Abstract
Purpose
To analyze the role of lymph node dissection (LND) in patients with large renal tumors.
Methods
We performed a retrospective study of patients with renal cell carcinoma ≥7 cm in size undergoing surgery between 1990 and 2012. Primary outcome measures were recurrence-free and overall survival of patients who did and did not undergo LND. Cox proportional hazards regression models were created to account for known risk factors for recurrence and survival. Secondary outcomes were recurrence-free and overall survival by lymph node status, lymph node template and number of lymph nodes removed.
Results
Of 524 patients, 164 had disease recurrence and 197 died. Median follow-up was 5 and 5.5 years for patients who did not die or have a recurrence, respectively. A total of 334 (64 %) patients underwent LND, and node-positive disease was identified in 26 (8 %). For patients who did and did not undergo LND, 5-year recurrence-free survival was 64 and 77 %, respectively. Five-year overall survival was 75 and 78 %, respectively. LND was not a predictor of recurrence or survival in multivariate analysis. Node-positive disease was associated with recurrence (p < 0.0005) and mortality (p = 0.032), although node-positive patients had a 5-year overall survival of 65 %.
Conclusions
We did not find a difference in recurrence-free or overall survival in patients with ≥7-cm tumors whether or not they underwent LND. Node-positive disease was associated with worse outcomes, suggesting that LND provides important staging information that can be important in the design of adjuvant clinical trials.
Keywords: Carcinoma, renal cell, Nephrectomy, Lymph node dissection
Introduction
Ten to 28 % of all patients with renal cell carcinoma (RCC) will experience local or distant recurrence following nephrectomy [1], with patterns of recurrence related closely to histology and stage [2]. Regional disease involving retroperitoneal lymph nodes is associated with higher pathological stage and larger tumor size and has an adverse prognosis similar to that of patients with pulmonary metastases [3, 4]. While lymph node dissection (LND) was part of the original description of radical nephrectomy by Robson [5], there is no definitive evidence that LND provides a therapeutic advantage [3, 6]. Due to this lack of a clear therapeutic effect, urological surgeons are inconsistent in their use of regional LND. Currently, the most accepted rationale for performing LND is for more complete pathological staging. However, in other urologic cancers, such as prostate, penile and bladder cancers, LND is associated with a small but real therapeutic advantage in patients with minimal metastatic disease [7–9].
Retrospective data from centers of excellence also suggest similar benefits of LND in RCC patients with lymph node-positive disease, although the selection criteria for LND were unclear [10–13]. The two prospective studies examining LND have been criticized: one study for lacking important clinical data [14] and the other for including a large proportion of patients with low-stage disease [15]. In the latter study, the EORTC 30881 randomized-controlled trial, LND did not impact survival. The overall incidence of node-positive disease in this study was only 4 %, compared to an incidence of 12–37 % in patients with pT3–4 disease cited in the literature [3]. With a low proportion of node-positive disease, this study was criticized as being underpowered [16]. The benefits of LND for patients who are more likely to have node-positive disease are unknown. Therefore, our goal was to evaluate recurrence-free and overall survival in patients with large renal tumors who did or did not undergo LND.
Materials and methods
Following institutional review board approval, we identified 524 patients who underwent partial or radical nephrectomy for histologically confirmed RCC between 1990 and 2012 at Memorial Sloan-Kettering Cancer Center. High-risk disease was defined as tumors ≥7 cm in pathological size in the absence of distant metastatic disease. We excluded patients with a history of previous RCC or synchronous bilateral tumors.
Chart review was performed to determine whether lymphadenopathy was diagnosed preoperatively on cross-sectional radiographic imaging or described by surgeons in their operative notes. Radiographic lymphadenopathy was defined as any retroperitoneal lymph node ≥1 cm in longest dimension. Operative and pathological reports were reviewed to confirm that LND was performed, the number of lymph nodes removed and the lymph node template performed. Number of nodes removed was categorized as 0–3, 4–7 or ≥8. Lymph node template was categorized as hilar only versus other, which included paracaval, precaval, retrocaval and interaortocaval for right-sided tumors and paraaortic, preaortic and interaortocaval for left sided tumors. Patients who had only incidental perinephric nodal tissue discovered in the nephrectomy specimen were counted as having no LND. Performance of LND and the extent of dissection were not standardized but based on the surgeon’s discretion. Some surgeons perform an extended LND in nearly all cases, while others perform LND more selectively, either for staging or in the presence of surgically resectable lymphadenopathy.
Our primary objective was to describe the differences between recurrence-free and overall survival in patients who did or did not undergo LND. Cox proportional hazards regression models were used for both outcomes and included pathological stage (pT2, pT3 or pT4 by AJCC 2010), tumor size (cm), tumor histology (clear cell, chromophobe or papillary), presenting symptoms (incidental, local or systemic) ASA score (I/II vs. III/IV), sarcomatoid histology (yes or no), surgery type (radical vs. partial), LND (yes or no) and lymphadenopathy (yes or no).
Secondary analyses were performed for recurrence-free and overall survival by lymph node status (positive or negative), number of nodes removed and LND template. All statistical analyses were performed using Stata 12.0 (Stata Corp., College Station, TX, USA).
Results
Characteristics of the study population are summarized in Table 1. LND was associated with younger age (p = 0.005). Pathological features associated with LND were higher stage (p = 0.001), larger tumor size (p < 0.0001) and lymphadenopathy (p < 0.0001). Rates of LND for minimally invasive versus open surgery were similar (p = 0.9), but patients undergoing radical nephrectomy were more likely to undergo LND than those undergoing partial nephrectomy LND (p < 0.0001).
Table 1.
Patient characteristics. All values are median (interquartile range) or frequency (%)
| No. LND (N = 190) | LND (N = 334) | p value | |
|---|---|---|---|
| Age | 62.9 (53.7, 70.4) | 59.2 (51.3, 67.4) | 0.005 |
| Gender | 0.5 | ||
| Female | 63 (33 %) | 102 (31 %) | |
| Male | 127 (67 %) | 232 (69 %) | |
| Race | 0.12 | ||
| White | 17 (8.9 %) | 45 (13 %) | |
| Other | 173 (91 %) | 289 (87 %) | |
| ASA | 0.8 | ||
| I/II | 116 (61 %) | 200 (60 %) | |
| III/IV | 74 (39 %) | 134 (40 %) | |
| Presenting symptoms | 0.6 | ||
| Incidental | 97 (51 %) | 157 (47 %) | |
| Local | 75 (40 %) | 146 (44 %) | |
| Systemic | 17 (9.0 %) | 31 (9.3 %) | |
| Pathological T stage | 0.001 | ||
| T2 | 84 (44 %) | 95 (28 %) | |
| T3 | 101 (53 %) | 227 (68 %) | |
| T4 | 5 (2.6 %) | 12 (3.6 %) | |
| Pathological N stage | |||
| pN0 | – | 308 (92 %) | |
| pN1 | – | 26 (7.8 %) | |
| Tumor size (cm) | 9.2 (8.0, 10.9) | 10.1 (8.5, 12.2) | <0.0001 |
| Histology | 0.10 | ||
| Clear cell | 134 (71 %) | 262 (78 %) | |
| Chromophobe | 35 (18 %) | 49 (15 %) | |
| Papillary | 21 (11 %) | 23 (6.9 %) | |
| Location | 0.12 | ||
| Right | 105 (55 %) | 161 (48 %) | |
| Left | 85 (45 %) | 173 (52 %) | |
| Lymphadenopathy | <0.0001 | ||
| No | 173 (91 %) | 223 (67 %) | |
| Yes | 17 (8.9 %) | 111 (33 %) | |
| Nodal template | |||
| Hilar | – | 40 (20 %) | |
| Other | – | 162 (80 %) | |
| Sarcomatoid | 8 (4.2 %) | 25 (7.5 %) | 0.14 |
| Procedure type | 0.9 | ||
| Open | 171 (90 %) | 301 (90 %) | |
| Laparoscopic | 19 (10 %) | 33 (9.9 %) | |
| Surgery type | <0.0001 | ||
| Radical | 166 (87 %) | 326 (98 %) | |
| Partial | 24 (13 %) | 8 (2.4 %) |
In total, 164 patients developed disease recurrence and there were 197 deaths from all causes. Median follow-up time was 5 and 5.5 years for patients who did not die or have a recurrence, respectively. A total of 334 (64 %) patients underwent LND, and node-positive disease was identified in 26 (8 %) patients.
Kaplan–Meier curves for recurrence-free survival and overall survival stratified by LND are presented in Figs. 1 and 2, respectively. Five-year recurrence-free survival rates were 64 and 77 %, respectively, for patients who did and did not undergo LND. Five-year overall survival rates were 75 % and 78 %, respectively.
Fig. 1.
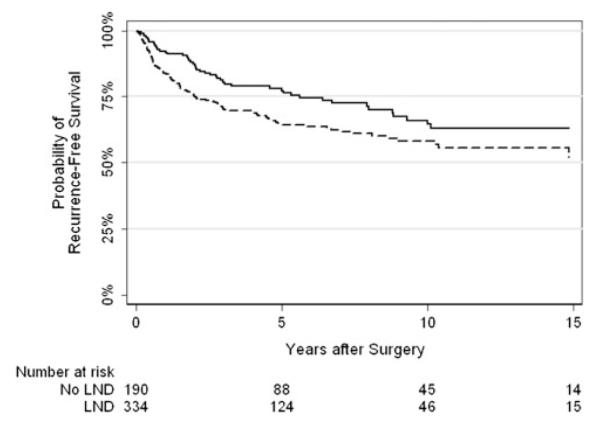
Kaplan–Meier curve for recurrence-free survival by lymph node dissection (solid line—no lymph node dissection, dashed—lymph node dissection)
Fig. 2.
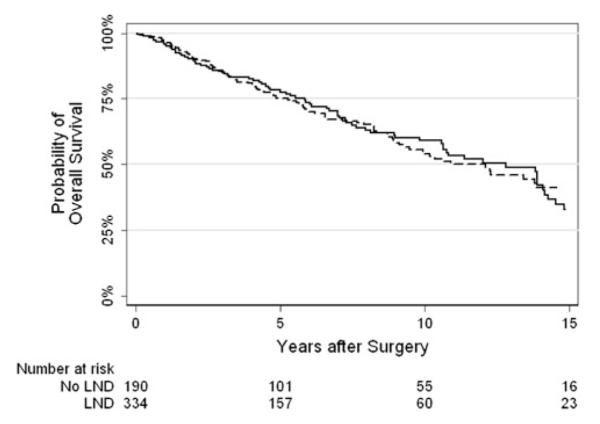
Kaplan–Meier curve for overall survival by lymph node dissection (solid line—no lymph node dissection, dashed—lymph node dissection)
Table 2 displays our Cox models for recurrence and survival rates. When controlling for pathological differences and surgical technique, LND was not associated with a significant difference in recurrence-free (p = 0.4) or overall survival (p = 0.3).
Table 2.
Multivariate Cox proportional hazards regression models for the outcomes of recurrence and survival
| Recurrence |
Survival |
|||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Lymphadenectomy | 1.16 (0.81, 1.66) | 0.4 | 0.84 (0.61, 1.15) | 0.3 |
| Pathological T stage | 0.013 | 0.024 | ||
| T2 | Ref. | – | Ref. | – |
| T3 | 1.72 (1.16, 2.55) | 0.007 | 1.56 (1.11, 2.19) | 0.010 |
| T4 | 2.45 (1.12, 5.37) | 0.025 | 1.99 (0.96, 4.10) | 0.063 |
| Histology | 0.015 | 0.24 | ||
| Clear cell | Ref. | – | Ref. | |
| Chromophobe | 0.42 (0.23, 0.76) | 0.004 | 0.67 (0.41, 1.08) | – |
| Papillary | 0.76 (0.38, 1.52) | 0.4 | 1.04 (0.59, 1.83) | – |
| Presentation | 0.16 | 0.003 | ||
| Incidental | Ref. | – | Ref. | – |
| Local | 1.12 (0.79, 1.58) | – | 0.93 (0.68, 1.29) | 0.7 |
| Systemic | 1.62 (0.99, 2.67) | – | 1.93 (1.26, 2.96) | 0.003 |
| ASA (III/IV vs. I/II) | 1.27 (0.91, 1.76) | 0.2 | 1.59 (1.17, 2.15) | 0.003 |
| Sarcomatoid | 2.46 (1.47, 4.11) | 0.001 | 2.77 (1.69, 4.56) | <0.0001 |
| Surgery type (partial vs. radical) | 1.00 (0.40, 2.53) | 0.9 | 0.42 (0.10, 1.74) | 0.2 |
| Lymphadenopathy | 1.35 (0.95, 1.91) | 0.090 | 1.19 (0.86, 1.66) | 0.3 |
| Tumor size (cm) | 1.04 (0.99, 1.10) | 0.14 | 1.02 (0.97, 1.07) | 0.4 |
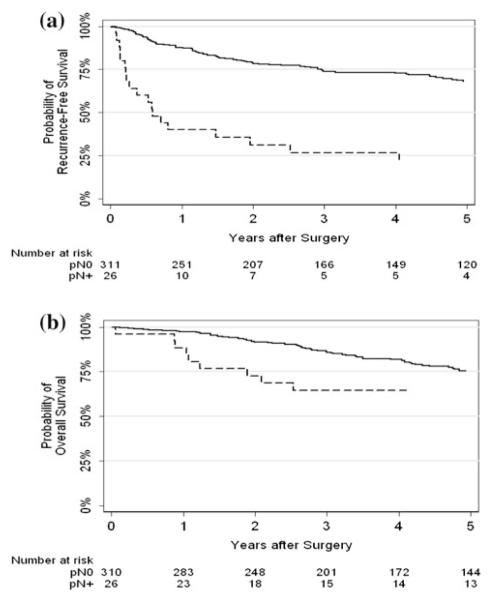
Kaplan–Meier curves for recurrence-free and overall survival stratified by lymph node status (positive vs. negative) are presented in Fig. 3. We found a statistically significant difference between lymph node status and recurrence-free survival (p < 0.0005). Five-year recurrence-free survival was 21 and 68 % for positive and negative nodes, respectively. We also found a statistically significant difference between lymph node status and overall survival (p = 0.032). Five-year overall survival was 65 and 75 % for positive and negative nodes, respectively.
Fig. 3.
a Recurrence-free survival by lymph node status; and b Overall survival by lymph node status (solid line—negative, dashed—positive)
We did not find a significant difference between number of nodes removed and recurrence (p = 0.3) or survival (p = 0.8). In addition, we did not find a significant difference between lymph node template and recurrence (p = 0.9) or survival (p = 0.5).
Discussion
The surgical management of RCC is unique among solid tumors. There is substantial evidence that resection of the primary tumor in metastatic disease and resection of synchronous or metachronous metastatic disease can provide a small but real survival benefit in selected patients [17]. Therefore, we would hypothesize that removing all regional lymph nodes at the time of nephrectomy would also provide a benefit if regional disease was present either grossly or microscopically.
This study was undertaken because of criticisms that EORTC 30881 involved a large proportion of low-stage or low-grade patients who were clinically node-negative [15,16, 18]. We therefore focused our analysis on patients with tumors ≥7 cm in size and included patients with lymphadenopathy. Although our rate of node-positive disease was double that of EORTC 30881 (8 vs. 4 %), we also found no association between LND and survival.
In fact, we found that 5-year disease-free and overall survival rates were lower in patients who underwent LND. This can likely be attributed to pathological differences between the two groups, as patients undergoing LND had larger, higher-stage tumors suggesting a surgical selection bias. When we controlled for pathological differences, we found no difference in recurrence-free or overall survival. However, whereas a randomized study design can limit confounding differences between groups, the current study is limited by such selection bias. Although we controlled for known pathological differences, there may be other unmeasured confounding variables that surgeons used to select patients for LND. Like the measured variables, unmeasured confounding variables would be likely to show worse disease in the LND group, which would underestimate the benefit of this procedure.
Our findings are in contrast to other retrospective series that have demonstrated a benefit of LND [12, 19]. Pantuck et al. [12] compared 129 patients with clinically node-positive disease who underwent nephrectomy. The 112 patients who underwent LND had approximately 5-month survival advantage over the 17 patients who did not undergo LND (p = 0.0002). Despite the impact on survival reported, a similar proportion of patients received immunotherapy and there was no difference in local or systemic recurrence rates between the two groups. The authors also found no difference in survival for patients with clinically node-negative disease and, therefore, recommended against LND in these patients. Canfield et al. subsequently argued that extended LND is an important staging procedure. In 40 patients with pathological node-positive disease that underwent extended LND, 17.5 % were clinically node-negative. Unfortunately, the authors did not report the denominator of patients that were clinically node-negative and underwent extended LND.
In the current series in which we selected only high-risk patients, 8 (3.5 %) patients without lymphadenopathy who underwent LND had node-positive disease. In contrast, of the 111 patients with lymphadenopathy who underwent LND, node-positive disease was identified in 18 (16 %). The proportion of patients with lymphadenopathy who were node-negative is remarkably lower than the 40 % previously reported by Studer et al. [20] and may reflect the absence of imaging re-review by radiologists in the current study or a clinical stage migration secondary to imaging performed for other reasons. These numbers are also likely inflated by the selection bias inherent to any retrospective study and may not reflect the true incidence of node-positive disease in this population.
Another argument supporting LND has been durable disease-free or overall survival of patients with node-positive, non-metastatic disease [10, 11]. It has been reported that 5–12 % of patients with positive lymph nodes have no other sites of metastasis and would theoretically benefit from LND [21]. In a multi-institutional study of 171 patients with node-positive disease, Karakiewicz et al. [10] reported a 39 % 5-year survival rate. Interestingly, in this population, stage, grade, tumor size and histology were not associated with cancer-specific mortality, but systemic symptoms at presentation were a harbinger of poor outcomes. In the MD Anderson experience, Delacroix et al. [11] reported the 5-year recurrence-free and overall survival of node-positive patients was 22 and 37 %, respectively. Neither of these studies reported outcomes of patients that did not undergo LND. In the current series, we found a 5-year recurrence-free and overall survival of 21 and 65 %, respectively. The vast improvement in overall survival compared to previous studies is promising and could be linked to the widespread use of targeted tyrosine kinase and mTOR inhibitor therapies.
Considering that we were unable to show an overall difference with or without LND, we were not surprised that there was no difference in the number of nodes removed or lymph node template. Two previous reports suggest that increasing number of nodes is associated with greater likelihood of finding positive nodes, [22, 23] but neither of these studies reported disease outcomes. On the other hand, a population-based study reported that increasing number of nodes removed was associated with worse cancer-specific survival on univariate, but not multivariate, analysis [24]. This paradoxical finding is similar to our results and can be explained by selection criteria for LND. There is also some evidence that the extended dissection should be favored due to the unpredictability of positive node landing sites [3], but we found no differences between hilar only dissection and more extensive dissections. Importantly, neither number of nodes or template of dissection has been evaluated in a prospective manner.
The current study highlights the difficulties in interpreting retrospective data for a procedure that is performed selectively without well-defined standards. Although we were unable to show a difference between LND and no LND, these results may underestimate the effect of LND performed in high-risk patients. Although the therapeutic impact of LND is not obvious based on this or other studies, the enhanced clinical staging provided by LND can be valuable for stratification in ongoing adjuvant trials of new systemic targeted therapies. Considering the limitations of the current literature, we suggest a well-designed, prospective study with a sufficient number of patients examining the role of LND in high-risk RCC.
Conclusion
We did not find a difference in recurrence-free or overall survival in patients with ≥7-cm tumors whether or not they underwent LND and regardless of the extent of LND. This should be considered in the context of the retrospective design of our study. Node-positive disease was associated with worse outcomes, suggesting that LND provides important staging information that can be important in the design of adjuvant clinical trials.
Acknowledgments
This investigation was supported by the Hanson Family Renal Cancer Research Fund, the Sidney Kimmel Center for Prostate and Urologic Cancers, and the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 CA082088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest The authors have no conflicts of interest.
Contributor Information
Michael A. Feuerstein, Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
Matthew Kent, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Wassim M. Bazzi, Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
Melanie Bernstein, Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Paul Russo, Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
References
- 1.Kim SP, Crispen PL, Thompson RH, et al. Assessment of the pathologic inclusion criteria from contemporary adjuvant clinical trials for predicting disease progression after nephrectomy for renal cell carcinoma. Cancer. 2012;15(118):4412–4420. doi: 10.1002/cncr.26695. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172:58–62. doi: 10.1097/01.ju.0000132126.85812.7d. [DOI] [PubMed] [Google Scholar]
- 3.Capitanio U, Becker F, Blute ML, et al. Lymph node dissection in renal cell carcinoma. Eur Urol. 2011;60:1212–1220. doi: 10.1016/j.eururo.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;1(20):4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 5.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37–42. doi: 10.1016/S0022-5347(17)64494-X. [DOI] [PubMed] [Google Scholar]
- 6.Godoy G, O’Malley RL, Taneja SS. Lymph node dissection during the surgical treatment of renal cancer in the modern era. Int Braz J Urol. 2008;34:132–142. doi: 10.1590/s1677-55382008000200002. [DOI] [PubMed] [Google Scholar]
- 7.Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol. 2001;165:62–64. doi: 10.1097/00005392-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Palapattu GS, Allaf ME, Trock BJ, Epstein JI, Walsh PC. Prostate specific antigen progression in men with lymph node metastases following radical prostatectomy: results of long-term followup. J Urol. 2004;172:1860–1864. doi: 10.1097/01.ju.0000139886.25848.4a. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WF., Jr Penile cancer: relation of extent of nodal metastasis to survival. J Urol. 1987;137:880–882. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Trinh QD, Bhojani N, et al. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease: prognostic indicators of disease-specific survival. Eur Urol. 2007;51:1616–1624. doi: 10.1016/j.eururo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Delacroix SE, Jr, Chapin BF, Chen JJ, et al. Can a durable disease-free survival be achieved with surgical resection in patients with pathological node positive renal cell carcinoma? J Urol. 2011;186:1236–1241. doi: 10.1016/j.juro.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 12.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol. 2003;169:2076–2083. doi: 10.1097/01.ju.0000066130.27119.1c. [DOI] [PubMed] [Google Scholar]
- 13.Schafhauser W, Ebert A, Brod J, Petsch S, Schrott KM. Lymph node involvement in renal cell carcinoma and survival chance by systematic lymphadenectomy. Anticancer Res. 1999;19:1573–1578. [PubMed] [Google Scholar]
- 14.Herrlinger A, Schrott KM, Schott G, Sigel A. What are the benefits of extended dissection of the regional renal lymph nodes in the therapy of renal cell carcinoma. J Urol. 1991;146:1224–1227. doi: 10.1016/s0022-5347(17)38052-7. [DOI] [PubMed] [Google Scholar]
- 15.Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European organization for research and treatment of cancer (EORTC) randomized phase three trial 30881. Eur Urol. 2009;55:28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Studer UE, Birkhauser FD. Lymphadenectomy combined with radical nephrectomy: to do or not to do? Eur Urol. 2009;55:35–37. doi: 10.1016/j.eururo.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 17.Karam JA, Wood CG. The role of surgery in advanced renal cell carcinoma: cytoreductive nephrectomy and metastasectomy. Hematol Oncol Clin North Am. 2011;25:753–764. doi: 10.1016/j.hoc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery JS, Leibovich BC. Lymph node excision for renal cancer. J Urol. 2013;189:419–421. doi: 10.1016/j.juro.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 19.Canfield SE, Kamat AM, Sanchez-Ortiz RF, Detry M, Swanson DA, Wood CG. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease (clinical stage TxN1-2M0): the impact of aggressive surgical resection on patient outcome. J Urol. 2006;175:864–869. doi: 10.1016/S0022-5347(05)00334-4. [DOI] [PubMed] [Google Scholar]
- 20.Studer UE, Scherz S, Scheidegger J, et al. Enlargement of regional lymph nodes in renal cell carcinoma is often not due to metastases. J Urol. 1990;144:243–245. doi: 10.1016/s0022-5347(17)39422-3. [DOI] [PubMed] [Google Scholar]
- 21.Capitanio U, Jeldres C, Patard JJ, et al. Stage-specific effect of nodal metastases on survival in patients with non-metastatic renal cell carcinoma. BJU Int. 2009;103:33–37. doi: 10.1111/j.1464-410X.2008.08014.x. [DOI] [PubMed] [Google Scholar]
- 22.Capitanio U, Suardi N, Matloob R, et al. Staging lymphadenectomy in renal cell carcinoma must be extended: a sensitivity curve analysis. BJU international. 2012 doi: 10.1111/j.1464-410X.2012.11313.x. [DOI] [PubMed] [Google Scholar]
- 23.Terrone C, Guercio S, De Luca S, et al. The number of lymph nodes examined and staging accuracy in renal cell carcinoma. BJU Int. 2003;91:37–40. doi: 10.1046/j.1464-410x.2003.04017.x. [DOI] [PubMed] [Google Scholar]
- 24.Joslyn SA, Sirintrapun SJ, Konety BR. Impact of lymphadenectomy and nodal burden in renal cell carcinoma: retrospective analysis of the National surveillance, epidemiology, and end results database. Urology. 2005;65:675–680. doi: 10.1016/j.urology.2004.10.068. [DOI] [PubMed] [Google Scholar]