Abstract
Purpose:
Pre-transplant malignancy (PTM) is a relative contraindication to organ transplantation. Studies examining the impact of PTM on outcomes after lung transplantation (LTx) or orthotopic heart transplantation (OHT) are limited. We evaluated the impact of PTM on outcomes after LTx and OHT.
Methods:
We retrospectively reviewed primary adult LTx and OHT recipients in the United Network for Organ Sharing database. Primary and secondary stratification were by PTM and tumor type, respectively. Matched cohorts (2:1) as well as multivariable Cox proportional hazards regression models evaluated mortality.
Results:
From 2000-2011, 13,613 adults underwent LTx and 19,817 adults underwent OHT. 740 (5.4%) LTx patients and 1,117 (5.6%) OHT patients had PTM. On unadjusted analysis, both LTx patients and OHT patients with PTM had similar 30 day, 1-year and 5-year survivals (p>0.05) compared to no PTM. These findings persisted after risk-adjustment. No tumor types were associated with increased mortality in LTx patients. Patients with leukemia/lymphoma/myeloma (LLM) had a significant increase in univariate mortality at 30 days (HR:1.82, p=0.04), 1-year (HR:1.93, p<0.001) and 5-years (HR:1.54, p=0.01) in OHT patients. Matched cohort analysis revealed comparable outcomes in LTx patients, but confirmed increased univariate 1-year mortality (HR:1.89, p=0.006) in OHT patients with LLM.
Conclusions:
In the largest study evaluating the effects of PTM on LTx and OHT, the incidence of PTM is 5.4% and 5.6%, respectively. In general, PTM does not increase mortality in either cohort. However, OHT patients with LLM have an increased hazard of mortality. Therefore, carefully selected patients with PTM should not be excluded from LTx or OHT.
Keywords: Pre-Transplant Malignancy, Transplantation (Heart), Transplantation (Lung)
Introduction
Organ transplantation has historically been reserved for individuals without concomitant, irreversible disorders which would limit survival or prohibit subsequent immunosuppression.[1, 2] For this reason, pre-transplant malignancy(PTM) has long been considered a relative contraindication. The primary concern in these patients is the potential for recurrent, or de novo, malignancy due to the associated immunosuppression necessary to prevent allograft failure.[1-9] Currently, there is limited data on the impact of PTM on outcomes after lung transplantation(LTx) or orthotopic heart transplantation(OHT). The United Network for Organ Sharing(UNOS) database offers a unique opportunity to evaluate the impact of PTM on outcomes following LTx and OHT in a large modern cohort.
Methods
Study Design
This is a retrospective study of all adult patients(≥18 years) who underwent LTx or OHT from 2000-2011 in the UNOS registry. Patients undergoing re-transplantation, multi-visceral organ transplantation, and patients missing PTM data were excluded from the analysis. Primary stratification was by the presence of PTM with secondary stratification by type of PTM(melanoma, non-melanoma skin cancer, solid organ tumor, hematologic tumor [lymphoma, leukemia and myeloma], multiple tumors and other). In LTx patients, sub-group analysis was performed to compare patients before and after the implementation of the Lung Allocation Score(LAS) in May 2005. Institutional Review Board Approval was attained for this study.
Analysis and Outcomes
Study analysis included examination of all pertinent covariates including recipient data, donor data and transplant variables. Primary outcomes were 30-day, 1-year and 5-year mortality.
Statistical Analysis
Continuous variables were compared using the Student’s t test(parametric) and the Wilcoxon rank-sum test(non-parametric). Categorical variables were compared using Chi-squared analysis or Fisher’s exact test as appropriate. Survival was estimated by the Kaplan-Meier method.
Multivariable Cox proportional hazards regression models were created to estimate the risk of death associated with designated covariates. Covariates were initially examined in a univariate fashion. Associated variables on exploratory analysis(p<0.20), those with biological plausibility and those with previously reported significance were included in a forward stepwise fashion into the model. Akaike’s information criterion and the likelihood ratio test were utilized in a nested approach to identify the model with the greatest explanatory power.
In addition to multivariable Cox models, 2:1 cohort matching was utilized to assess the impact of PTM while further minimizing the effect of confounding factors.[10] A multivariable propensity model was created as described above. Though discriminant analysis may be used to create a propensity model, it assumes a normal distribution of all covariates. Logistic regression analysis does not make this assumption [11] and was utilized in our study. The matched cohort was then generated using 2:1 nearest neighbor matching without replacement with the created propensity model. As this matching was performed in a k:1 manner, all data was treated as though created via randomization[12], and all subsequent analysis of this cohort was performed in an unmatched manner utilizing the various statistical methods previously mentioned.
In all comparisons, p <0.05(2-tailed) was considered statistically significant. Mean values are displayed with their standard deviations while median values are displayed with their interquartile ranges. Hazard ratios(HR) are presented with their 95% confidence intervals(CI). Statistical analysis was performed using STATA 11.2(StataCorp LP, College Station, TX).
Results
Lung Cohort Statistics
During our study period, after applying exclusion criteria, a final LTx cohort of 13,613 patients was produced.
The mean age of the LTx cohort was 53±13 years and 7,577 patients(56%) were male. Idiopathic pulmonary fibrosis(IPF;n=3961[29%]) was the most common indication for transplantation. 740 patients(5%) had PTM. Of the 8,503 patients(62%) transplanted in the LAS era, the mean LAS score was 45±16.
Heart Cohort Statistics
25,376 patients underwent OHT during our study period. Applying exclusion criteria resulted in a final OHT cohort of 19,817 patients.
The mean age of the OHT cohort was 52±12 years and 15,060 patients(76%) were male. 8,800 patients(44%) underwent OHT due to dilated cardiomyopathy. 1,117 patients(6%) had PTM.
Lung Propensity Score Matched Cohort Statistics
After utilizing logistic regression analysis to generate a propensity score, 632/740(85%) patients with PTM were matched in a 2:1 fashion with 1,264 control patients for a total matched LTx cohort of 1,896 patients. The mean age of this matched cohort was 59±10 years and 1,106 patients(58%) were male. 1,490 patients(79%) were transplanted in the post-LAS era and the mean LAS score of this population was 45±15.
Heart Propensity Score Matched Cohort Statistics
Likewise, 891/1,117(80%) OHT patients with PTM were matched in a 2:1 fashion with 1,782 control patients to generate a total matched cohort of 2,673 patients. The mean age of this cohort was 55±11 years, and 1,598 patients(60%) were male. 1,080 patients(40%) underwent OHT due to ischemic cardiomyopathy.
Lung Cohort Baseline Characteristics
After stratification by the presence of PTM, several differences were noted(Table 1). PTM patients were older, more likely to be Caucasian, have hypertension, and be college graduates. Additionally, they were more likely to have IPF and require ventilator assistance. PTM patient donors were older and CMV positive. PTM patients were less likely to get bilateral lung transplants, had a shorter waitlist time, and were more likely to have their transplant at high volume centers. While other variables were statistically significant, their absolute differences were small and unlikely to be clinically relevant. The prevalence of PTM increased in the post-LAS era(3.3% vs 6.7%, p<0.001), with significant increases in all etiologies(Supplement).
Table 1.
Baseline Characteristics of LTx Patients Stratified by Pre-transplant Malignancy (PTM)
| Variable | No PTM (N=12,873) | PTM (N=740) | p value |
|---|---|---|---|
| Recipient demographics and comorbidities | |||
| Age (years) | 52.4 ± 13.0 | 58.6 ± 10.9 | < 0.01 |
| Male | 7,172/12,873 (55.7%) | 405/740 (54.7%) | 0.60 |
| Race | |||
| White | 10,965/12,872 (85.2%) | 695/740 (93.9%) | <0.01 |
| Black | 1,071/12,872 (8.3%) | 28/740 (3.8%) | <0.01 |
| Hispanic | 615/12,872 (4.8%) | 9/740 (1.2%) | <0.01 |
| Other race | 221/12,872 (1.7%) | 8/740 (1.1%) | 0.24 |
| Hypertension | 1,245/6,779 (18.4%) | 82/286 (28.7%) | <0.01 |
| Diabetes mellitus | 1,894/12,822 (14.8%) | 95/738 (12.9%) | 0.16 |
| Creatinine (mg/ dL) | 0.88 ± 0.5 | 0.88 ± 0.6 | 0.83 |
| Pre-operative dialysis | 9/6,797 (0.1%) | 0/286 (0%) | 1.00 |
| BMI (kg/m2) | 24.8 ± 7.3 | 24.8 ± 4.5 | 0.95 |
| CMV positive | 7,043/10,223 (68.9%) | 397/583 (68.1%) | 0.69 |
| Private insurance | 8,137/12,873 (63.2%) | 442/740 (59.7%) | 0.06 |
| College level education | 5,927/11,108 (53.4%) | 417/682 (61.1%) | <0.01 |
| Etiology of respiratory failure | |||
| Pulmonary hypertension (Primary) |
317/12,873 (2.5%) | 10/740 (1.4%) | 0.06 |
| Cystic Fibrosis | 1,844/12,873 (14.3%) | 14/740 (1.9%) | <0.01 |
| Idiopathic pulmonary fibrosis |
3,712/12,873 (28.8%) | 249/740 (33.6%) | <0.01 |
| Other diagnosis | 2,291/12,873 (17.8%) | 148/740 (20.0%) | 0.13 |
| Recipient hemodynamics, pulmonary function and acuity | |||
| Cardiac output (L/min) | 5.3 ± 1.5 | 5.3 ± 1.3 | 0.80 |
| Mean PA pressure | 27.4 ± 11.0 | 25.4 ± 9.7 | <0.01 |
| Trans-pulmonary gradient | 16.1 ± 10.1 | 14.6 ± 9.01 | <0.01 |
| FEV1 (L) | 36.5 ± 21.0 | 39.0 ± 21.5 | <0.01 |
| FVC (L) | 49.6 ± 17.8 | 50.7 ± 18.1 | 0.11 |
| Hospitalized | 1,566/12,873 (12.2%) | 80/735 (10.9%) | 0.30 |
| ICU | 719/12,873 (5.6%) | 43/735 (5.9%) | 0.76 |
| Ventilator support | 529/12,873 (4.1%) | 50/740 (6.8%) | <0.01 |
| ECMO support | 98/12,873 (0.8%) | 7/740 (1.0%) | 0.52 |
| LAS | 44.6 ± 15.6 | 44.2 ± 15.2 | 0.52 |
| Donor and transplant variables | |||
| Age (years) | 33.2 ± 14.1 | 34.4 ± 14.7 | 0.02 |
| Male | 7,775/12,873 (60.4%) | 429/740 (58.0%) | 0.19 |
| CMV positive | 7,779/12,807 (60.7%) | 475/736 (64.5%) | 0.04 |
| Bilateral LTx | 7,653/12,873 (59.5%) | 406/740 (54.9%) | <0.01 |
| Ischemic time (hours) | 4.9 ± 1.7 | 4.9 ± 1.8 | 0.75 |
| Waitlist time (days) | 137 [37-414] | 79 [25-248] | <0.01 |
| Sex matching | 8,804/12,873 (68.4%) | 512/740 (69.2%) | 0.65 |
| HLA matching | 6,329/10,811 (58.5%) | 393/648 (60.7%) | 0.29 |
| Annual center volume | |||
| ≤ 18 LTx | 3,305/12,873 (25.7%) | 165/740 (22.3%) | 0.04 |
| 19-31 LTx | 3,450/12,873 (26.8%) | 155/740 (21.0%) | <0.01 |
| 32-46 LTx | 3,004/12,873 (23.3%) | 156/740 (21.1%) | 0.16 |
| ≥ 47 LTx | 3,114/12,873 (24.2%) | 264/740 (35.7%) | <0.01 |
LTx = lung transplantation, PTM = pre-transplant malignancy, BMI = body mass index, CMV = cytomegalovirus, PA = pulmonary artery, FEV1 = forced expiratory volume in 1 sec, FVC = forced vital capacity, ICU = intensive care unit, ECMO = extra-corporeal membrane oxygenation, LAS = lung allocation score, HLA = human leukocyte antigen
Heart Cohort Baseline Characteristics
OHT patient stratification by the presence of PTM(Table 2) revealed that PTM patients were older, female and Caucasian. They had an elevated creatinine and a lower BMI than non-PTM patients. PTM patients were more likely to have a college level education, were more likely to have dilated cardiomyopathy and other diagnoses as the etiology of their heart failure. PTM patients had lower cardiac outputs, were more likely to be on inotropic support and less likely to require VAD support. Their donors were older and were more likely to be female. PTM patients had shorter waitlist times, but were less likely to receive sex-matched organs. Solid organ tumors were the most common type of PTM, affecting 448 patients(40%).
Table 2.
Baseline Characteristics of OHT Patients Stratified by Pre-transplant Malignancy (PTM)
| Variable | No PTM (N=18,700) | PTM (N=1,117) | p value |
|---|---|---|---|
| Recipient demographics and comorbidities | |||
| Age (years) | 51.9 ±12.2 | 55.3 ± 11.5 | <0.01 |
| Male | 14,369/18,700 (76.8%) | 691/1,117 (61.9%) | <0.01 |
| Race | |||
| White | 13,492/18,700 (72.2%) | 883/1,117 (79.1%) | <0.01 |
| Black | 3,203/18,700 (17.1%) | 152/1,117 (13.6%) | <0.01 |
| Hispanic | 1,356/18,700 (7.3%) | 55/1,117 (4.9%) | <0.01 |
| Other race | 649/18,700 (3.5%) | 27/1,117 (2.4%) | 0.06 |
| Hypertension | 4,458/11,068 (40.3%) | 209/552 (37.9%) | 0.26 |
| Diabetes mellitus | 4,419/18,621 (23.7%) | 252/1,112 (22.7%) | 0.42 |
| Creatinine (mg/ dL) | 1.3 ± 0.8 | 1.4 ± 0.9 | 0.04 |
| Pre-operative dialysis | 165/11,239 (1.5%) | 14/560 (2.5%) | 0.05 |
| BMI (kg/m2) | 26.4 [23.3-29.8] | 25.7 [22.7-29.1] | <0.01 |
| CMV positive | 10,949/15,307 (71.5%) | 632/887 (71.3%) | 0.89 |
| Private insurance | 11,061/18,700 (59.2%) | 690/1,117 (61.8%) | 0.08 |
| College level education | 7,512/14,828 (50.7%) | 531/923 (57.5%) | <0.01 |
| Etiology of heart failure | |||
| Dilated cardiomyopathy | 8,222/18,700 (44%) | 578/1,117 (51.8%) | <0.01 |
| Ischemic heart disease | 8,309/18,700 (44.4%) | 410/1,117 (36.7%) | <0.01 |
| Congenital heart disease | 513/18,700 (2.7%) | 11/1,117 (1%) | <0.01 |
| Other diagnosis | 1,163/18,700 (6.2%) | 86/1,117 (7.7%) | 0.048 |
| Recipient hemodynamics and acuity | |||
| Cardiac output (L/min) | 4.5 ± 1.5 | 4.3 ± 1.4 | <0.01 |
| Mean PA pressure | 28.6 ± 10.2 | 28.6 ± 10.1 | 0.99 |
| Hospitalized | 8,896/18,699 (47.6%) | 529/1,113 (47.5%) | 0.98 |
| ICU | 5,512/18,699 (29.5%) | 319/1,113 (28.7%) | 0.56 |
| Ventilator support | 494/18,700 (2.6%) | 23/1,117 (2.1%) | 0.24 |
| Inotropic support | 8,089/18,700 (43.3%) | 552/1,117 (49.4%) | <0.01 |
| VAD support | 8,560/18,700 (45.8%) | 432/1,117 (38.7%) | <0.01 |
| Intra-aortic balloon pump | 980/18,700 (5.2%) | 56/1,117 (5.0%) | 0.74 |
| Pacemaker | 658/18,497 (3.6%) | 30/1,091 (2.8%) | 0.16 |
| Donor and transplant variables | |||
| Age (years) | 31.4 ± 12.2 | 32.7 ± 12.7 | <0.01 |
| Male | 13,470/18,700 (72.0%) | 731/1,117 (65.4%) | <0.01 |
| CMV positive | 11,454/18,638 (61.5%) | 670/1,112 (60.3%) | 0.42 |
| Ischemic time (hours) | 3.2 ± 1.1 | 3.2 ± 1.0 | 0.15 |
| Waitlist time (days) | 83 [25-232] | 71 [22-212] | 0.01 |
| Sex matching | 13,557/18,700 (72.5%) | 773/1,117 (69.2%) | 0.02 |
| HLA matching | 9,341/15,863 (58.9%) | 542/946 (57.3%) | 0.33 |
| Annual center volume | |||
| ≤ 13 OHT | 5,247/18,700 (28.1%) | 305/1,117 (27.3%) | 0.59 |
| 14-21 OHT | 4,624/18,700 (24.7%) | 267/1,117 (23.9%) | 0.54 |
| 22-33 OHT | 4,305/18,700 (23.0%) | 264/1,117 (23.6%) | 0.64 |
| ≥ 34 OHT | 4,524/18,700 (24.2%) | 281/1,117 (25.2%) | 0.47 |
OHT = orthotopic heart transplantation, PTM = pre-transplant malignancy, BMI = body mass index, CMV = cytomegalovirus, PA = pulmonary artery, ICU = intensive care unit, VAD = ventricular assist device, HLA = human leukocyte antigen
Lung Propensity Score Matched Cohort Baseline Characteristics
In the matched cohort, stratification by PTM revealed similar differences in baseline characteristics. Again, PTM patients were more likely to be Caucasian and had hypertension. Cystic fibrosis was unlikely to be the cause of respiratory failure in PTM patients, as was IPF. PTM patients were more likely to receive CMV positive organs. The overall PTM incidence did not change before or after LAS in the matched cohort.
Heart Propensity Score Matched Cohort Baseline Characteristics
When stratified by PTM in the matched cohort, PTM patients were more likely to be Caucasian and more likely to be transplanted due to dilated cardiomyopathy. PTM patients were more likely to have a college level education and less likely to require VAD support. Solid organ tumors remained the most common type of PTM, affecting 381 patients(42.8%).
Lung Cohort Survival
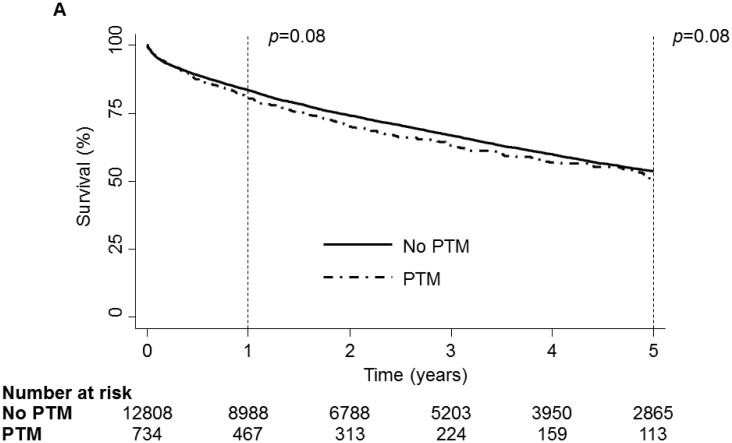
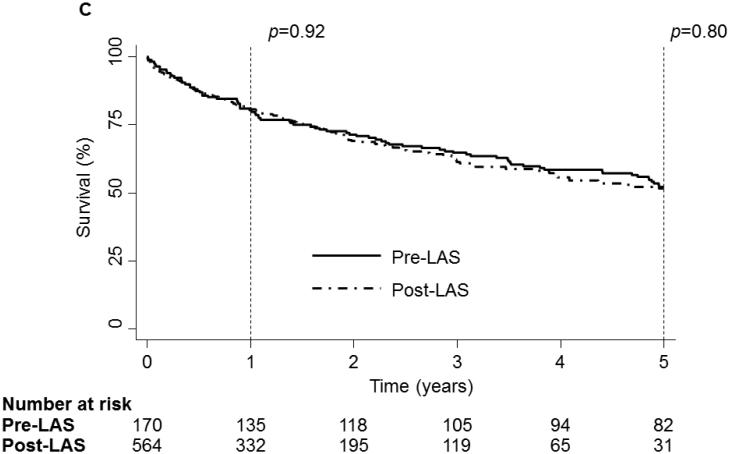
On unadjusted analysis, neither PTM nor any specific tumor type were associated with an increase in mortality[Fig 1:a,b]. Though the incidence of PTM increased in the post-LAS era, PTM was associated with similar survival before and after the onset of LAS[Fig 1c].
Figure 1.
1 and 5 year Kaplan-Meier survival curve of the LTx Cohort stratified by Pre-Transplant Malignancy (PTM). Patients without PTM are depicted as a solid line while patients with PTM are depicted with a dash-dot line. (B) 1 and 5 year Kaplan-Meier survival curve of the LTx Cohort stratified by type of malignancy. Patients without cancer are depicted with a dark blue line, patients with melanoma are depicted with a purple line, patients with non-melanoma skin cancer are depicted with a dark green line, patients with a solid organ tumor are depicted with an orange line, patients with a hematologic tumor are depicted with a light green line, patients with multiple tumors are depicted with a red line and patients with other tumors are depicted with a light blue line. (C) 1 and 5 year Kaplan-Meier survival curve of the LTx Cohort stratified by the presence of the Lung Allocation Score (LAS). The solid line depicts the era before the LAS and the dash-dot line depicts the era after the LAS. p values were determined by the log-rank test.
On adjusted analysis, PTM was not associated with an increased hazard of mortality at any time point[30-day:HR-0.95,p=0.84; 1-year:HR-0.97,p=0.83; 5-year:HR-1.07,p=0.51]. No specific tumor type was associated with a significant change in hazard of mortality at any time point(Table 3). In all models, increasing annual center volume was protective while increasing age, increasing serum creatinine, and the need for ICU care prior to LTx were associated with an increased hazard of mortality.
Table 3.
Cox Proportional Hazards Model of 5-Year LTx Mortality
| Variable | Multivariate HR (95% CI) | p value |
|---|---|---|
| Type of malignancy | ||
| No cancer | 1 (Reference) | |
| Melanoma | 0.99 (0.50-2.01) | 1.00 |
| Non-melanoma skin cancer | 1.10 (0.74-1.62) | 0.65 |
| Solid organ tumor | 1.03 (0.77-1.38) | 0.84 |
| Hematologic tumor | 1.12 (0.58-2.17) | 0.74 |
| Multiple tumors | 1.14 (0.37-3.56) | 0.82 |
| Other | 0.58 (0.24-1.39) | 0.22 |
| Recipient demographics and comorbidities | ||
| Age≥60 years | 1.32 (1.18-1.48) | <0.01 |
| Male | 1.07 (0.96-1.19) | 0.24 |
| BMI, per kg/m2 | 1.01 (1.00-1.02) | 0.01 |
| Creatinine, per mg/dL | 1.11 (1.03-1.19) | <0.01 |
| Bilirubin, per mg/dL | 1.06 (1.04-1.09) | <0.01 |
| Diabetes | 1.08 (0.94-1.24) | 0.26 |
| Pulmonary hypertension | 0.92 (0.81-1.03) | 0.16 |
| Private insurance | 0.96 (0.86-1.06) | 0.40 |
| College level education | 0.99 (0.90-1.11) | 0.96 |
| Recipient hemodynamics and acuity | ||
| FEV1, per liter | 0.99 (0.99-1.00) | 0.88 |
| Inhaled nitric oxide | 0.77 (0.29-2.10) | 0.62 |
| Trans-pulmonary gradient | 1.01 (1.00-1.02) | <0.01 |
| Hospitalized | 1.38 (1.15-1.66) | <0.01 |
| ICU | 1.89 (1.45-2.48) | <0.01 |
| Ventilator support | 1.04 (0.80-1.34) | 0.79 |
| ECMO support | 1.29 (0.75-2.23) | 0.36 |
| LAS era | 0.97 (0.83-1.13) | 0.67 |
| Donor and transplant variables | ||
| Age, per year | 0.99 (0.99-1.00) | 0.55 |
| Cigarette usage | 1.12 (0.97-1.30) | 0.13 |
| Diabetes | 1.39 (1.15-1.70) | <0.01 |
| Inotrope usage | 0.94 (0.85-1.04) | 0.22 |
| Bilateral lung transplant | 0.87 (0.77-0.99) | 0.03 |
| HLA mismatch | 0.94 (0.85-1.05) | 0.26 |
| Race matching | 0.85 (0.77-0.95) | <0.01 |
| Ischemic time, per hour | 0.98 (0.95-1.02) | 0.34 |
| Biopsy proven rejection | 1.32 (0.84-2.08) | 0.23 |
| Annual center volume | ||
| ≤ 18 LTx | 1 (Reference) | |
| 19-31 LTx | 0.77 (0.67-0.89) | <0.01 |
| 32-46 LTx | 0.72 (0.62-0.84) | <0.01 |
| ≥ 47 LTx | 0.71 (0.61-0.82) | <0.01 |
LTx = lung transplantation, HR = hazard ratio, CI = confidence interval, BMI = body mass index, FEV1 = forced expiratory volume in 1 sec, ECMO = extra-corporeal membrane oxygenation, ICU = intensive care unit, HLA = human leukocyte antigen, LAS = lung allocation score
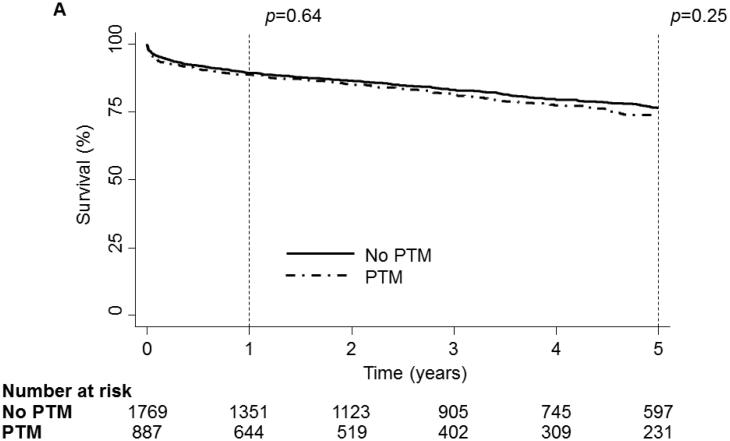
Heart Cohort Survival
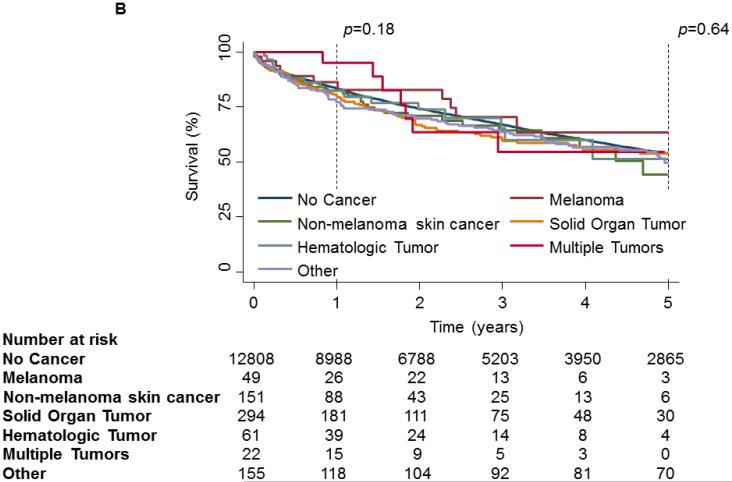
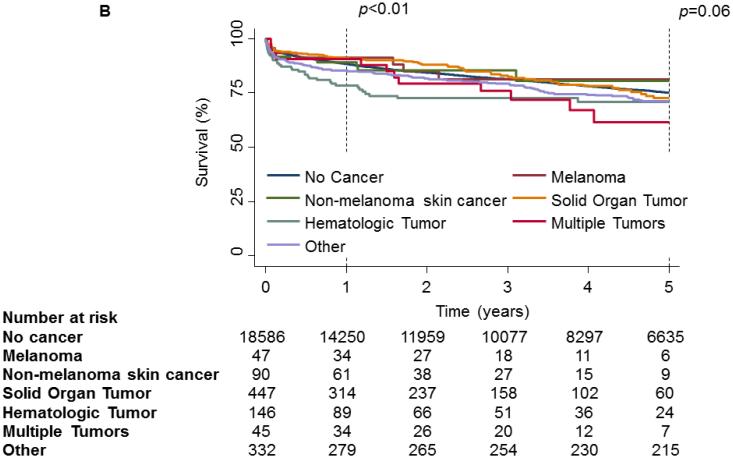
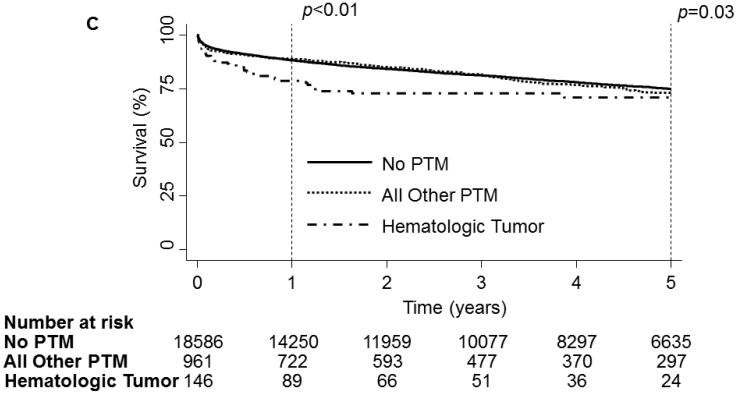
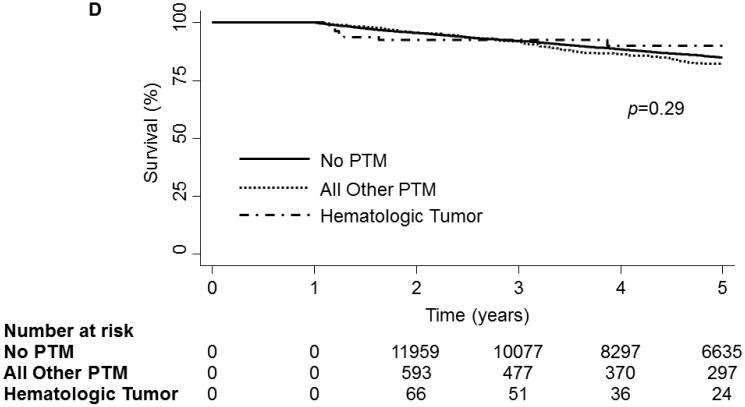
On unadjusted analysis, PTM was not associated with any decrease in survival[Fig 2a]. When examining survival by tumor type, no significance was observed at the 30-day(p=0.17) time point. However, at 1-year a significant divergence could be seen(p<0.01) but could not be maintained to the 5-year time point(p=0.06,Fig 2b). Further analysis of hematologic malignancies against all other tumor types combined revealed a significant decrease in survival at 1-year(p<0.01) and 5-years(p=0.03, Fig 2c). However, when conditioned on survival to 1-year, 5-year mortality was not significantly different(Fig 2d).
Figure 2.
1 and 5 year Kaplan-Meier survival curve of the OHT Cohort stratified by Pre-Transplant Malignancy (PTM). Patients without PTM are depicted as a solid line while patients with PTM are depicted with a dash-dot line. (B) 1 and 5 year Kaplan-Meier survival curve of the OHT Cohort stratified by type of malignancy. Patients without cancer are depicted with a dark blue line, patients with melanoma are depicted with a purple line, patients with non-melanoma skin cancer are depicted with a dark green line, patients with a solid organ tumor are depicted with an orange line, patients with a hematologic tumor are depicted with a light green line, patients with multiple tumors are depicted with a red line and patients with other tumors are depicted with a light blue line. (C) 1 and 5 year Kaplan-Meier survival curve of the OHT Cohort stratified by the type of malignancy. The solid line depicts patients without PTM, the dotted line depicts patients with other types of malignancy and the dash-dot line depicts patients with hematologic tumors. (D) 5 year Kaplan-Meier survival curve of the OHT Cohort conditional on 1 year survival, stratified by the type of malignancy. The solid line depicts patients without PTM, the dotted line depicts patients with other types of malignancy and the dash-dot line depicts patients with hematologic tumors. p values were determined by the log-rank test.
Adjusted analysis revealed that overall PTM was not associated with an increased hazard of mortality at 30-days[HR-1.19,p=0.40], 1-year[HR-0.87,p=0.35] or 5-years[HR-0.99,p=0.98]. However in examining specific tumor types, hematologic tumors were associated with an increased hazard of mortality at 30-days[HR-2.00(1.10-3.64),p=0.02], 1-year[HR-1.97(1.30-2.97),p<0.01] and 5-years[HR-1.65(1.15-2.36),p<0.01;Table 4]. In all models, new onset dialysis, age≥60 years and the need for ventilator support were associated with an increased hazard of mortality, while increasing annual center volume was protective.
Table 4.
Cox Proportional Hazards Model of 5-Year OHT Mortality
| Variable | Multivariate HR (95% CI) | p value |
|---|---|---|
| Type of malignancy | ||
| No cancer | 1 (Reference) | |
| Melanoma | 0.96 (0.40-2.31) | 0.93 |
| Non-melanoma skin cancer | 0.96 (0.51-1.79) | 0.90 |
| Solid organ tumor | 0.82 (0.63-1.06) | 0.13 |
| Hematologic tumor | 1.65 (1.15-2.36) | <0.01 |
| Multiple tumors | 0.99 (0.54-1.80) | 0.97 |
| Other | 0.99 (0.79-1.26) | 1.00 |
| Recipient demographics and comorbidities | ||
| Age≥60 years | 1.18 (1.10-1.28) | <0.01 |
| Male | 0.90 (0.83-0.98) | 0.02 |
| Race | ||
| White | 1 (Reference) | |
| Black | 1.45 (1.31-1.60) | <0.01 |
| Hispanic | 1.11 (0.97-1.27) | 0.12 |
| Other | 1.03 (0.84-1.27) | 0.78 |
| Creatinine, per mg/dL | 1.07 (1.04-1.11) | <0.01 |
| Diabetes | 1.05 (0.97-1.14) | 0.23 |
| Private insurance | 0.80 (0.74-0.85) | <0.01 |
| Etiology of heart failure | ||
| Dilated cardiomyopathy | 1 (Reference) | |
| Ischemic cardiomyopathy | 1.17 (1.08-1.27) | <0.01 |
| Congenital disease | 1.59 (1.32-1.92) | <0.01 |
| Other | 1.07 (0.94-1.21) | 0.30 |
| Recipient hemodynamics and acuity | ||
| New onset dialysis | 4.18 (3.84-4.55) | <0.01 |
| Hospitalized | 1.13 (1.06-1.22) | <0.01 |
| Ventilator support | 1.62 (1.34-1.94) | <0.01 |
| IABP support | 0.90 (0.78-1.05) | 0.20 |
| VAD support type | ||
| No VAD | 1 (Reference) | |
| Extracorporeal/temporary | 1.67 (1.31-2.13) | <0.01 |
| Pulsatile VAD | 1.09 (0.99-1.20) | 0.07 |
| Continuous flow (except HMII) | 1.03 (0.64-1.63) | 0.91 |
| HMII | 1.14 (0.98-1.33) | 0.10 |
| Donor and transplant variables | ||
| Age, per year | 1.01 (1.01-1.01) | <0.01 |
| Cigarette usage | 1.16 (1.08-1.26) | <0.01 |
| CMV positive | 1.13 (1.05-1.21) | <0.01 |
| Sex matching | 0.88 (0.82-0.95) | <0.01 |
| Race matching | 0.95 (0.88-1.03) | 0.18 |
| Ischemic time, per hour | 1.08 (1.05-1.12) | <0.01 |
| Annual center volume | ||
| ≤ 13 OHT | 1 (Reference) | |
| 14-21 OHT | 0.88 (0.80-0.96) | <0.01 |
| 22-33 OHT | 0.85 (0.77-0.93) | <0.01 |
| ≥ 34 OHT | 0.77 (0.70-0.85) | <0.01 |
OHT = orthotopic heart transplantation, HR = hazard ratio, IABP = intra-aortic balloon pump, VAD = ventricular assist device, HMII = heartmate II, CMV = cytomegalovirus
Lung Propensity Score Matched Cohort Survival
As with the overall LTx cohort, neither PTM nor specific tumor types significantly affected survival at any time on unadjusted analysis. Additionally, PTM was not associated with an increase in mortality before or after the advent of the LAS at any time point.
Despite adjustment, PTM and specific tumor types remained insignificant predictors of mortality. Only the need for an ICU prior to LTx was associated with an increased hazard of mortality across all models.
Heart Propensity Score Matched Cohort Survival
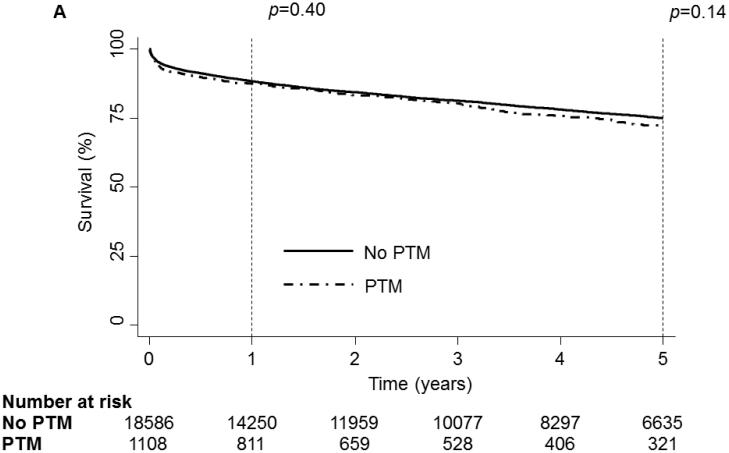
Again, on unadjusted analysis, PTM was not associated with any decrease in survival(Fig 3a). No specific tumor type was associated with a significant decrease in survival at 30-days(p=0.15) or 5-years(p=0.13). However, at 1-year significant divergence in survival was again demonstrated(p=0.048,Fig 3b). Analysis of hematologic malignancies against all other tumor types revealed a significant decrease in survival at 1-year(p=0.01) but not at any other time(30-day:p=0.07, 5-year:p=0.11;Fig 3c).
Figure 3.
1 and 5 year Kaplan-Meier survival curve of the Matched OHT Cohort stratified by Pre-Transplant Malignancy (PTM). Patients without PTM are depicted as a solid line while patients with PTM are depicted with a dash-dot line. (B) 1 and 5 year Kaplan-Meier survival curve of the Matched OHT Cohort stratified by type of malignancy. Patients without cancer are depicted with a dark blue line, patients with melanoma are depicted with a purple line, patients with non-melanoma skin cancer are depicted with a dark green line, patients with a solid organ tumor are depicted with an orange line, patients with a hematologic tumor are depicted with a light green line, patients with multiple tumors are depicted with a red line and patients with other tumors are depicted with a light blue line. (C) 1 and 5 year Kaplan-Meier survival curve of the Matched OHT Cohort stratified by the type of malignancy. The solid line depicts patients without PTM, the dotted line depicts patients with other types of malignancy and the dash-dot line depicts patients with hematologic tumors. p values were determined by the log-rank test.
Multivariate adjusted analysis revealed that PTM was not associated with an increased hazard of mortality at any time point[30-days:HR-1.26,p=0.37; 1-year:HR-0.93,p=0.72; 5-year:HR-1.07,p=0.57]. Hematologic tumors were the only specific tumor type to demonstrate an increased hazard of mortality[30-day:HR-2.46,p=0.01; 1-year:HR-2.02,p<0.01; 5-year:HR-1.64,p=0.02]. New onset dialysis was associated with an increased hazard of mortality in all models.
Discussion
In this analysis of 13,613 LTx patients and 19,817 OHT patients, PTM was not associated with any increase in mortality in either adjusted or unadjusted analysis. When accounting for specific tumor types, hematologic malignancies were associated with an increase in the hazard of mortality in OHT patients, but not in LTx patients. The onset of the LAS did not demonstrate any variation in PTM mortality, but was associated with an increase in PTM prevalence. Propensity score matched cohort analysis of both LTx and OHT patients confirmed these findings.
Early guidelines in thoracic organ transplantation (specifically OHT) were extrapolated from the renal transplant experience.[2, 5] As such, many centers utilized the work of Penn and colleagues [13] in determining suitable candidates for transplantation. From this experience, pre-transplant malignancy became known as a relative contraindication.[3, 9] In one study, recurrence rates of PTM in renal transplant patients reached 21%, with 88% of these recurrences occurring in the first 5 years, potentially affecting long term survival from transplantation.[14] Recurrence rates from 0-33% have been recorded in the OHT literature, with all recurrences occurring in the first 5 years after transplantation. [1, 2, 5-8]. Rates of recurrence in the LTx literature range from 0-57%, again with all recurrences occurring in the first 5 years after transplantation. [9, 15] In addition to the risk of recurrence associated with PTM, de novo tumor formation may occur in all immunosuppressed patients after transplantation. According to Engels et al, the standardized incidence ratio for malignancy after solid organ transplantation, compared to the population at large, is 2.10 [16]. Furthermore, 14% of OHT patients and 15% of LTx patients without PTM develop a post-transplant malignancy within 5 years [17, 18]. While this paper does not address the issue of de novo tumor formation or recurrence associated with PTM, it does address the correlate issue associated with recurrence, namely the effect of PTM on mortality.
It is clear that mortality is only affected by a subset of PTM (hematologic malignancies) in OHT patients. One reason may be improvement in the effectiveness of treatments for many malignancies, including advanced and personalized chemotherapy regimens [19, 20], aggressive surgical intervention [21], and autologous stem cell transplantation [22]. These medical advancements may provide longer disease free intervals and more curative treatments than previously described. In addition, improvements in disease surveillance have resulted in earlier detection of recurrence with subsequent therapy. Perhaps the most important reason may be related to patient selection. Evidence suggests that surgeons are becoming more exclusive in the types of PTM patients that are accepted into transplant programs [1, 5, 8]. In addition to standard exclusion criteria, careful attention is paid to the type of PTM, the natural history of the disease, the life expectancy with optimal treatment, the extent of tumor involvement, and the disease-free survival. Many of these decisions are made in an interdisciplinary fashion alongside oncologists [8]. While current patient selection techniques appear to be quite successful based on our findings, standardization of PTM patient selection criteria, specifically with hematologic malignancies in OHT candidates, may improve outcomes.
Another factor discussed in the thoracic PTM literature is the immunosuppressive regimen and how it may vary in patients with PTM. An article by Dillon et al found that no variations from standard immunosuppressive regimens were necessary to maintain graft function in PTM patients undergoing OHT, with only 1 patient (1/7[14%]) having a recurrence of disease [1]. Similar data was reported by Pechet et al. In this study of pediatric LTx in PTM patients, only one child (1/19[5%]) had a variation in their post-transplant regimen, with no patients having a recurrence of their disease [9]. While our current study did not examine the specifics of the immunosuppressive regimens of the included patients, these data suggest that courses tailored specifically for PTM patients are not necessary.
This study has revealed several significant differences between patients with and without PTM. While many of these differences are intuitive, such as PTM patients being older than their counterparts, some have been surprising. In both the LTx and OHT cohorts, donor age was significantly different between the PTM and non-PTM populations. Additionally, other donor criteria were significantly different in at least one cohort. However, these differences were small and though significant, are likely not clinically relevant.
In this study, the relative hazard of mortality for hematologic malignancies was significantly increased for OHT patients. Moreover, survival was significantly decreased in this cohort, though this decrease did not develop until 1-year post-operatively. While survival was also significantly lower at 5-years, this finding was not maintained when conditioned on 1-year survival. We speculate that this correlation between mortality and time from operation is likely due to variations in immunosuppression. These periods of lability lead to opportunities for “over”-immunosuppression, which may facilitate recurrent malignancy, and are more likely to occur during the first year. However, no other tumor type was found to have a significant effect on mortality in either the LTx or OHT cohort. This may be intrinsic to the nature of hematologic malignancies. Perhaps this is due to the challenging nature of treating these systemic malignancies and the inherent difficulty in removing all disease burden. In the previously reported OHT literature, malignancy associated deaths occurred among patients with multiple tumor types including various adenocarcinomas, lymphomas, leukemias and cardiac sarcomas [5-8]. In a study by Koerner et al, 3 deaths (3/8[38%]) occurred in patients with histories of “plasmocytoma” [7]. Likewise, 1 death (1/2[50%]) in an article by Edwards et al occurred in a patient with a history of acute lymphoblastic leukemia [5]. Finally, 2 deaths (2/3[67%]) occurred in patients with a history of leukemia or lymphoma in an article by Goldstein et al [6]. These data represent small series; however, the fact that this elevated mortality rate among hematologic malignancies is reinforced in several studies lends credibility to the findings presented here.
Interestingly, hematologic malignancies were not associated with an increased hazard in LTx patients at any time, despite LTx patients typically requiring higher doses of immunosuppression than their OHT counterparts. While previous reports have cited malignancy associated deaths in LTx patients due to leukemia [9], bronchioloalveolar carcinoma [15] and medulloblastoma [23], this literature is primarily composed of case reports or small series which make detailed analysis of tumor-associated risk difficult to ascertain. A possible explanation for the hazard difference seen in OHT and LTx patients in this study may lie in the inherent mortality differences between the populations, as well as variations in selection criteria, though undoubtedly this does not account for all variation. Further study of this topic is required.
Limitations
Several limitations are associated with this study. First, the UNOS database does not provide certain clinically relevant PTM information, such as data on disease-free survival prior to transplantation. Second, our study does not assess recurrence data, de novo tumor formation data or immunosuppression data, and can make no comment on these covariates or their relation to mortality outcomes. Additionally, our matched cohort analysis is dependent upon the fit of our propensity score to our patient population. As not all patients will fit the score, all patients are not equally included in this analysis, introducing selection bias. However, since this bias is generally towards the null hypothesis[24], our finding of increased mortality in the OHT matched cohort with hematologic malignancies is strengthened further. Finally, the UNOS dataset is composed of many variables available for analysis, however the possibility remains that potentially influential variables are not included in this analysis. Furthermore, large registry databases depend heavily on accurate and honest coding. In this study, we have assumed that all coding errors or missing data in the database are random and are thus unlikely to render any bias. If this assumption is false, residual bias may exist.
Conclusions
In the largest study to date of the effects of PTM on outcomes following LTx and OHT, PTM is not associated with any decrease in survival. Secondary stratification by tumor type reveals an increased hazard of mortality in OHT patients with hematologic malignancies. Sub-group analysis stratified by LAS reveals no significant effect on outcomes. These results were verified by propensity score matched cohort analysis of both LTx and OHT patients. Further analysis is needed to investigate the roles of de novo tumor formation, tumor recurrence and immunosuppressive regimens. Thoracic organ transplantation in carefully selected patients with PTM can be performed with equivalent outcomes.
Supplementary Material
Acknowledgements
Dr. Beaty is the Irene Piccinini Investigator in Cardiac Surgery. Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. This research was supported in part by a National Institutes of Health Grant: T32CA126607. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. There are no relevant financial disclosures.
Footnotes
Conflicts: The authors have no relevant conflicts to disclose.
Presentation: The contents of this manuscript were presented at the 32nd Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation
References
- 1.Dillon TA, et al. Cardiac transplantation in patients with preexisting malignancies. Transplantation. 1991;52(1):82–5. doi: 10.1097/00007890-199107000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ladowski SD, et al. Long-term follow-up of hearttransplant recipients with pre-transplant malignancies. Tex Heart Inst J. 2006;33(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JM, et al. Heart transplantation in patients with malignant disease. J Heart Transplant. 1990;9(6):627–9. discussion 630. [PubMed] [Google Scholar]
- 4.Copeland JG, et al. Selection of patients for cardiac transplantation. Circulation. 1987;75(1):2–9. doi: 10.1161/01.cir.75.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BS, et al. Cardiac transplantation in patients with preexisting neoplastic diseases. Am J Cardiol. 1990;65(7):501–4. doi: 10.1016/0002-9149(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DJ, et al. Orthotopic heart transplantation in patients with treated malignancies. Am J Cardiol. 1995;75(14):968–71. doi: 10.1016/s0002-9149(99)80704-8. [DOI] [PubMed] [Google Scholar]
- 7.Koerner MM, et al. Results of heart transplantation in patients with preexisting malignancies. Am J Cardiol. 1997;79(7):988–91. doi: 10.1016/s0002-9149(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 8.Oechslin E, et al. Pretransplant malignancy in candidates and posttransplant malignancy in recipients of cardiac transplantation. Ann Oncol. 1996;7(10):1059–63. doi: 10.1093/oxfordjournals.annonc.a010499. [DOI] [PubMed] [Google Scholar]
- 9.Pechet TV, et al. Lung transplantation in children following treatment for malignancy. J Heart Lung Transplant. 2003;22(2):154–60. doi: 10.1016/s1053-2498(02)00671-x. [DOI] [PubMed] [Google Scholar]
- 10.Elwood JM. Critical appraisal of epidemiological studies and clinical trials. 3rd. Oxford University Press; Oxford ; New York: 2007. pp. xiii–570. Oxford medical publications. [Google Scholar]
- 11.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penn I. Kidney transplantation following treatment of tumors. Transplant Proc. 1986;18(Suppl 3):16–20. [Google Scholar]
- 14.Penn I. Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant. 1997;2(4):14–7. [PubMed] [Google Scholar]
- 15.Garver RI, Jr., et al. Recurrence of bronchioloalveolar carcinoma in transplanted lungs. N Engl J Med. 1999;340(14):1071–4. doi: 10.1056/NEJM199904083401403. [DOI] [PubMed] [Google Scholar]
- 16.Engels EA, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(10):1186–91. doi: 10.1016/j.healun.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini C, et al. Incidence of neoplastic disease following lung transplantation: a 17-year single-center experience. Transplant Proc. 2011;43(4):1156–8. doi: 10.1016/j.transproceed.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 19.Dancey JE, et al. The genetic basis for cancer treatment decisions. Cell. 2012;148(3):409–20. doi: 10.1016/j.cell.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23. doi: 10.1186/1741-7015-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua TC, et al. Viewing metastatic colorectal cancer as a curable chronic disease. Am J Clin Oncol. 2012;35(1):77–80. doi: 10.1097/COC.0b013e3181fe4444. [DOI] [PubMed] [Google Scholar]
- 22.Ramchandren R. Advances in the Treatment of Relapsed or Refractory Hodgkin's Lymphoma. Oncologist. 2012 doi: 10.1634/theoncologist.2011-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor S, Kurland G, Jakacki R. Recurrent medulloblastoma following pediatric double-lung transplant. J Heart Lung Transplant. 2000;19(10):1011–3. doi: 10.1016/s1053-2498(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S, Lash TL. 3rd Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. Modern epidemiology; pp. x–758. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.