 0.17 |
| Big picture comparisons | 3.84 (0.85) | 4.14 (0.81) | 0.12 |
| Weighing the options | 3.75 (0.75) | 4.13 (0.62) | 0.02 |
| Seeking and using information | 3.76 (0.70) | 4.06 (0.55) | 0.05 |
| Spirituality and inner strength | 3.78 (0.81) | 3.79 (0.79) | 0.97 |
| Access to active treatment | 3.68 (0.71) | 3.77 (0.74) | 0.55 |
| Side‐effects | 3.66 (0.84) | 3.79 (0.80) | 0.50 |
| Family concerns | 3.67 (0.82) | 3.74 (0.78) | 0.71 |
Discussion and conclusion
The NIH Consensus Conference recommended that men with localized low‐risk prostate cancer be offered the option of AS. Active surveillance has been neglected in decision aids or not distinguished from watchful waiting,8, 9 although this gap is beginning to be addressed. Therefore, in this study, we used concept mapping to elicit perspectives of men and partners related to active treatment and AS for low‐risk prostate cancer.
According to the concept map, participants distinguish overall and detailed comparisons of AS and treatment. They also see a contrast between ideas related to how to gather information and how to engage with their physicians to find the needed information for decision making. The topic of side‐effects of treatment was also seen in the conceptual framework.
The higher importance of more factually oriented clusters suggests that men and partners define factual information as being a key component of the treatment decision. This finding validates the use of up‐to‐date information in patient decision aids about the nature of prostate cancer, the options available to the patient, the potential benefits and harms of the options, and how likely they are to occur. This is consistent with the information function of a decision aid, in which options are clearly described and contrasted.32 Side‐by‐side formats showing attributes of the options and how likely they are to occur would be particularly valuable to men and their partners when making a treatment decisions.
Decision aids should also have content or features about how to engage in the decision, for example, how to interact with the physician to get necessary information and suggestions for how to express his preferences for particular outcomes. Prompts about questions to ask your doctor and messages designed to reinforce the importance of sharing one's preferences would be helpful in a decision aid. This type of information is also represented in the clusters and is consistent with the deliberative function of patient decision aids.32
A final point should be made about the role of decision aids in distinguishing AS from watchful waiting. When describing the treatment options for localized prostate cancer, it is important for decision aids to clearly describe the process, intent and the kinds of patients for whom AS is appropriate. There is potential reassurance value for the patient and partner in knowing that the cancer is being closely monitored, and if there is progression, there will still be time for cure.
The conceptual framework also shows the relation of family and spiritual values in the treatment decision. Partners anticipated having a role in the treatment decision, describing themselves as finding things out, taking notes or helping the man to remember and encouraging him to be an active participant in the decision. Men and partners do not necessarily expect to have the same views. Nevertheless, the high correlation of the sorts suggests that men and their partners do not greatly vary in how statements are grouped although there may be some differences in the level of importance of statements.
Eliciting the conceptual framework is an important step in constructing decision aids that resonate with the decision makers.33 The concept map was built on a rich base of qualitative information from ethnically diverse men and partners. The participants engaged in the statement generating discussions, sorted and rated statements and interpreted the concept maps.
While our study had sufficient numbers of participants in the three race/ethnicity groups to conduct comparative analyses, we selected a combined map. There was high cross‐gender and ethnic group similarity in the sorting and reasonably similar importance ratings of statements. While designers of decision aids may wish to target certain features of their product to different ethnic groups, these analyses suggest that major modifications in content are not important. Participation by ethnic minority men and partners in these formative steps does help ensure that any resulting decision aid has a better chance of resonating with their concerns.
One limitation of the study was that it was conducted with men with no history of prostate cancer. We tried to compensate for their relative lack of information by providing an overview of treatments and AS. Participants thus had more understanding of the outcomes of treatments and AS, but they did not have strong views associated with the treatment experience, for example, preference for their own treatment or decisional regret.34 However, it is possible that men facing a diagnosis of prostate cancer may have different views of the treatment options. To address these concerns, we are investigating conceptualizations of AS among men who chose AS or immediate treatment for localized prostate cancer.
Conclusion
We used concept mapping to identify a conceptual framework incorporating AS and other treatments for early‐stage prostate cancer. Eliciting the conceptual framework is an important step in constructing decision aids. This application of concept mapping has been useful in showing knowledge and processes to support decisions for localized prostate cancer. It addresses gaps in covering AS.
The 2011 NIH Consensus Conference recommended that men with localized low‐risk prostate cancer be offered AS.3 We used concept mapping to examine the conceptual frameworks of an ethnically diverse group of men and partners related to the management of localized prostate cancer. Major modifications in content for members of different ethnic groups do not appear to be required based on this analysis. The findings from the study will help design interventions to promote informed management decisions for patients with localized prostate cancer where AS may be an option.
Funding sources
Funding was provided by the U.S. Centers for Disease Control and Prevention, #1U48DP001949‐01, SIP 09‐15. Dr. Le was supported by a National Cancer Institute (NCI) postdoctoral fellowship at the University of Texas School of Public Health Cancer Education and Career Development Program (NCI R25 CA57712). Dr. Torres‐Vigil was supported in part by the National Cancer Institute (5K01CA151785‐04).
Acknowledgements
We are grateful to the Informed Medical Decisions Foundation for permission to use a segment of their shared decision programme Treatment Choices for Prostate Cancer.
References
- 1. Harlan S, Cooperberg M, Elkin E et al Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. The Journal of Urology, 2003; 170: 1804–1807. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone A, Krapcho M et al SEER cancer statistics review, 1975–2010 [serial on the Internet], 2013. Available at: http://seer.cancer.gov/csr/1975_2010/, accessed 24 January 2014.
- 3. Ganz P, Barry J, Burke W et al National Institutes of Health State‐of‐the‐Science Conference: role of active surveillance in the management of men with localized prostate cancer. Annals of Internal Medicine, 2012; 156: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu‐Yao G, Yao S. Population‐based study of long‐term survival in patients with clinically localised prostate cancer. The Lancet, 1997; 349: 906–910. [DOI] [PubMed] [Google Scholar]
- 5. Bill‐Axelson A, Holmberg L, Ruutu M et al Radical prostatectomy versus watchful waiting in early prostate cancer. The New England Journal of Medicine, 2011; 364: 1708–1717. [DOI] [PubMed] [Google Scholar]
- 6. Wilt T, Brawer M, Jones K et al Radical prostatectomy versus observation for localized prostate cancer. The New England Journal of Medicine, 2012; 367: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin G, Aaronson D, Knight S, Carroll P, Dudley R. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA: A Cancer Journal for Clinicians, 2009; 59: 379–390. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman R. Improving the communication of benefits and harms of treatment strategies: decision AIDS for localized prostate cancer treatment decisions. Journal of the National Cancer Institute Monograph, 2012; 2012: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmes‐Rovner M, Stableford S, Fagerlin A et al Evidence‐based patient choice: a prostate cancer decision aid in plain language. BMC Medical Informatics and Decision Making, 2005; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeliadt S, Ramsey S, Penson D et al Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer, 2006; 106: 1865–1874. [DOI] [PubMed] [Google Scholar]
- 11. Anandadas C, Clarke N, Davidson S et al Early prostate cancer–which treatment do men prefer and why? BJU International, 2011; 107: 1762–1768. [DOI] [PubMed] [Google Scholar]
- 12. Song L, Chen R, Bensen J et al Who makes the decision regarding the treatment of clinically localized prostate cancer–the patient or physician?: results from a population‐based study. Cancer, 2013; 119: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dall'era M, Albertsen P, Bangma C et al Active surveillance for prostate cancer: a systematic review of the literature. European Urology, 2012; 62: 976–983. [DOI] [PubMed] [Google Scholar]
- 14. Martin R, Gunnell D, Hamdy F et al Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. The Journal of Urology, 2006; 176: 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davison B, Oliffe J, Pickles T, Mroz L. Factors influencing men undertaking active surveillance for the management of low‐risk prostate cancer. Oncology Nursing Forum, 2009; 36: 89–96. [DOI] [PubMed] [Google Scholar]
- 16. Donovan J. Presenting treatment options to men with clinically localized prostate cancer: the acceptability of active surveillance/monitoring. Journal of the National Cancer Institute Monograph, 2012; 2012: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byers T, Wolf H, Bauer K et al The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer, 2008; 113: 582–591. [DOI] [PubMed] [Google Scholar]
- 18. Shavers V, Brown M, Klabunde C et al Race/ethnicity and the intensity of medical monitoring under ‘watchful waiting’ for prostate cancer. Medical Care, 2004; 42: 239–250. [DOI] [PubMed] [Google Scholar]
- 19. Volk R, Cantor S, Cass A et al Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. Journal of General Internal Medicine, 2004; 19: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai S, Lai H, Krongrad A et al Radical prostatectomy: geographic and demographic variation. Urology, 2000; 56: 108–115. [DOI] [PubMed] [Google Scholar]
- 21. Shavers V, Brown M, Potosky A et al Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. Journal of General Internal Medicine, 2004; 19: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke J, O'Campo P, Peak G et al An introduction to concept mapping as a participatory public health research method. Qualitative Health Research, 2005; 15: 1392–1410. [DOI] [PubMed] [Google Scholar]
- 23. Kane M, Trochim W. Concept Mapping for Planning and Evaluation. Thousand Oaks, CA: Sage, 2007. [Google Scholar]
- 24. Trochim W. An introduction to concept mapping for planning and evaluation. Evaluation and Program Planning, 1989; 12: 1–16. [Google Scholar]
- 25. Cheraghi‐Sohi S, Bower P, Mead N et al What are the key attributes of primary care for patients? Building a conceptual ‘map’ of patient preferences. Health Expectations, 2006; 9: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McFall SL, Ureda J, Byrd TL et al What is needed for informed decisions about prostate cancer screening: perspectives of African‐American and Hispanic men. Health Education Research, 2009; 24: 280–291. [DOI] [PubMed] [Google Scholar]
- 27. Volk RJ, Jibaja‐Weiss ML, Hawley ST et al Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Education and Counseling, 2008; 73: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Informed Medical Decisions Foundation/Health Dialogue . Treatment choices for prostate cancer, 2009. Available at: http://www.informedmedicaldecisions.org/, accessed 24 January 2014.
- 29. Concept Systems, Inc . The Concept System 4.0, 4th edn Ithaca, NY: Concept System, Inc., 2004. [Google Scholar]
- 30. Kruskal J, Wish M. Multidimensional Scaling. Beverly Hills, CA: Sage, 1978. [Google Scholar]
- 31. Everitt B. Cluster Analysis, 5th edn Hoboken: Wiley, 2011. [Google Scholar]
- 32. Elwyn G, Frosch D, Volandes A, Edwards A, Montori V. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Medical Decision Making, 2010; 30: 701–711. [DOI] [PubMed] [Google Scholar]
- 33. Coulter C, Kryworuchko J, Mullen P et al Using a systematic development process In: Volk R, Llwellyn‐Thomas H. (eds) 2012 Update of the International Patient Decision Aid Standards (IPDAS) Collaborations Background Document: http://ipdas.ohri.ca/resources.html, 2012. [Google Scholar]
- 34. Steginga S, Occhipinti S, Gardiner R, Yaxley J, Heathcote P. Making decisions about treatment for localized prostate cancer. BJU International, 2002; 89: 255–260. [DOI] [PubMed] [Google Scholar]


