Abstract
The role of antibody-mediated rejection (AMR) in acute and chronic rejection after lung transplantation is poorly understood. We report the case of a prior single lung transplant (SLT) recipient developing acute AMR isolated to her new, contralateral SLT. A 44 year-old woman six years status post SLT for idiopathic pulmonary fibrosis underwent a second SLT for bronchiolitis obliterans syndrome. Despite a negative crossmatch, she subsequently developed severe AMR to her new allograft within six days of transplantation. The process of allograft sensitization is dynamic and further study is warranted to better understand this process.
Keywords: Transplantation, Lung, Rejection (Lung)
INTRODUCTION
In lung transplantation (LTx), antibody-mediated rejection (AMR) remains an enigmatic and controversial finding. While prior transplants and multiparity contribute to the generation of recipient anti-human leukocyte antigen (anti-HLA) antibodies, the dynamic character of sensitization is poorly understood. We report the case of a prior single lung transplant (SLT) recipient developing acute AMR isolated to her new contralateral SLT.
CASE REPORT
A 44 year-old woman 5 years status post a left SLT for end-stage idiopathic pulmonary fibrosis, presented with advanced bronchiolitis obliterans syndrome (BOS) and increasing right lung infiltrates necessitating re-listing for LTx. Her prior transplant was notable for significant preoperative anti-HLA sensitization to 48% of class I and 63% class II antigens (A1, A36, A9, DR3 and DR52) as measured by solid phase luminex assay (Luminex Corporation-Austin, TX). After her initial LTx, despite a negative virtual crossmatch and a negative retrospective flow cytometric crossmatch, she developed 3rd party antibodies and donor specific antibodies (DSA) requiring a single session of peri-operative plasmapheresis. Her prior history is also notable for two children, receipt of several blood transfusions, reflux, cytomegalovirus viremia and a vague family history of autoimmune disease.
One month prior to re-transplantation, luminex testing demonstrated a calculated panel reactive antibody (PRA) level of 64% with HLA-specific antibodies to antigens A1, A36, B8, DR17, DR18, DR11, DR13, and DR14.
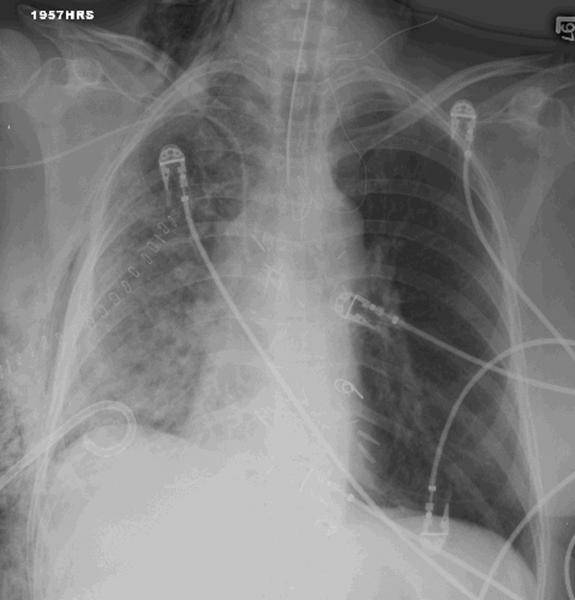
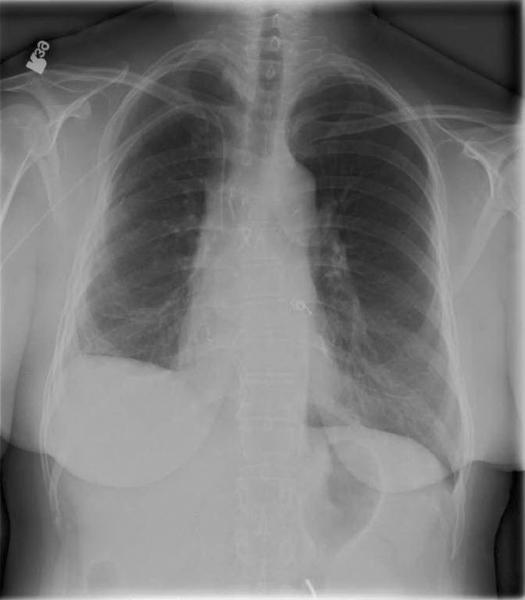
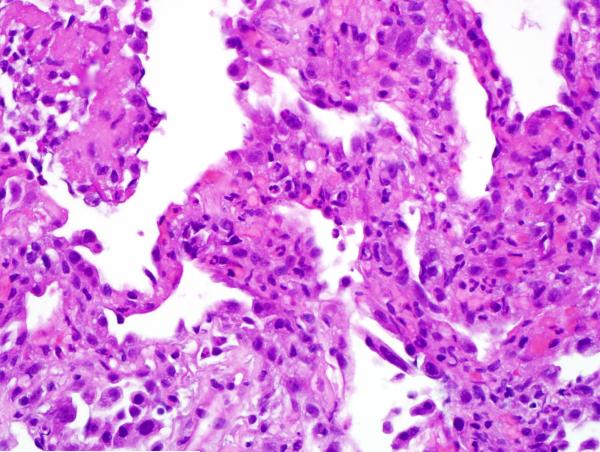
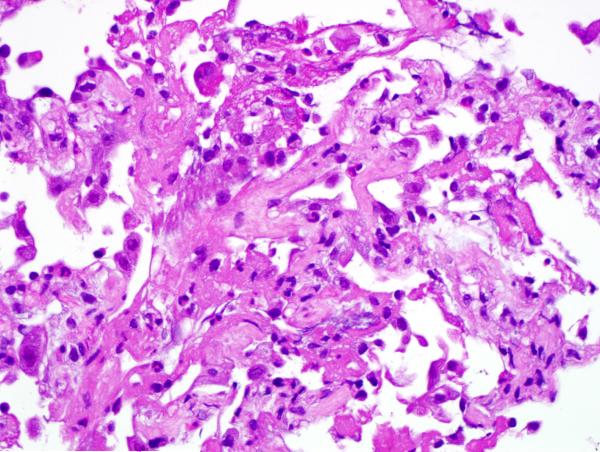
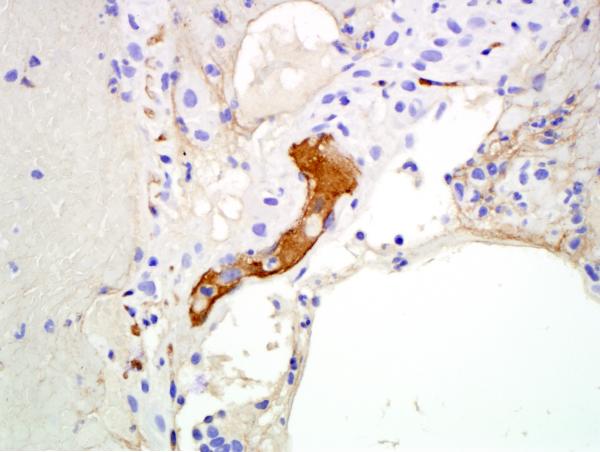
Given the patient’s sensitization, a virtual crossmatch was undertaken. Once a virtual crossmatch negative donor was found, the patient underwent an uneventful right SLT with a total ischemic time of 71 minutes (warm-32 minutes). Despite a negative retrospective crossmatch, she was empirically treated with rituximab (Rituxan-Genentech Incorporated-San Francisco, CA) peri-operatively followed by three cycles of plasmapheresis and intravenous immunoglobulin (IVIG), in addition to her primary immunosuppressive regimen of hydrocortisone, tacrolimus and mycophenolate mofetil. Despite any evidence of infection, on postoperative day 6 the patient experienced an acute decline in respiratory function. Her chest x-ray revealed infiltrates throughout her new allograft with sparing of the previous transplant (Figure 1). Luminex testing revealed a significant increase in anti-HLA antibodies specific to her new allograft to a level that would yield a strongly positive cytotoxic crossmatch. Bronchoscopic biopsy demonstrated acute alveolar damage, capillaritis, positive immunostaining for C4d and no evidence of acute cellular rejection, highly suggestive of AMR (Figure 2).
Figure 1.
A) CXR on POD 6 showing right-sided infiltrates B) CXR post-discharge showing infiltrate resolution
Figure 2.
A) Hematoxylin and eosin stained section shows alveolated parenchyma, septal widening and an interstitial neutrophilic infiltrate consistent with capillaritis. The type 2 pneumocytes show marked reactive atypia. B) Hematoxylin and eosin stained section shows alveolated parenchyma, acute lung injury pattern, sloughing type 2 pneumocytes and intra-alveolar fibrinous exudate. No diagnostic hyaline membrane noted. C) An immunostain for C4d demonstrates strong labeling of hyperplastic endothelial cells in an alveolar septal capillary.
The patient underwent daily plasmapheresis for 16 days, with concomitant IVIG to treat DSA. After nearly a month of treatment, her DSA levels declined and her clinical status improved, allowing for her IVIG treatments to be gradually spaced out to once a week and her plasmapheresis treatments to stop altogether. Repeat trans-bronchial biopsy on postoperative day 44 revealed evidence of focal resolving organizing lung injury and subsequent C4d staining was negative. After approximately 3 months, her DSA levels were low enough to no longer yield a positive cytometric crossmatch. Subsequent trans-bronchial biopsies on postoperative day 91 and 221 showed focal organization in one of five alveolated fragments and no evidence of acute cellular rejection, respectively.
The patient is now 14 months post SLT and continues to do well, with her most recent luminex test negative for DSA. Her current pulmonary function tests include a forced vital capacity (FVC) of 78% and a forced expiratory volume in 1 second (FEV1) to FVC ratio of 69%. By comparison, her FVC and FEV1/FVC ratio immediately prior to re-transplantation were 46% and 67% respectively. She is maintained on an immunosuppressive regimen of prednisone, tacrolimus and mycophenolate mofetil, and except for an episode of asymptomatic cytomegalovirus viremia, her outpatient course to date has been uneventful.
DISCUSSION
Although AMR is a well-defined cause of allograft dysfunction in kidney and heart transplantation, its role in LTx is controversial.[1] While several studies have suggested that highly sensitized patients have decreased survival following LTx, the evidence is conflicting.[2, 3]
We report a case of SLT, followed six years later by a contralateral SLT in a highly sensitized patient, who subsequently underwent severe acute humoral rejection with circulating antibody, C4d deposition, tissue pathology and graft dysfunction.[4, 5] Interestingly, AMR was directed only at the new allograft with radiographic sparing of the older allograft.
Previous studies have identified prior transplants, blood transfusions, multiparity, and autoimmune diseases as potentially sensitizing events.[2, 3] Our patient experienced nearly all of these events. Given her history of DSA directed against her original LTx, one would expect that subsequent episodes of AMR would include anti-HLA antibodies against her first allograft. In this case however, severe humoral rejection developed exclusively against her new allograft.
Cases in which AMR occurs at the site of a newly transplanted lung allograft have been documented without much understanding of the mechanism.[5] Rapid and devastating AMR in the form of hyperacute rejection is a rare but well documented example.[6] A specific case documented AMR occurring one month after a patient’s lung transplantation.[5] Preoperative negative crossmatches have also been known to result in AMR postoperatively, as virtual crossmatch sensitivity for B cell cytotoxicity is 84%.[6, 7] No literature exists, however, which addresses a case in which a patient with a previous SLT develops isolated AMR in her second SLT. This result is not intuitive and more research in graft sensitization is necessary to fully explain this phenomenon.
Our patient demonstrated a broad sensitization to a number of common human leukocyte antigens. Anti-HLA antibodies among LTx recipients have been recognized as a significant risk factor for acute rejection and for BOS.[5] Therefore, despite a single negative virtual crossmatch, she underwent empiric perioperative desensitization with plasmapheresis, rituximab, and IVIG. Despite these precautions, she experienced a rapid decline in her pulmonary function accompanied by a rapid increase in her DSA levels. Desensitization protocols utilizing both bortezomib (Velcade-Takeda Pharmaceutical Company Limited-Osaka, Japan) and rituximab have been explored. These studies have shown impressive short term reductions in antibody levels during treatment, but rebound antibody titers to pre-treatment levels or higher appear to be significant.[8] It is unknown whether this combined regimen may have avoided the complications of AMR in our patient.
The rapid change in her clinical status and precipitous rise in her DSA levels, despite negative retrospective and virtual crossmatches as well as empiric desensitization, highlight the static nature of antibody detection contrasted with the dynamic nature of sensitization. This dynamic nature stresses the importance of repetitive antibody screenings.
CONCLUSION
AMR in LTx remains poorly defined and less well understood than in other solid organ transplantation. This case illustrates several key issues. First, humoral rejection can occur despite an initial negative crossmatch; validating the need to view AMR as a dynamic process requiring multiple post-operative antibody screening exams. Second, the ideal desensitization regimen is unknown. Finally, humoral rejection is treatable with excellent outcomes.
Abbreviations
- AMR
antibody-mediated rejection
- Anti-HLA
anti-human leukocyte antigen
- BOS
bronchiolitis obliterans syndrome
- DSA
donor specific antibodies
- FVC
forced vital capacity
- FEV1
forced expiratory volume in one second
- IVIG
intravenous immunoglobulin
- LTx
lung transplantation
- PRA
panel reactive antibody
- SLT
single lung transplant
Footnotes
Conflicts: None.
Presentations: None.
REFERENCES
- 1.Glanville AR. Antibody-mediated rejection in lung transplantation: myth or reality? J Heart Lung Transplant. 2010;29(4):395–400. doi: 10.1016/j.healun.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Gammie JS, et al. Influence of panel-reactive antibody on survival and rejection after lung transplantation. J Heart Lung Transplant. 1997;16(4):408–15. [PubMed] [Google Scholar]
- 3.Hadjiliadis D, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24(7 Suppl):S249–54. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto SK, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–41. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrell MR, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2009;28(1):96–100. doi: 10.1016/j.healun.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest. 1996;110(2):559–62. doi: 10.1378/chest.110.2.559. [DOI] [PubMed] [Google Scholar]
- 7.Ellis TM, et al. Diagnostic accuracy of solid phase HLA antibody assays for prediction of crossmatch strength. Hum Immunol. 2012;73(7):706–10. doi: 10.1016/j.humimm.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Weston M, et al. Desensitization protocol using bortezomib for highly sensitized patients awaiting heart or lung transplants. Clin Transpl. 2009:393–9. [PubMed] [Google Scholar]