Abstract
Introduction
Open repair for chronic aortic dissection remains a challenging surgical option. Different centers report diverse experiences and outcomes pertaining to thoracoabdominal aortic aneurysm repair (TAAAR) for chronic type B dissection. We highlight our center’s experience and results on a background of published literature and current evidence.
Methods
We reviewed 214 open TAAAR performed between October 1998 and February 2014. Of these, chronic type B dissection was present in 62 (29.0%) patients. We reviewed these patients in terms of demographics, operative characteristics and outcomes. Thirteen (21.0%) patients had surgery on the descending thoracic aorta [Category A =2 (3.2%), B =0 (0%), C =11 (17.7%)] and 49 (79.0%) in the thoracoabdominal thoracic aorta [Crawford extent I =5 (8.1%), extent II =39 (62.9%), extent III =4 (6.5%), extent IV =1 (1.6%)]. Left heart bypass was used in 12 (19.4%) patients.
Results
The composite in-hospital endpoint, adverse outcome—defined as operative death, renal failure necessitating dialysis at discharge, stroke, or permanent paraplegia or paraparesis—occurred after 28 (45.2%) procedures. There were 14 (22.6%) operative deaths. In-hospital mortality was seven (16.3%) out of 43 elective patients, and increased to seven (36.8%) of the 19 non-elective ones. Permanent paraplegia or paraparesis occurred after two (3.2%) cases, stroke occurred after seven (11.3%) and renal failure requiring dialysis occurred after 16 (25.8%). Mean follow-up time was 3.2 years and actuarial 5-year mortality was 27.4% [nine (14.5%) elective and eight (12.9%) non-elective patients].
Conclusions
TAAAR in chronic type B dissection carries a substantial risk of early adverse outcomes. The results could be well alleviated with cases directed towards specialized regional and supra-regional centers. Although the endovascular approaches offer relatively low mortality and morbidity, there is a lack of long-term data and guidelines on their use. There is a need for a multidisciplinary international registry on the management of thoracoabdominal aortic aneurysms and dissection. This would provide a degree of guidance on relevant clinical and surgical judgments and outcomes.
Keywords: Aneurysm, thoracoabdominal aneurysm, aorta, long-term survival, chronic type B dissection
Introduction
Open repair of thoracoabdominal aortic aneurysm repair (TAAAR) has evolved over the past two decades. Historically, open repair for chronic aortic dissection had a mortality of 27% in reported large series for elective procedures (1). The current reported mortality in specialized centers is around 10% (2-4). This improvement can be attributed to strategies which have revolutionized the delivery and performance of this procedure. They include left heart bypass, cardiopulmonary bypass with hypothermia, cerebral protection, improved understanding of thoracoabdominal aortic aneurysmal pathology, cerebral spinal drainage and spinal cord protection strategies such as reimplantation of intercostal arteries for adequate revascularization. However, open repair of chronic aortic dissection still presents many challenges. Neurological complications, severe post-operative morbidity and mortality, and incremental costs of open repair and service delivery provided an incentive for the evaluation of open surgical strategies and the ensuing for a better alternative that could standardize outcomes. Endovascular repair emerged as a promising option for TAAAR and chronic aortic dissection. However, the lack of long-term results and treatment failure rates as high as 40% have raised doubts about the superiority of this approach (5-7).
Methods
Patient population
We identified 214 consecutive patients who underwent TAAAR between 1998 and 2014 at our institution. Sixty-two (29.0%) had chronic type B dissection. All relevant clinical data was collected prospectively and entered into a local hospital database from which, periodically, core datasets were validated and submitted to The Society for Cardiothoracic Surgery (UK). In brief, a dataset was collected for each operation that included relevant demographics, indicators of disease severity, acuity, comorbidities, procedural details and all relevant in-hospital outcomes. Outcomes evaluated for the purposes of this study include neurological and renal complications, postoperative ventilation times, and in-hospital and follow-up mortality.
Study variables
Preoperative variables
Pre-operative variables included current smoker, diabetes, hypercholesterolemia, hypertension, cerebrovascular disease, respiratory disease, peripheral vascular disease, and renal failure. These are defined in Table 1. Patient pre-operative variables are shown in Table 2, and operative variables are shown in Table 3.
Table 1. Definition of patient preoperative study variables.
| Preoperative variable | Definition |
|---|---|
| Current smoker | Smoking within six weeks of the operation |
| Diabetes | Diagnosis of diet, tablet or insulin controlled diabetes |
| Hypercholesterolemia | Diagnosed with cholesterol over 5.0 mmol/L or on drug treatment |
| Hypertension | Diagnosed with hypertension (BP 139/89 mmHg) or on antihypertensive treatment |
| Cerebrovascular disease | The presence of carotid artery disease, chronic neurological injury, or a history of transient ischemic attack or cerebrovascular accident |
| Respiratory disease | Diagnosed with a respiratory disease (i.e., asthma, emphysema, bronchiectasis, chronic obstructive pulmonary disease), on treatment (i.e., inhalers) or impaired pulmonary function tests |
| Peripheral vascular disease | Diagnosed with peripheral vascular disease on the basis of symptoms (claudication), previous intervention, or evidence of stenotic disease |
| Renal disease | Definition has changed over time. Over the past 3 years, we have recorded estimated glomerular filtration rate (eGFR) as well as the presence of established renal failure and dialysis. An eGFR of 89 mL/min/1.73 m2 is considered as renal dysfunction (CKD2). Prior to this, we recorded renal dysfunction as a creatinine value greater than 200 mol/L and as established renal failure with dialysis |
Table 2. Preoperative characteristics of patients who underwent thoracoabdominal aortic surgery for chronic dissection.
| Demographics | Chronic dissections (n=62) |
|---|---|
| Age at operation (years) | 52.4±14.4 |
| Body mass index (kg/m2) | 26.5±5.2 |
| Female gender | 17 (27.4) |
| Angina class IV | 2 (3.2) |
| Myocardial infarction within the last 30 days | 0 (0) |
| Current smoker | 10 (16.1) |
| NYHA class ≥ III | 6 (9.7) |
| Diabetes | 0 (0) |
| Hypercholesterolemia | 19 (30.7) |
| Hypertension | 37 (59.7) |
| Respiratory disease | 5 (8.1) |
| Cerebrovascular disease | 1 (1.6) |
| Peripheral vascular disease | 3 (4.8) |
| Renal dysfunction | 6 (9.7) |
| Non elective | 19 (30.7) |
| Left ventricular ejection fraction <30% | 1 (1.6) |
Table 3. Operative details for all chronic dissection patients.
| Operative details | Chronic dissections (n=62) |
|---|---|
| Descending thoracic aorta | 13 (21.0) |
| DTA A | 2 (3.2) |
| DTA B | 0 (0) |
| DTA C | 11 (17.7) |
| Thoracoabdominal aorta | 49 (79.0) |
| TAAA I | 5 (8.1) |
| TAAA II | 39 (62.9) |
| TAAA III | 4 (6.5) |
| TAAA IV | 1 (1.6) |
| TAAA V | 0 (0) |
| Left heart bypass | 12 (19.4) |
TAAA, thoracoabdominal aortic aneurysm; DTA, descending thoracic aorta.
Urgency of intervention
In patients with aortic dissection, dissection was considered acute when surgery was performed within 14 days of onset, subacute when surgery was performed 15-60 days after onset, and chronic when surgery was performed more than 60 days after onset. The maximum TAAA diameter was based on measurements of all segments of the descending thoracic aorta and the abdominal aorta. The largest of these measurements was used as the maximum diameter.
Postoperative variables
Post-operative variables included intubation time, ITU stay, post-operative stay, acute renal failure, in-hospital mortality and follow-up mortality. The definitions are described in Table 4.
Table 4. Definition of patient post-operative study variables.
| Postoperative variable | Definition |
|---|---|
| Intubation time (hours) | Presence of endotracheal tube with supported respiratory effort |
| ITU stay (days) | Care within our Critical Care Area which includes 1:1 nursing care as “intensive care” or 2:1 nursing as “high dependency care” |
| Postoperative stay (days) | Number of days within the hospital setting from the day after surgery |
| Acute renal failure | Postoperative requirement for hemofiltration |
| In hospital mortality | Operative mortality that took place within our institution |
| Follow-up mortality | Patients were tracked using the national Demographics Batch Service (DBS) which is a mechanism that allows the UK National Health Service and other organizations to trace patient information, including dates of death, using an upload file that is linked to current information using patient identifiers (http://nww.hscic.gov.uk/demographics/dbs/index_html) |
ITU, intensive therapy unit.
Extent of aortic aneurysm repair
The extent of aortic repair was classified according to the system described by Crawford et al. (8). Extent I repairs were defined as repairs that involved most or all of the descending thoracic aorta and the upper abdominal aorta but not the infrarenal aorta. Extent II repairs involved most or the entire descending thoracic aorta and extended into the infrarenal abdominal aorta. Extent III repairs involved the distal half, or less, of the descending thoracic aorta (beginning below the 6th rib) and involved varying portions of the abdominal aorta. Extent IV repairs involved most or the entire abdominal aorta beginning at the diaphragm.
Results
The average age of the cohort was 52.4 years ±14.4, 17 (27.4%) patients were female, 10 (16.1%) were current smokers, five (8.1%) suffered from respiratory disease and one (1.6%) from cerebrovascular disease. There were no diabetic patients in the cohort. Nineteen (30.7%) patients presented as non-elective. Pre-operative variables are shown in Table 1. Thirteen (21%) patients suffered a chronic dissection in the descending thoracic aorta and 49 (79%) in the thoracoabdominal aorta. Twelve (19.4%) underwent left heart bypass. Operative variables are shown in Table 2.
Elective vs. non-elective
Pre-operative, operative and post-operative outcomes have been stratified by presenting priority as shown in Tables 1, 2 and 3. Due to low numbers in these groups, we cannot make any erroneous statistical inferences via the attendant P values. But we can, whilst bearing the low numbers in mind, note some potentially useful clinical trends. Non-elective patients tend to have a lower BMI than elective patients (24.5±4.7 vs. 27.8±5.7; P=0.052), and they are also considerably less likely to be a current smoker (5.3% vs. 20.9%; P=0.16) or have hypertension (47.4% vs. 65.1%; P=0.19). Non-elective patients are more likely to have a dissection located in the descending aortic segment (26.3% vs. 18.6%; P=0.51) and are less likely to undergo left heart bypass (15.8% vs. 20.9%; P=0.74).
Outcomes
Post-operative outcomes for the cohort of chronic dissection patients are shown in Table 5. Overall, the composite outcome of in-hospital death, post-operative renal failure, stroke or new paraplegia or paraparesis occurred in 28 (45.2%) patients. In-hospital mortality occurred in 14 (22.6%) of patients, of which seven (16.3%) were elective patients and the remaining seven (36.8%) non-elective. The most common post-operative complication was renal failure, which occurred in 16 (15.8%) patients. Stroke occurred in seven (11.3%) patients. Paraplegia or paraparesis had the lowest incidence within the composite outcome of two (3.2%) patients. Amongst other in-hospital outcomes, return to theatre for bleeding occurred in seven (11.3%) cases and prolonged ventilation (>48 hours) in 14 (22.6%) cases. The median days spent on ICU were six (IQR =4-21 days) and the median post-operative length of stay was 13.5 days (IQR =9-29 days). Post-operative outcomes are shown in Table 5. On average, non-elective patients’ length of stay was shorter than that of elective patients (11 vs. 14 days; P=0.19). This difference in hospital stay should be viewed in the context of the greatly increased post-operative risk of death at 30 days observed in the non-elective group (36.8% vs. 14.0%; P=0.09). However, when other co-morbidities are taken into account with the composite adverse outcome, priority has less of an effect (47.4% vs. 44.2%; P=0.82). Follow-up mortality figures also show non-elective patients being at increased risk, although post-discharge the mortality rate does not increase significantly in either group (1 year mortality: 42.1% vs. 16.3%; P=0.051. 3-year mortality: 42.1% vs. 20.9%; P=0.08. 5-year mortality: 42.1% vs. 20.9%; P=0.08). Kaplan-Meier charts for mid-term mortality are shown in Figures 1 and 2. Mean follow up time was 3.6 years and actuarial 5-year mortality occurred in 17 (27.4%) patients. Of these, nine (14.5%) were elective and eight (12.9%) non-elective. Using the log-rank test to assess the difference in follow-up mortality between elective and non-elective patients showed no significant difference (P=0.08). Again, caution should be taken with the interpretation of these results due to the low numbers.
Table 5. In-hospital and mid-term outcomes for all chronic dissection patients.
| Outcomes | Chronic dissections (n=62) |
|---|---|
| ITU LOS (days) | 6 [4, 21] |
| Post-operative LOS (days) | 13.5 [9, 29] |
| Reoperation for bleeding | 7 (11.3) |
| Prolonged ventilation (>48 hrs) | 14 (22.6) |
| Post-operative renal failure | 16 (25.8) |
| Stroke | 7 (11.3) |
| Paraplegia or paraparesis | 2 (3.2) |
| In-hospital mortality | 14 (22.6) |
| 30-day mortality | 13 (21.0) |
| Composite in-hospital adverse outcome | 28 (45.2) |
| 5-year mortality | 17 (27.4) |
| Elective patients | 9 (14.5) |
| Non-elective patients | 8 (12.9) |
ITU, intensive therapy unit; LOS, length of stay.
Figure 1.
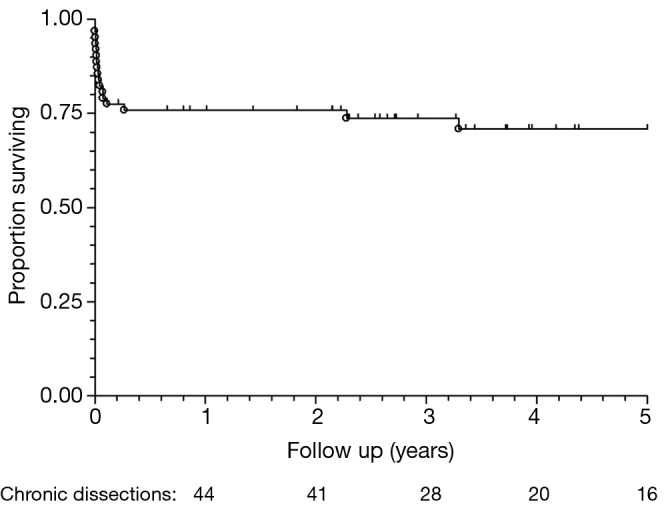
Kaplan-Meier survival chart for all chronic dissection patients (n=62).
Figure 2.
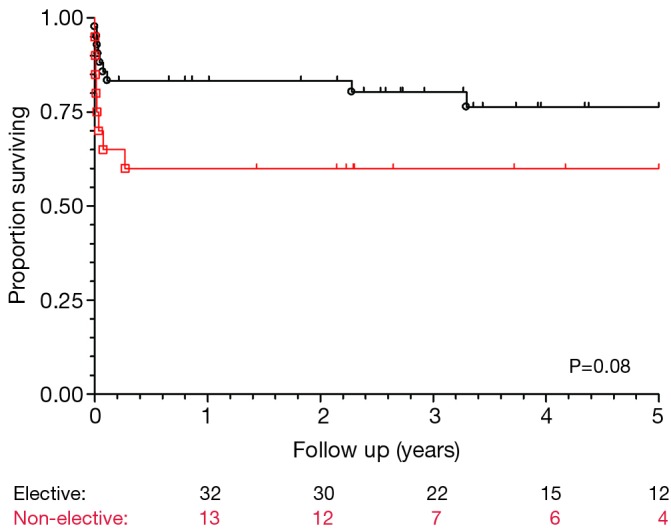
Kaplan-Meier survival chart for all chronic dissection patients, stratified by priority.
Discussion
Medical management of TAAAR for chronic type B dissection
Medical management plays a major role in patients following acute type B aortic dissection, as long as there is no evidence of malperfusion syndrome and/or the onset of intractable pain, which can necessitate urgent or emergency repair. The same applies to chronic distal dissection resulting either from conservatively treated type B dissection or after successful operations for type A dissection. However, some of the affected aortas do progress to aneurysmal dilatation (9-12). Control of hypertension and beta-blocker therapy are imperative for patients surviving acute dissection, but nevertheless a high incidence of enlargement during the follow-up period has been reported (12), with a growth rate in the thoracic aorta as great as 4 mm/year (13). There is a trend in the literature suggesting that it is best to adopt the ‘complication-specific approach. That is, reserving surgical replacement of the descending aorta for patients with rupture, organ ischemia, refractory pain, uncontrollable hypertension, sizable dilatation of the false lumen, or other life-threatening conditions. Even with this approach however, along with aggressive medical therapy and close surveillance with serial imaging, almost 20% of patients surviving the acute phase of type B dissection will develop fatal rupture (14). Approximately 25% of patients presenting with acute type B aortic dissection are complicated at admission by malperfusion syndrome or hemodynamic instability, resulting in a high risk of early death if untreated (15-17). According to the interdisciplinary expert consensus document on management of type B aortic dissection (18), outcome data on 1,529 patients with acute complicated type B aortic dissection submitted to open surgical repair was studied. The pooled early mortality rate was 17.5% and rates for early stroke and spinal cord ischemia after treatment were 5.9% and 3.3% respectively. Five-year survival rates ranged from 44% to 64.8%. Freedom from aortic events and reintervention ranged from 58.7% to 68% at 5 years. There is a need for similar consensus on the timing of intervention, rate of aneurysm growth, and size and method of intervention for chronic type B dissection among those patients that present following conservative management or a previous type A aortic dissection repair. On the other hand, there is strong evidence to suggest that endovascular intervention does not deliver a superior option to medical therapy. The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) Trial was the first prospective randomized study of elective stent graft placement in survivors of uncomplicated chronic type B aortic dissection. It demonstrated that thoracic stent graft placement failed to improve the rates of 2-year survival and adverse events when compared with optimal medical therapy. The trial included 140 patients in a stable condition at least two weeks after index dissection. They were randomly subjected to elective stent-graft placement in addition to medical therapy (n=72) or optimal medical therapy alone (n=68) with surveillance. However, a major concern regarding this trial is the fact that it was underpowered to evaluate the mortality end-point, as was acknowledged by the authors in their article. For the study to have adequate power, 28 events needed to be observed, but only 11 events were observed. Thus, the significance of the negative results of this study must be called into question. Extending the follow-up of these patients would potentially provide further time points to allow for a more meaningful analysis of the data (19,20).
Open vs. endovascular repair of TAAAR for chronic type B dissection
Chronic dissection presents a significant challenge and an overall increased risk of intra-operative and postoperative complications after distal aortic resection. The timing of intervention after dissection onset and complications is not uniformly understood. Patients assigned to medical treatment, TEVAR, or open surgery often significantly differ in baseline comorbidities and severity of the disease, making direct comparisons among treatment strategies difficult. LeMaire et al. (3) revealed independent predictors of adverse outcome included increasing age, pulmonary disease, cerebrovascular disease, and aneurysm rupture; extent IV TAAA repair was associated with a lower risk of adverse outcome. A report by Estrera et al. (21) of 182 patients who underwent repair of thoracic aortic aneurysms showed that almost 50% had chronic dissection; their hospital mortality was 8.8%. Techniques of TAAA repair have evolved from the original “clamp and sew” technique to modern perfusion-assisted techniques with varying degrees of hypothermia. Recently reported outcome rates range from 3.4% to 8.9% for 30-day mortality, 3.4% to 12.3% for in-hospital mortality, 1.5% to 5.8% for paraplegia, 3.7% to 6.3% for stroke, and 1.7% to 14.3% for renal failure (22-30). Open repair remains a durable option and the long-term survival is well-defined and acceptable. The Mount Sinai group published their results in TAAAR among the patients aged 60 years or younger to assess the value of conventional repair in younger patients. In their report, 107 of 294 TAAA operations were in patients [75 men (70%)] aged a mean of 48±9 years. The most common indication for operation was chronic dissection, in 60 (56%); five (4.7%) had acute dissection, and rupture was present in six (5.6%). Eleven percent had Marfan syndrome. Overall 30-day mortality was 4.7%. Stroke occurred in four patients (3.7%) and paraplegia in one (0.9%). Survival at 1, 5, and 8 years was 90.5%, 89.4% and 80.5%, respectively. They concluded that early mortality and neurologic complication rates are similar, if not superior, to endovascular repair for descending aortic and TAAAs. Open repair has proven durability and a very low rate of required re-intervention, in contrast with endovascular repair. Hence, open repair should be the modality of choice.
Yet, endovascular surgeons demanded a shift in the paradigm and requested a selective, safer surgical approach and strategies. This move was supported by service commissioners, leading to an increase in the threshold for considering endovascular intervention over open surgical repair. Moreover, some centers even advocated routine stenting of uncomplicated type B dissections. Subsequently, many of the published reports with endovascular interventions had varying proportions of patients who would not be considered appropriate candidates for open surgery. Consequently, it would seem logical that there should be better results expected after endovascular interventions than after open surgery. Some authors are now proposing endovascular stenting as a substitute for surgery in patients with distal chronic dissections who need intervention, asserting that it provides benefits of cost minimization, and reduced length of stay in intensive care and hospital overall that will counterbalance the eventual need for further surgeries in the future. Although this is frequently circulated in closed ended debates, there is not yet much robust evidence to support this and no provisional health economic evaluation to confirm the cost-benefit element. The expert consensus on the treatment of descending thoracic aortic disease using endovascular stent-grafts (31) suggested that stent graft treatment of patients with chronic aortic dissection does not reduce the risk of aortic rupture or increase life expectancy. Despite this, the aforementioned enthusiasm for endovascular stenting remains unaccounted for. This enthusiasm requires further evaluation and convention needs to be established for medium-long term outcomes, long-term survival, and the overall efficacy of surgical repair of chronic distal dissection. It is difficult to evaluate this using current literature due to the challenges of attaining comparable patient populations for the purpose of comparison. The St. George’s vascular group published (32) a systematic review on mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. In their 17 studies with a population of 567 patients, the results reported a technical success rate of 89.9% (range, 77.6-100%). Mid-term mortality was 9.2% (46/499) and survival ranged from 59.1% to 100% in studies with a median follow-up of 24 months. Endoleak, predominantly type I, developed in 8.1% of patients (25/309). Re-intervention rates ranged from 0% to 60% in studies with a median follow-up of 31 months. Aneurysms of the distal aorta or continued false lumen perfusion with aneurysmal dilatation occurred in 7.8% of patients (26/332). Rare complications included delayed retrograde type A dissection (0.67%), aorto-oesophageal fistula (0.22%) and neurological complications (paraplegia 2/447, 0.45%; stroke 7/475, 1.5%). They concluded on this basis that the absolute benefit of TEVAR over alternative treatments for chronic B-AD remains uncertain, due to lack of natural history data for medically treated cases, significant heterogeneity in case selection and absence of consensus in the reporting standards for intervention. They subsequently recommended the development of registries and clinical trials to address these challenges.
Zoli et al. (30) conceptualized on the rationale behind an aggressive endovascular approach and stated that its purpose of sealing the proximal entry point between true and false lumen will decrease blood flow into the false lumen, thus promoting thrombosis and stabilization of the downstream aorta. Unfortunately, endovascular stent grafting for type B dissection does not appear to deliver the expected results. Guangqi and colleagues (33) published their experience with 121 consecutive patients undergoing endovascular repair for acute and chronic type B dissection. In that series, postoperative endoleaks and 30-day mortality of 22% and 8.2%, respectively.
Conclusions
TAAAR continues to carry a substantial risk of early adverse outcomes. It is unlikely that surgeons will face better comorbidities and young population cohorts who undergo TAAAR. The results could be well alleviated with cases directed toward specialized regional and supra-regional centers. While endovascular approaches also promise immediate solutions, the long-term data is lacking and there is no convention on its use. There is a need for a multidisciplinary international registry on the management of thoracoabdominal aneurysms and dissection, which will provide meaningful information and guide clinical and surgical judgment and outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.DeBakey ME, McCollum CH, Crawford ES, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery 1982;92:1118-34 [PubMed] [Google Scholar]
- 2.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404 [DOI] [PubMed] [Google Scholar]
- 3.LeMaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:286-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehrenbacher JW, Corvera JS. Best surgical option for thoracoabdominal aneurysm repair - the open approach. Ann Cardiothorac Surg 2012;1:334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5 [DOI] [PubMed] [Google Scholar]
- 6.Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81 [DOI] [PubMed] [Google Scholar]
- 7.Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35 [DOI] [PubMed] [Google Scholar]
- 8.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404 [DOI] [PubMed] [Google Scholar]
- 9.Haverich A, Miller DC, Scott WC, et al. Acute and chronic aortic dissections--determinants of long-term outcome for operative survivors. Circulation 1985;72:II22-34 [PubMed] [Google Scholar]
- 10.Masuda Y, Yamada Z, Morooka N, et al. Prognosis of patients with medically treated aortic dissections. Circulation 1991;84:III7-13 [PubMed] [Google Scholar]
- 11.Glower DD, Fann JI, Speier RH, et al. Comparison of medical and surgical therapy for uncomplicated descending aortic dissection. Circulation 1990;82:IV39-46 [PubMed] [Google Scholar]
- 12.Genoni M, Paul M, Jenni R, et al. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg 2001;19:606-10 [DOI] [PubMed] [Google Scholar]
- 13.Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110:II256-61 [DOI] [PubMed] [Google Scholar]
- 14.Juvonen T, Ergin MA, Galla JD, et al. Risk factors for rupture of chronic type B dissections. J Thorac Cardiovasc Surg 1999;117:776-86 [DOI] [PubMed] [Google Scholar]
- 15.Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402 [DOI] [PubMed] [Google Scholar]
- 16.Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 2006;114:2226-31 [DOI] [PubMed] [Google Scholar]
- 17.Trimarchi S, Eagle KA, Nienaber CA, et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2010;122:1283-9 [DOI] [PubMed] [Google Scholar]
- 18.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78 [DOI] [PubMed] [Google Scholar]
- 19.Gysi J, Schaffner T, Mohacsi P, et al. Early and late outcome of operated and non-operated acute dissection of the descending aorta. Eur J Cardiothorac Surg 1997;11:1163-9; discussion 1169-70 [DOI] [PubMed] [Google Scholar]
- 20.Kwolek CJ, Watkins MT. The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial: the need for ongoing analysis. Circulation 2009;120:2513-4 [DOI] [PubMed] [Google Scholar]
- 21.Estrera AL, Rubenstein FS, Miller CC, 3rd, et al. Descending thoracic aortic aneurysm: surgical approach and treatment using the adjuncts cerebrospinal fluid drainage and distal aortic perfusion. Ann Thorac Surg 2001;72:481-6 [DOI] [PubMed] [Google Scholar]
- 22.Zoli S, Etz CD, Roder F, et al. Long-term survival after open repair of chronic distal aortic dissection. Ann Thorac Surg 2010;89:1458-66 [DOI] [PubMed] [Google Scholar]
- 23.Lemaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:286-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Luozzo G, Geisbüsch S, Lin HM, et al. Open repair of descending and thoracoabdominal aortic aneurysms and dissections in patients aged younger than 60 years: superior to endovascular repair? Ann Thorac Surg 2013;95:12-9; discussion 19 [DOI] [PubMed] [Google Scholar]
- 25.Bisdas T, Redwan A, Wilhelmi M, et al. Less-invasive perfusion techniques may improve outcome in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2010;140:1319-24 [DOI] [PubMed] [Google Scholar]
- 26.Keyhani K, Miller CC, 3rd, Estrera AL, et al. Analysis of motor and somatosensory evoked potentials during thoracic and thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:36-41 [DOI] [PubMed] [Google Scholar]
- 27.Lima B, Nowicki ER, Blackstone EH, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg 2012;143:945-952.e1. [DOI] [PubMed]
- 28.Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2011;141:953-60 [DOI] [PubMed] [Google Scholar]
- 29.Patel VI, Ergul E, Conrad MF, et al. Continued favorable results with open surgical repair of type IV thoracoabdominal aortic aneurysms. J Vasc Surg 2011;53:1492-8 [DOI] [PubMed] [Google Scholar]
- 30.Zoli S, Roder F, Etz CD, et al. Predicting the risk of paraplegia after thoracic and thoracoabdominal aneurysm repair. Ann Thorac Surg 2010;90:1237-44; discussion 1245 [DOI] [PubMed] [Google Scholar]
- 31.Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41 [DOI] [PubMed] [Google Scholar]
- 32.Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47 [DOI] [PubMed] [Google Scholar]
- 33.Guangqi C, Xiaoxi L, Wei C, et al. Endovascular repair of Stanford type B aortic dissection: early and mid-term outcomes of 121 cases. Eur J Vasc Endovasc Surg 2009;38:422-6 [DOI] [PubMed] [Google Scholar]


