Abstract
The 2013 Pennington Biomedical Research Center’s Scientific Symposium focused on the treatment and management of pediatric obesity and was designed to (i) review recent scientific advances in the prevention, clinical treatment and management of pediatric obesity, (ii) integrate the latest published and unpublished findings and (iii) explore how these advances can be integrated into clinical and public health approaches. The symposium provided an overview of important new advances in the field, which led to several recommendations for incorporating the scientific evidence into practice. The science presented covered a range of topics related to pediatric obesity, including the role of genetic differences, epigenetic events influenced by in utero development, pre-pregnancy maternal obesity status, maternal nutrition and maternal weight gain on developmental programming of adiposity in offspring. Finally, the relative merits of a range of various behavioral approaches targeted at pediatric obesity were covered, together with the specific roles of pharmacotherapy and bariatric surgery in pediatric populations. In summary, pediatric obesity is a very challenging problem that is unprecedented in evolutionary terms; one which has the capacity to negate many of the health benefits that have contributed to the increased longevity observed in the developed world.
Keywords: prevention, treatment, children, adolescents, overweight, therapy
INTRODUCTION
Pediatric obesity is a major public health problem in developed countries and is also becoming an issue in many developing nations.1 In addition to being at increased risk of remaining obese as an adult,2 obese children have lower health-related quality of life,3,4 an increased risk of having metabolic disorders and adverse cardiovascular disease risk factors,5,6 and a greater chance of psychological and social problems such as being the victim or perpetrator of bullying.7,8 The economic and health-care costs associated with pediatric obesity are high and are predicted to rise in the United States unless the prevalence can be reduced through effective prevention and treatment.9,10
Pediatric obesity is rarely due to single-gene defects transmitted from parents to offspring or arising de novo. It develops in the presence of complex and generally subtle biological predispositions that become manifest under environmental and social conditions where obesogenic behaviors are adopted. Thus, understanding the additive and multiplicative interactions between obesogenic behaviors and biology on the modulation of adiposity and the risk of pediatric obesity is an urgent public health priority. Consequently, pediatric obesity prevention and treatment can span the continuum from modifications to the policy and built environment, to behavioral and pharmacological interventions and surgical approaches.
On 27–29 October 2013, the Pennington Biomedical Research Center convened a scientific symposium on the scientific basis for the prevention, management and treatment of childhood obesity. The specific objectives of the symposium were to (i) review recent scientific advances in the prevention, clinical treatment and management of pediatric obesity, (ii) integrate the latest published and unpublished findings and (iii) explore how these advances can be integrated into clinical and public health approaches. The scientific program was developed to review both preclinical and clinical data on the genetic, epigenetic and behavioral influences on pediatric obesity such as those observed during gestation and early childhood development. In addition, the relative merits of various behavioral approaches in the treatment of pediatric obesity, and the specific roles of pharmacotherapy and bariatric surgery in pediatric populations were discussed. The purpose of this article is to summarize the evidence discussed and provide recommendations for implementing evidence-based solutions in clinical and public health settings.
EPIDEMIOLOGY OF PEDIATRIC OBESITY AND SEVERE OBESITY
Most population health surveillance systems rely on the body mass index (BMI; kg m−2) as an indicator of the presence of overweight or obesity in both adults and children. Among children and adolescents, cutoff criteria are typically based on national or international pediatric reference data. For example, the World Health Organization has published growth standards for infants and children from birth to 5 years of age11 and growth reference curves for BMI for children 5–19 years of age.12 Similarly, using an international sample of children and adolescents, the International Obesity Task Force produced BMI growth curves using least median of squares (LMS) regression set to adult overweight (25 kg m−2) and obesity thresholds (30 kg m−2) at 18 years of age.13 These reference curves were recently updated to include thresholds for underweight.14 In the United States, the most commonly used reference data are the Centers for Disease Control and Prevention (CDC) BMI growth charts,15 and children and adolescents are considered overweight if their BMI is ≥85th percentile and obese if their BMI is ≥95th percentile. In addition, more severe or extreme levels of pediatric obesity have been defined using the 97th and 99th percentiles, or 120% of the 95th percentile.16–18
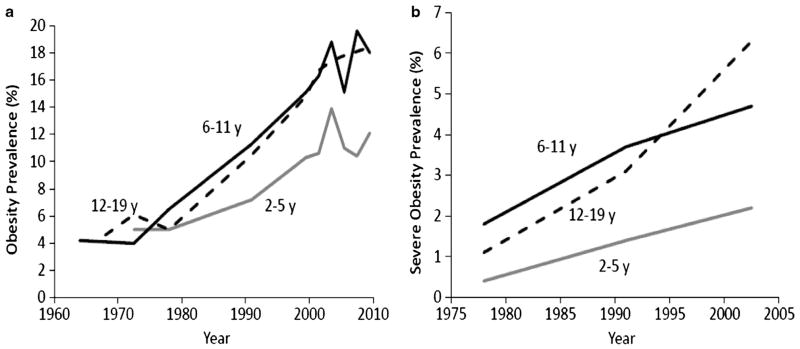
The global prevalence of pediatric obesity increased considerably in recent decades and remains high1,19 In the United States, the prevalence of obesity among children and adolescents increased from 5% in 1971–1974 to 16.9% in 2009–2010.16,20 The prevalence of obesity has increased in all age groups, but the rise in 2- to 5-year old has not been as great as in older children and adolescents (Figure 1a). Despite the rapid and significant increase in pediatric obesity since the 1970s, there is some evidence that the prevalence may be stabilizing in some countries. For example, an analysis of data collected between 1995 and 2008 from nine countries (Australia, China, England, France, The Netherlands, New Zealand, Sweden, Switzerland and the United States) demonstrated that the rate of change in obesity over that time frame was −0.01%.21
Figure 1.
Temporal trends in (a) obesity (BMI ≥95th percentile) and (b) severe obesity (BMI ≥120% of 95th percentile or ≥35 kg m−2) among 2–5, 6–11 and 12–19 years old in the United States. Results in panel a are from Ogden and Carroll20 and Ogden et al.16 Results in panel b are from Wang et al.18
In addition to the observed increases in overall obesity prevalence, more severe forms of pediatric obesity have also increased, possibly at a greater rate. For example, between 1971–1974 and 2009–2010, the prevalence of pediatric obesity increased 3.3-fold;16,20 whereas for severe pediatric obesity this rose 4-fold between 1976–1980 and 1999–2006.18 Similar to the trends in overall obesity, severe obesity has increased at a slower rate among 2- to 5-year-old children compared with older children and adolescents (Figure 1b). Conversely, comparable to reported trends of a plateauing of overall pediatric obesity prevalence, a recent study has indicated that extreme obesity in pre-school children increased between 1998 and 2003 but then declined from 2003 to 2010.22
In summary, the epidemiological evidence indicates that pediatric obesity and severe obesity has increased substantially in recent decades. Although there is some evidence that the increases may be slowing in recent years, the prevalence remains historically high.
THE HUMAN GENOME: ONE OF THE DRIVERS OF PEDIATRIC OBESITY
Two decades of research on the nature of the conditions that led to the obesity epidemic have allowed us to conclude that there are multiple social, environmental, behavioral and biological determinants of what has been termed ′a constellation of obesogenic conditions.’ These determinants exhibit not only statistically significant main effects but also interactive and multiplicative influences on the risk of gaining weight and becoming obese. Although there are a few exceptions, an important property of most of these determinants is that, when taken individually, they generally are characterized by small effect sizes.
The genetic component of obesity arises mainly as a function of DNA sequence differences and other genomic features such as chromosomal anomalies. Furthermore, although epigenomic mechanisms are of great interest, they do not contribute to the heritability of adiposity or obesity except when the epigenomic signature at a given chromatin site persists across generations.23 Major defects in single genes (generally homozygotes for recessive alleles) in syndromic and nonsyndromic cases account for >5% of severe obesity cases with an early age of onset.24–26 Thus far, at least 10 genes have been causally implicated in congenital generalized lipodystrophies and familial partial lipodystrophies.27 These single-gene cases have helped delineate some of the biological mechanisms contributing to positive energy balance and the risk of obesity.
There is also a genetic component to the common form of pediatric obesity; however, the exact magnitude of the heritability levels of traits such as BMI, adiposity or risk of obesity remains a matter of debate. What is particularly important for the risk of pediatric obesity is the narrow-sense heritability, that is, the proportion of the trait variance that can be accounted for by additive genetic effects. In humans, this is a difficult parameter to quantify. The simplest approach is to derive the heritability coefficient from the regression of offspring on their parent’s phenotype.28 Heritability levels derived from this approach are markedly lower than those obtained by other methods, particularly those based on the comparison of monozygotic and dizygotic twins raised together or apart. The latter coefficients typically include not only additive effects, but also dominance, gene–gene interaction and gene–environment effects, and such heritability coefficients are referred to as broad-sense heritability. The difference between narrow- and broad-sense coefficients can be substantial, and this has considerable implications for our understanding of obesity genotypes.
Based on parent and offspring data, it is not clear yet whether the familial risk of obesity, as assessed by the number of parents who are obese and the severity of their obesity, is stronger during infancy and early childhood as opposed to adolescence and early adulthood. What is clear is that the offspring birth weight is influenced by both maternal and paternal birth weight, as evidenced by a large Norwegian study of >67 000 mother–father–child trios, in which the mother birth weight–baby birth weight correlation (r = 0.23) was higher than the father birth weight–baby birth weight correlation (r = 0.13).29 It is also increasingly recognized on the basis of relatively large cohort data that the mother–child correlation for BMI is slightly higher than the father–child coefficient, although the difference is small.30
The search for the DNA variants that explain human variation in adiposity and the risk of obesity is ongoing. Thus far, about 60 single-nucleotide polymorphisms (SNPs) have been found to be associated with obesity traits at a genome-wide significance level (P ≤5 × 10−8).31,32 Three observations can be made based on these early results. First, the SNPs shown to be associated with obesity traits in adults are generally replicated in children and adolescents. Second, the new SNPs that are progressively being discovered have small effect sizes, and it takes an increasingly large sample size to be able to uncover them. Third, the fraction of the heritability accounted for by the aggregate of these SNPs remains small when conventional models of analysis are used. However, the missing heritability may not be as large as currently estimated if the more accurate approach of using narrow-sense heritability as the denominator instead of the broad-sense coefficient is used.33,34 Moreover, the genetic variance accounted for by SNPs may in fact be much larger when the genome-wide association data are analyzed under a different set of assumptions.35,36
Little has been reported on gene–gene interactions and their impact on the risk of human obesity. However, research on model organisms, such as yeast, strongly suggests that such interactions are likely having a significant role in trait variance.37 In contrast, there is a growing body of evidence on gene–behavior interactions and their influences on adiposity and risk of obesity. Controlled intervention studies with pairs of monozygotic twins have strongly suggested that there are powerful gene–overfeeding38 and gene–caloric restriction39 interaction effects. In a 2010 study, Li et al.40 estimated that a physically active lifestyle was associated with a 40% reduction in the genetic predisposition to obesity, as estimated by the number of risk alleles at 12 genome-wide association study-identified SNPs. In a very large study of the same question (N = 218 166 adults), the true reduction in risk was estimated to be on the order of 27%.41 Studies on gene–nutrient interactions have also been reported.42 To date, most studies on gene–behavior interaction effects have been based on observational cross-sectional or longitudinal data. These designs allow for large sample sizes, which is a fundamental condition for the detection of gene–behavior interaction effects. However, it would be more productive in the future to favor intervention studies in which the targeted behavior is systematically manipulated in order to remove the effects of confounders as much as possible and to reveal to what extent DNA sequence differences modulate the response to a change in behavior.
Although it may appear that little progress is being made on our understanding of the genetics of pediatric obesity, foundations are being laid for solid progress to be achieved in the coming decade.
ADIPOSE TISSUE DEVELOPMENT AND EPIGENETIC EFFECTS ON PEDIATRIC OBESITY
Adipose tissue is a primary target in our understanding of pediatric obesity. It undergoes pronounced developmental changes during fetal, neonatal and postnatal life that have the potential to determine an individual’s lifetime adiposity and susceptibility to obesity.43 Fat cell number increases with obesity from the earliest stage of infancy at which such measurements can be made,44 persisting into adulthood.45 The important impact of accelerated growth in early life is exemplified by the recent finding that in some rare genetic mutations that result in obesity, the metabolic phenotype is only apparent in early life (for example, for kinase suppressor of Ras 2).46
It is now recognized that there are three types of adipose tissue: brown adipose tissue (BAT), beige (or brown in white) and white, each having different molecular markers that can be depot specific.47 BAT is the least abundant fat in the body but is characterized as possessing the unique uncoupling protein 1, which has the capacity to generate 300 times more heat than any other tissue.48 This occurs as a result of the free flow of protons across the inner mitochondrial membrane without the need for adenosine triphosphate synthesis, resulting in the rapid dissipation of chemical energy as heat.49 Adipose tissue is one of the last tissues to appear in the fetus and BAT in particular has the essential feature of enabling the newborn to effectively adapt to cold exposure in the extrauterine environment.43 A major factor determining adipose tissue function at birth is maturation of the hypothalamic–pituitary axis, which in small mammals only matures after birth,50 with the result that thermogenesis in BAT does not commence until at least 1 week after birth.51
Significant depots of BAT are present both around central organs such as the kidney and heart and also in the neck or supraclavicular region,52 for which the latter region is the major depot. This is a primary site of thermogenesis that in childhood is influenced by BMI and ethnicity.53 The extent to which these BAT depots are replaced by white adipose tissue or are transformed to a mix of beige and white adipocytes remains a current focus of academic debate.54 These processes can be manipulated by environmental challenges to the fetus and/or neonate, which offer the potential to promote BAT function in the newborn as well as into later life.55 The potential influence of maternal body weight and glycemic status on BAT remains to be described,56 as does the substantial change in dietary and activity patterns over the past two decades, coincident with the obesity epidemic.57
To date, the main processes in adipose tissue considered to be most strongly influenced by epigenetics relate to the actions of microRNAs58 and long noncoding RNAs in the regulation of adipogenesis.59 Fat depots have their own specific gene expression profiles60 as well as developmental growth trajectories,61 which currently do not appear to be driven by epigenetic changes.62 Neither examining methylation profiles in blood nor looking at fractional changes in methylation of a gene provides many insights into obesity and related disease processes.63,64 It is also apparent that the impact of pediatric obesity is strongly influenced by gender,65 with evidence that females are apparently being protected from the adverse effects of excess adiposity on a range of metabolic processes including inflammatory responses within the kidney.66
Advances in our ability to understand tissue- or depot-specific roles of fat together with its impact on whole body energy regulation67 will be critical in developing effective early life strategies designed to prevent obesity. To this end, a technique of thermal imaging has been developed to be able to quantify potential changes in BAT activity throughout the life cycle in free-living subjects.68 We are now beginning to further explore the impact of other factors on BAT function including diet and genes.69 There is now a new opportunity to both quantify and manipulate BAT development in early life in order to not only promote survival of the newborn but also to potentially prevent excess adiposity in later life.70
THE INTRAUTERINE ENVIRONMENT AND PEDIATRIC OBESITY
Based on the concept of developmental programming, that is, that environmental events can reset physiological development of the embryo and fetus, pre-pregnancy overweight and obesity in women of reproductive age is a significant risk factor for pediatric obesity. In the United States, 32% of reproductive age women are obese (BMI ≥30 kg m−2) and 56% are overweight or obese (BMI ≥25 kg m−2).71 In overweight and obese women, between one-half to two-thirds have excessive weight gain during pregnancy, which is a significant risk factor for post-partum weight retention and increased risk of obesity in a subsequent pregnancy.72 Concurrently, there has also been an increase in pediatric obesity with 12.3% of children and adolescents from 2 to 19 years of age having a BMI ≥97th percentile.16 An increase in weight has been observed even at birth, with a mean 114 g increase in birth weight from 1975 through 2005 in one US city.73 The potential impact of this increase in birth weight on body composition is unknown but greater fetal adiposity at birth has a significant positive correlation with childhood obesity.74
At birth the human neonate has between 12 and 18% body fat depending on the methodology used to estimate body composition, and as detailed above, this will compromise a combination of BAT, beige and white adipose tissue.47 Fetal fat mass represents almost 50% of the variance in birth weight at term.75 There are multiple factors associated with neonatal adiposity, with higher pre-pregnancy BMI and gestational weight gain being the most frequently cited.72 Maternal pre-pregnancy BMI is the stronger correlate in overweight and obese women, whereas excessive gestational weight gain accounts for more of the variance in normal weight women.76 Both higher maternal glucose and lipids have also been associated with fetal/neonatal adiposity.77
Nutrient availability for feto-placental growth is a function of the metabolic alterations that occur during pregnancy. There is a 50–60% decrease in maternal insulin sensitivity during pregnancy primarily affecting maternal carbohydrate and lipid metabolism.78 Both nutrients have a significant correlation with fetal growth and adiposity.79 This decrease in insulin sensitivity appears to be related to increased inflammation affecting the post-receptor signaling pathways in skeletal muscle and adipose tissue.80 The meta-inflammation of pregnancy is related to cytokines in both maternal adipose tissue and placenta.81
There is evidence for changes in gene expression in placenta and maternal adipose tissue early in the first trimester of pregnancy82 before any significant physiological adaptations. This may account, in part, for the relative lack of success of lifestyle interventions during later pregnancy designed to prevent excess maternal weight gain, as well as for clinical observations that maternal pre-pregnancy BMI remains a strong correlate of fetal growth and adiposity. Therefore, planning of pregnancy, including prior optimization of maternal weight and metabolic condition, offers a safe means to initiate the prevention rather than treatment of pediatric obesity.
MATERNAL NUTRITION AND DEVELOPMENTAL PROGRAMMING OF OBESITY IN OFFSPRING
The ‘developmental origins of health and disease’ hypothesis has highlighted the link between the periconceptual, fetal and early infant phases of life and the subsequent development of adult obesity and related metabolic disorders. One interpretation of this relationship is that the fetus makes ‘predictive adaptations’ in response to intrauterine cues, resulting in permanent adjustments in homeostatic systems to aid immediate survival and improve success in an adverse postnatal environment. However, inappropriate interpretations of prenatal cues or changes to that immediate environment may result in a mismatch between ‘prenatal predictions’ and later life experiences. As a result, these adaptations, known as predictive adaptive responses,83,84 may ultimately be disadvantageous in postnatal life, leading to an increased risk of chronic diseases in adulthood and potentially a cycle of disease transmission across generations. Whether such a concept is applicable to a fetus that grows with an excess intrauterine environment and is then similarly exposed to plentiful nutrition postnatally remains to be clarified.
Using this type of conceptual framework, it is not surprising that at both extremes of the maternal nutritional spectrum, similar phenotypic outcomes are observed in offspring. Both maternal under- and overnutrition lead to increased adiposity and related metabolic disorders in offspring, which in animal models, are exacerbated in the presence of a postnatal high-fat diet. A number of animal studies have now shown that the effects of developmental programming on metabolic disorders may also manifest in future generations via either the paternal or maternal line without further suboptimal environmental exposures.85 It has also been shown that, at least in experimental models with rodents, developmental programming of metabolic disorders in offspring is potentially reversible by nutritional or targeted therapeutic interventions during critical periods of developmental plasticity. For example, neonatal administration of the glucagon-like peptide 1 analog Exendin-4 can ameliorate the consequences of fetal growth restriction induced by uterine artery ligation, whereas pre-weaning administration of either leptin or growth hormone can reverse some of the metabolic abnormalities in offspring associated with reduced birth weight induced by more severe maternal undernutrition.86–88
There is also an increasing interest in the role of epigenetics in developmental programming of obesity and related metabolic disorders. For example, Exendin-4 has been shown to increase histone acetylase activity and reverse epigenetic modifications that silence Pdx1 in the pancreas of growth-restricted rats.89 It has also been shown that the effects of neonatal leptin treatment on gene expression and methylation status of some hepatic genes in adulthood are directionally dependent on the animal’s nutritional status in utero. There are also data from rodents suggesting that methyl donors including folic acid, glycine and choline may have beneficial effects in offspring following imbalanced maternal nutrition, particularly as it relates to cardiovascular outcomes.90–93
In the setting of maternal obesity, dietary intervention and exercise have both been reported to have beneficial effects on offspring metabolic outcomes in animal studies in which fetal overgrowth does not occur.94,95 However, leptin administration to rodent offspring of mothers fed a standard rodent laboratory diet may elicit an adverse phenotype in males.96 Translation of the preclinical findings to the clinical setting remains a clear challenge, as whether comparable effects are seen in large-for-date offspring and/or species born after a long gestation with a mature hypothalamic–pituitary axis has yet to be established.
MATERNAL WEIGHT GAIN AND PEDIATRIC OBESITY
The amount of weight gained during pregnancy is highly variable among women, with the majority of the variance, especially the increase in weight gain, being accounted for by increases in fat mass.97 Globally, it was the issue of undernutrition that spurred the first international guidelines for weight gain in pregnant women in 1990. However, with the increased prevalence of obesity in reproductive aged women and the observation that many women were exceeding the previous Institute of Medicine (IOM) recommendations, the IOM reconvened and revised the guidelines in 2009.72 It is recommended that to prevent adverse maternal and infant outcomes, normal weight women limit total weight gain in pregnancy to 11.5–16 kg (25–35 lbs), overweight women to 7–11.5 kg (15–25 lbs) and obese women (all classes) to 5–9 kg (11–20 lbs).98
Weight gain in pregnancy is the result of accrual of water, protein (fat-free mass) and fat in the fetus, placenta, uterus, blood, amniotic fluid, mammary gland and maternal adipose tissue. The majority of the weight gained in excess of the IOM guidelines is deposited in maternal adipose tissue; however, limited data are available on changes in adipose tissue distribution. These studies show that in women with a normal pregravid BMI, the majority of the adipose tissue is deposited in the subcutaneous depots of the hips and thighs,99,100 but some visceral fat is accumulated in late pregnancy.101 However, obese women, who have more subcutaneous fat before conception, tend to accumulate more visceral fat.99 Understanding how fat is deposited in maternal tissues during pregnancy is important because the metabolic and inflammatory characteristics of fat stored in different depots may contribute to undesirable metabolic outcomes in pregnancy (for example, gestational diabetes, dyslipidemia and hypertension) and risk for a more rapid onset of obesity-related diseases (for example, type 2 diabetes).
As is the case with non-pregnant women, the main determinants of weight gain in pregnant women are related to energy balance, with energy intake, energy metabolism and physical activity probably all having roles. Not surprisingly, irrespective of maternal BMI, energy intake was ~ 900 kcal day−1 higher in women exceeding the IOM recommendations compared with women with appropriate weight gain.102 The role of specific macronutrient intake has not been widely studied, while the change in metabolic rate through pregnancy is proportional to the changes in body composition indicating that a resetting of energetic efficiency does not explain excess gestational weight gain.102
Average weight gain in pregnancy, across all BMI categories, has increased over the last four decades from 10 to 15 kg (22–33 lbs).103 The most recent report from the CDC shows that >48% of all women exceed the 2009 IOM guidelines for appropriate weight gain during pregnancy.98 Excess gestational weight gain in the 2011 report was 38% for normal weight women and was 1.5 times higher in overweight and obese women at 59% and 56%, respectively. The prevalence of excess gestational weight gain within pregravid BMI categories has not shifted in the past decade, however, the number of women entering pregnancy as either overweight or obese has increased significantly from 30% in 1983 to 54% in 2011. As overweight and obese women are more likely to exceed the IOM guidelines, the prevalence of excess gestational weight gain can be forecast to continue to rise.
Excess weight gain during pregnancy is associated with a number of complications, including preterm birth,104 infants born large for gestational age (birth weight ≥90th percentile)105 and childhood obesity.106,107 Although pregravid obesity is the strongest predictor of obesity and metabolic dysfunction in children,74 weight gain during gestation is emerging as another critical predictor of childhood obesity. For example, data from the Southampton Women’s Study show that children born to women with excess gestational weight gain irrespective of BMI, had greater adiposity, but within the normal range, at birth and 4–6 years of age.108 Historical studies shown that adult offspring born to mothers with excess gestational weight gain have a higher percent body fat and waist circumference,109–112 but this has yet to be related to increased metabolic disease. Possible mechanisms linking excess gestational weight gain and large for gestational age offspring include gestational diabetes, and/or, metabolic adaptations to excess maternal adiposity that is reflected in raised pregravid BMI and maternal plasma leptin concentrations.111
Given the available evidence, it is important to manage maternal obesity and optimize gestational weight gain by intervening before and during pregnancy, especially as two-thirds of women have either inadequate or excessive weight gain in pregnancy. This is clearly a significant challenge as intervention studies have yet to yield consistent positive effects on gestational weight gain and in turn improved maternal and infant outcomes including a reduced prevalence of pediatric obesity.
BREASTFEEDING AND PEDIATRIC OBESITY
The American Academy of Pediatrics recommends exclusive breastfeeding for all infants for approximately 6 months, followed by the introduction of complementary foods while continuing to breastfeed until at least 1 year of age.113 This recommendation is due to the many benefits of breastfeeding, that could include the prevention of pediatric obesity, as a World Health Organization meta-analysis of 33 studies examining breastfeeding and pediatric obesity114 indicates children who were breastfed were less likely to be overweight or obese as compared with those who never breastfed (pooled odds ratio of 0.78; 95% confidence interval: 0.72–0.84). This conclusion was confirmed by a more recent meta-analysis of 10 studies comparing breast- and formula-fed infants.115
There are limitations to these epidemiological studies; most notably the problem of residual confounding, as breastfeeding is also related to other factors that influence child’s weight, such as maternal BMI, education, income and smoking during pregnancy. There are several studies that have attempted to control for these variables and breastfeeding usually remains protective against pediatric obesity. Li et al.116 examined the interaction between maternal obesity and duration of breastfeeding and weight at ages 2–14 years, although this comparison can be influenced by the reduced incidence of breastfeeding in women with a high BMI. Thirty-one percent of the children who never breastfed and had mothers with a BMI ≥30 kg m−2 were overweight, compared with 6% of those children who were breastfed for at least 4 months by mothers with a BMI <25 kg m−2. Bogen et al.117 reported a significantly lower odds ratio of 0.55 for those children who breastfed for >26 weeks, without supplemental formula, compared with those children never breastfed, even when adjusting for maternal BMI, age, education, parity, marital status, gender, birth weight and delivery method.
There are several hypotheses as to why there is a protective effect of breastfeeding on pediatric obesity. First, the composition of human milk and infant formula vary greatly. Human milk contains over 100 bioactive components and many, such as adiponectin, leptin and ghrelin, have effects on appetite regulation, metabolism and energy balance.118 In addition, the protein concentration of infant formula is higher than breast milk.119 This may contribute to a more rapid weight gain in the first year of life but whether this is associated with greater adiposity is yet to be determined.
Another reason for the increased risk of obesity among formula-fed infants may be due to the mode of delivery of the milk. For example, it has been postulated that receiving formula from a bottle may result in overfeeding and may diminish the infant’s ability to self-regulate energy intake.120 However, there has been a steady increase in the use of breast pumps and feeding expressed breast milk in a bottle to the infant. Li et al.121 analyzed data from the Infant Feeding Practices Study II and reported that for a small number of infants (that is, 13 out of a total study group of 1899) receiving breast milk only from the bottle gained 89 g more per month during the first year of life compared with infants fed only at the breast. Fifty-eight percent of mothers reported that their infants always or most of the time emptied the bottle of formula or breast milk.122 Infants who emptied bottles in early infancy were 69% more likely to have excess weight (weight for age >1 z-score) after 6 months of age, but whether fat mass is affected in the long term is not known.
In conclusion, breastfeeding may be a preventive factor against pediatric obesity, whereas bottle feeding may result in lack of self-regulation of appetite and increased rate of weight gain. In addition, formula feeding may increase the prevalence of obesity because of the higher protein concentrations, increasing insulin levels, stimulating fat deposition, and resulting in more rapid growth.120 Formula also lacks many bioactive compounds, including some that regulate appetite and metabolism.118 Based on current evidence, public health professionals should promote breastfeeding for its many benefits to mothers and children, including the protective effect against pediatric obesity. Bottle-feeding mothers should be taught the cues of infant hunger and satiety, allowing the infant to develop self-regulation of milk intake.
EFFICACY AND EFFECTIVENESS OF PHYSICAL ACTIVITY AND NUTRITION INTERVENTIONS IN PEDIATRIC OBESITY
To reduce the prevalence of pediatric obesity, efficacious research findings need to be translated into practice. Translation enhances implementation of evidence-based interventions (EBIs) into evidence-based practice in clinical and community settings. As a first step, EBIs are generally tested within efficacy trials, focusing on evaluating an intervention under ‘ideal’ conditions, where a high degree of control over implementation is exercised to maximize internal validity.123–125 The next step is to evaluate EBIs in real-world settings (that is, health care, community and so on)123–125 using effectiveness trials to assess enhanced external validity and sustainability.124,125 A key challenge in translation is balancing EBI fidelity with acceptability within clinical and community settings.123,124 Decreases in EBI fidelity reduce ability to achieve expected outcomes. However, failure to achieve acceptability of an EBI by providers and/or consumers may prevent the translation of EBI into evidence-based practice.124
To assist with translation, the US Preventive Services Task Force (USPSTF) identified effective behavioral pediatric weight management programs considered to be comprehensive, medium-to-high intensity (26–75 h of program contact) and primary-care relevant.126 Comprehensive programs were identified as including a dietary and physical activity component and using behavioral modification strategies. These programs significantly reduced BMI (−1.9 to −3.3 kg m−2) and improved metabolic outcomes in overweight and obese children. Although these programs were considered to be primary-care relevant, the USPSTF concluded that because of the content of the programs and amount of treatment contact, these programs would be feasible only in specialty settings.
There are several recommendations regarding modifications in intervention intensity, which include decreasing frequency and duration of contact, narrowing agent of change, decreasing comprehensiveness of dietary goals, focusing on dietary or leisure-time activity goals, and reducing number and/or use of behavioral strategies127,128 have been proposed to overcome translational challenges in pediatric obesity.128 As these modifications impact EBI fidelity and may reduce ability to achieve previously reported weight improvements, identifying clinically relevant BMI reductions is important for ascertaining modification effectiveness. EBIs for pediatric weight management have produced a reduction of approximately −1.0 in standardized BMI (zBMI).129 However, what reduction is needed to improve metabolic outcomes in children to provide clinically relevant results? Although little research has examined this question, two studies suggest that a zBMI decrease of −0.13 to −0.25 is needed to improve metabolic outcomes.130,131
Several studies have examined recommended modifications to EBIs for pediatric weight management (Table 1). One investigation reduced intensity of contact using a guided self-help approach.132 The guided-self-help condition provided all components of the comprehensive, behavioral interventions, which was delivered in 12 face-to-face sessions, totaling approximately 4.5 h of contact time, which was supplemented with parent and child self-help manuals. At intervention end, there was a significant difference in reduction of zBMI favoring the guided self-help condition. Another investigation reduced intensity of contact and narrowed the agent of change and decreased the comprehensiveness of the dietary goals.133 In this 4-month investigation, the behavioral interventions were delivered in 12 face-to-face sessions, totaling 18 h of contact time. At treatment end, the parent-only intervention had a greater reduction in zBMI than the wait-list control, which was the only significant difference found between conditions. Further modifications were examined in two trials that reduced intensity of contact combined with narrowing agent of change, decreasing comprehensiveness of dietary goals, and targeting dietary or physical activity/sedentary behavior goals.134 Each trial had two behavioral, family-based interventions delivered to the parent only, but differed in diet and leisure-time activity goals. Trial 1 focused on changing two dietary behaviors, while Trial 2 examined changing one dietary and one leisure-time activity behavior. Both trial interventions were delivered in 8 face-to-face sessions, totaling 6 h of contact time. At 12 months, both trials found a significant reduction in zBMI (Trial 1 = −0.12; Trial 2 = −0.16), with no difference between the three conditions in each trial.
Table 1.
Overview of selected studies that have tested modifications to EBIs for the treatment of pediatric obesity
| Study | Modifications | Description of sample | Description of intervention | Results |
|---|---|---|---|---|
| Boutelle et al.132 |
|
|
|
|
| Janicke et al.133 |
|
|
|
|
| Raynor et al.134 |
|
|
|
|
| Taveras et al.135 |
|
|
|
|
Abbreviations: BMI, body mass index; EBIs, evidence-based interventions; RCT, randomized controlled trial.
One cluster randomized controlled trial (RCT) examined all recommended intensity modifications.135 This investigation included 10 pediatric practices randomized to either usual care or a 12-month Chronic Care Model intervention. The 475 children in the trial were aged 2–6 years and ≥85th BMI percentile. Intervention was delivered to the parent only, used motivational interviewing and focused negotiation skills for behavior. The intervention was focused on changing a dietary or leisure-time activity behavior, and was provided in four face-to-face sessions, and three phone calls with the parents only, totaling 2.5 h of contact time. At 12 months, the reduction in zBMI was −0.05, with no difference between the conditions.
In summary, to assist with translation, many methods that reduce treatment intensity have been examined to increase acceptability. Critically increasing the number of intervention modifications reduces the ability to reduce BMI. Thus, research is needed to identify the effective components of family-based, comprehensive behavioral interventions that can be delivered at the lowest contact intensity to improve pediatric weight status of a magnitude that is clinically relevant.
FAMILY-BASED PEDIATRIC OBESITY TREATMENT PROGRAMS
Childhood represents an ideal time for lifestyle interventions,136 as lifestyle behaviors in youth are not yet ingrained and intervention can often occur before the onset of medical comorbidities that could complicate treatment. Intervening in childhood further capitalizes on the potential for height growth, such that small reductions in weight can have a significant, positive impact on obesity and related disease risk.137 Finally, parental involvement can be harnessed to provide ongoing support for healthy behavior change138 and demonstrate the largest effects.139
Family-based behavioral treatment is a behavioral weight-control intervention that concurrently targets children who are overweight and their parents.136,139–144 It focuses on successive changes using family support and utilizes strategies such as self-monitoring, reinforcement and stimulus control to facilitate behavior change. Multiple meta-analyses confirm that family-based behavioral treatment should be considered a first-line approach to treatment.126,136,139,144–146 Based on this evidence, the USPSTF has given a grade B recommendation (there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial) for children who are overweight and obese to receive specialty treatment of moderate-to-high intensity that includes dietary, physical activity and behavioral counseling components.147
Despite successful weight loss during treatment of children, maintaining these effects over the long term remains a challenge. Weight loss maintenance interventions (delivered after an initial family-based behavioral treatment weight loss phase) demonstrated improved short-term efficacy of weight loss, with social facilitation maintenance (SFM) treatment yielding the strongest effects.148 Although children sustained their levels of weight loss while in weight maintenance treatment, once contact ceased, some weight regain occurred. Nonetheless, there was an overall ‘net effect’ of weight maintenance treatment, even 20 months after all treatment cessation. Therefore, weight maintenance interventions that incorporate a socioenvironmental approach (targeting behaviors across social and environmental contexts) with the continued use of self-regulating behaviors may improve long-term weight outcomes in children.
Although the previous trial compared maintenance treatment with a no maintenance treatment control condition, the study design precluded evaluating whether specialized treatment content or continued contact contributed to maintenance effects. As a result, the SFM treatment was enhanced (SFM+), and is predicated on learning theory research, which suggests that previously learned behaviors and newly learned behaviors exist simultaneously within a behavioral repertoire, rather than new learning replacing old learning.149 In the context of obesity, weight regain is common, as environmental cues tend to trigger responses from previous learning (that is, unhealthy weight behavior). However, this finding also predicts that if new learning (that is, healthy weight behaviors) is practiced and reinforced intensively and across multiple contexts, the chance for relapse decreases. Accordingly, SFM+ utilizes a supportive, socioenvironmental approach by deliberately targeting the practice of new weight maintenance behaviors, including self-regulatory behaviors specific to weight loss maintenance, across contexts.
Biosimulation projections suggested that SFM+ is promising150 and this treatment is currently being compared with a credible health education program, matched for time and attention, in a large-scale RCT. Moreover, emerging research supports the importance of intervening on each socioenvironmental level (that is, individual, family, peer and community). For example, appetitive traits like food reinforcement and impulsivity influence response to behavioral treatment,151 parent weight loss predicts child weight loss,152 peers and friends increase overweight/obese youths’ motivation to be physically active,153 and the built environment impacts weight maintenance.154
Current research is now focused on (1) optimizing the duration and intensity of family-based weight loss maintenance treatments, (2) examining the mediating influences of peer interactions, social problems, parent weight loss, and feeding practices on children’s weight loss and weight maintenance and (3) evaluating cost-effective delivery models that can be readily scaled for widespread dissemination. Continued attention to identification and early intervention remains a priority, particularly regarding opportunities to tailor interventions specific to individuals’ and families’ risk and symptom profiles. Future efforts should target integrating family-based interventions in routine clinical practice, increasing training and credentialing programs for providers, and advocating for third-party reimbursement for pediatric obesity care.
MOBILE-HEALTH APPROACHES TO TREAT PEDIATRIC OBESITY
Mobile and wireless health (mHealth) technologies have developed at an exponential pace in recent years. For example, low-cost, real-time technologies to assess and/or intervene on disease, movement, images, behavior, social interactions, environmental toxins, hormones and other physiological variables, have made remarkable advances in the last decade because of increased computational sophistication, as well as reductions in size and power requirements.155 In particular, smartphones have many capabilities that can be harnessed for promoting health.156 Beyond basic capabilities for two-way communication (that is, voice or SMS), many mobile phones have built-in cameras, gigabytes of storage, an Internet browser, global positioning systems support, built-in accelerometers, connectivity to WiFi, and wireless communication with external devices and to outside networks via Bluetooth (for example, heart rate monitors, external accelerometers). In addition, mHealth can also take advantage of stationary data that can be correlated with movement through global positioning systems. In the past, behavior could only be measured by subjective observation and self-report, but today’s technologies provide opportunities to passively capture a digital ‘footprint’ that catalogues a person’s everyday behaviors, contexts and even states. Data streamed from a broad variety of sources, for example, sensors, social media, pictures and videos, location, purchase transactions, applications and Internet use, can, when aggregated, provide an in-depth view of the person as they interact with the world around them. Technologies are emerging for overarching, in situ measurement of behavior and intention using multi-modal sensing sources. These new mobile technologies offer heretofore unimagined opportunities for tracking and intervening on obesity-related behaviors (for example, physical activity, healthful eating, sleep and stress) and important physical outcomes (for example, weight and body composition).
The promise of mHealth is based on the premise that mobile technologies can allow us to develop behavioral monitoring and change systems that are personalized, adaptive and delivered in real-time.157–159 Available research suggests that immediate and contextualized feedback is superior to traditional approaches to promote behavior change160 as well as improve learning.161,162 These technologies also enhance the possibility of scaling surveillance, prevention and intervention efforts in ways that have been unthinkable with conventional face-to-face programs. Further, these devices have the ability to capitalize on small, but frequent intervention doses at timing that is optimized for the individual user. Wearable and deployable sensors and smart phones can provide rich, temporally dense contextualized data that allows for the personalization of instantaneous feedback, ensuring appropriate response, messaging, timing and a dose that is adaptive.163 More specifically, these adaptive technologies can be set to prompt for answers to questions or deliver intervention content based on current behaviors, behavior changes and locations, at random or in response to particular events.157
Early and ongoing uses of mobile technologies to intervene on obesity-related behaviors in youth include SMS text-messaging and Ecological Momentary Interventions.164,165 Although results are mixed, in general, SMS text-messaging interventions, compared with paper diaries, appear to be effective at promoting increased adherence to self-monitoring of physical activity,165 and tailored SMS messages appeared to help improve awareness of weight management166 in pediatric populations. SMS has also been used to successfully reduce dropout in pediatric obesity interventions.167 However, these types of interventions do not harness the true capabilities of mHealth technologies, such as ubiquitous measurement and real-time feedback.168 Newer systems are moving toward real-time data collection.168–173 These systems typically provide visual feedback to the user: for example, UbiFit, which uses a display of a garden that users can quickly glance at to convey information on physical activity,169 Fish’n’-Steps where the growth and happiness of a virtual fish is tied to daily step count166 or MOPET, which uses a virtual trainer to provide motivation and advice.172 Others incorporate social networking to connect users and encourage physical activity through competition or social support.164,166,167,171,173 A few mobile systems that involve some sensing capabilities, including gDitty (now Zamzee)174 and ChickClique,173 have been developed for children and adolescents; however, these require manual upload of data and therefore cannot be used for real-time, databased feedback. mHealth has yet to reach its full potential in pediatric obesity.168,172
KNOWME Networks for Hispanic youth is one of the first interventions to take advantage of a combination of ubiquitous and active data collection and real-time feedback.169,171 KNOWME is a suite of wireless, wearable sensors that monitor behavior and interface with a mobile phone to collect, store, analyze, display and transmit data to a secure web interface in real-time. KNOWME supports just-in-time adaptive interventions that respond to moment-by-moment contextualized behaviors. Development of the product included a youth advisory team made up of 20 minority youth (ages 12–17 years) that met at each design phase to determine wearability, messages and feedback, and interface design. Personalized algorithms using sensor and phone data streams were developed for each advisory member that recognized seven activities (lying down, sitting, standing, slow walk, brisk walk, active game play and running) with an overall accuracy of 94%.169,170 To examine wearability and test the web interface, 12 overweight Hispanic youth, (five females, 14.8 ± 1.9 years old, BMI percentile 97 ± 3) wore KNOWME for 2 weekend days. Participants wore KNOWME for 11.4 ± 2.0 h per day, sent a mean of 8 SMS messages to the research team per day and received a mean of 9 SMS messages in return per day. To test whether wearing KNOWME for a weekend could increase physical activity and decrease sedentary behavior, 10 Hispanic youth (mean age 16.3 ± 1.7 years, mean BMI percentile 97.2 ± 4.4, 50% female) wore an accelerometer for a 2.5-day weekend to determine baseline activity levels. Within 3 weeks from baseline, participants wore KNOWME for the full 2.5 days. KNOWME sent time-stamped participant activity data to a secure website, which displayed minute-to-minute participant data (physical activity, sensor functioning) and refreshed every 10 min. If a participant was sedentary for over 2 h, KNOWME automatically generated a ‘MOVE!’ message, and a research team member initiated an SMS conversation prompting the participant to be active. Sedentary behavior was significantly lower during KNOWME wear as compared with baseline wear (~171 min, P<0.1). Physical activity increased by 1.5 min day−1 (P<0.09) in this group that showed <1 min of moderate-to-vigorous physical activity per day at baseline. Approximately 75 SMS messages were sent between researchers and participants (43% from participants) during the weekend. Lagged mixed regression analysis showed that prompts sent to participants were associated with an increase in physical activity (P<0.0001) in the following 10-min period. SMS prompts sent to participants were post-hoc coded using motivational interviewing principles into four message types: affirmation, suggestion, neutral and prompting question. When prompting question messages were sent to participants, physical activity in the following 10-min period was significantly higher (2411 accelerometer counts) than when messages of that type were not sent (P<0.01). These findings indicate that using a mobile suite to collect real-time data can increase physical activity and decrease sedentary behavior in overweight Hispanic youth. Further, prompting adolescents to engage in physical activity with reflective SMS messages is a potentially effective intervention approach for overweight Hispanic youth. The next step for KNOWME is scaling up.
In summary, mHealth interventions show promise for the prevention and treatment of pediatric obesity, including gaming and entertainment experiences, new forms of real-time adaptive feedback and social networks.158
EXERGAMING AS A STRATEGY TO TREAT PEDIATRIC OBESITY
Screen-based entertainment that is highly popular among children may provide an innovative tool to treat pediatric obesity.175 Exergaming, which is video gaming that involves gross motor movement, is now recognized as a viable physical activity option by leading scientific and health organizations, including the American Heart Association and the Presidential Active Lifestyle Award. Systematic reviews and meta-analyses indicate that exergaming can reach criteria of moderate-intensity activity.176–178 Eighteen studies that examined youth exergame play found an increase in youths’ energy expenditure on average 222 ± 100% and increase in heart rate on average 64 ± 20%.177 Importantly, exergaming that involved lower body movement produces higher energy expenditure than exergaming that used just the arms.176 In addition, playing with or against a partner rather than alone induces higher energy expenditure.175
Group-based exergame programs supervised in a laboratory or school setting have shown promising results for improving behaviors and health outcomes. One key contributing factor is friend participation and social interaction, which encourage sustained exergame play. For instance, a 20-week RCT assigned overweight and obese adolescents to cooperative exergame play (that is, play in a team to earn points), competitive exergame play (that is, play against a partner to win) or a control condition.179 Those adolescents who played cooperatively (approximately 20 h over 20 weeks) lost significantly more weight compared with the control group, for a difference of 2.6 kg. In a separate study, adolescents who played a tennis exergame against a peer expended more energy than those who played alone, and the energy expended in social play was comparable to energy expended during a beginner’s tennis lesson on a tennis court.180 The Exergaming for Health group weight loss program included 10 1-h gaming sessions over 10 weeks and found a significant reduction in BMI z-score, increased weekly exercise of 2 h per week, and an 84% retention rate among 48 overweight and obese 8–16 years old.181 Finally, a study of 27 children aged 9–12 years found that those assigned to weekly multiplayer classes played twice as long and had a significantly lower dropout rate (15% vs 64%) compared with those who played only at home.182
Despite this emerging evidence of efficacy in exergaming interventions, many interventions do not provide sufficient frequency or duration of game play to produce weight loss. In fact, a bout of exergaming may be compensated with reduced physical activity or increased energy intake throughout the day, either of which would decrease or even counteract the potential weight loss from exergaming.183 Attenuating weight gain may be a more age-appropriate goal for overweight or obese adolescents, and this outcome was observed in four of six RCTs in a recent meta-analysis of exergames.183 After 30 dance exergaming sessions of 40–60 min each over 10 weeks, there was no observed BMI change among obese adolescents but higher perceived competence to exercise regularly compared with a no-game control group, which may help to encourage exercise and weight maintenance.184 Fifteen-minute bouts three times per week over 8 months did not change BMI or body fat but did enhance cardiorespiratory endurance in 208 school children, which may also contribute to weight maintenance.185
An additional consideration in exergaming is game play in the home without a structured intervention program, which has not achieved success in changing children’s body composition, largely due to high attrition and waning interest in game play.186–188 A notable exception is a 24-week home-based exergame intervention for 322 overweight and obese 10–14 years old that observed a significant decrease in BMI (−0.24) and a significant decrease in body fat (−0.83%).189 In addition, a 12-week dance exergame intervention in the home observed improved endothelial function and aerobic fitness among 35 overweight/obese children.190
In summary, exergaming has been repeatedly documented as producing moderate-intensity physical activity, and several recent trials have demonstrated weight loss in structured, supervised exergaming interventions. Given the popularity and novelty of playing video games for health, exergames may equip youth with a fun option for physical activity and weight loss under the appropriate conditions.
TREATMENT OF PEDIATRIC OBESITY IN HEALTH-CARE SETTINGS
Health care clearly has a function in both the prevention and treatment of pediatric obesity. However, effective obesity intervention strategies may not fit well in traditional health-care office visits, and so it is not straightforward. To provide guidance to health-care providers, the American Medical Association, the CDC, and the Maternal and Child Health Bureau convened an expert committee to make recommendations on assessment, prevention, and treatment of child and adolescent obesity.191 They summarized current publications on behaviors that are either associated with healthier weight (cross-sectional) or lead to improved weight when adopted. Unfortunately, the limited evidence focused on the target patient behaviors, and seldom addressed the effectiveness of the delivery of this information in the health-care office setting. The treatment committee therefore proposed a staged approach, beginning with low-intensity interventions, offered through the primary-care office and advancing to more intensive interventions with increasing use of specialized professionals and programs (Table 2).191
Table 2.
Four stages of treatment for child and adolescent obesity
| Stage 1: prevention plus | |
|---|---|
| Behaviors: 5+ Fruits and vegetables ≥2-h screen time ≤1-h of physical activity Etc. |
Delivery: Office based Trained office support MD, PNP< PA, RN Scheduled follow-up visits |
| Stage 2: structured weight management | |
| Behaviors: reduced calorie eating plan ≤1-h screen time >1-h physical activity Monitoring |
Delivery: RD, MD, RN with training in assessment and counseling Office based Support from referrals Monthly visits |
| Stage 3: comprehensive multidisciplinary intervention | |
| Behaviors: More frequent contact More structured monitoring, goal setting, feedback |
Delivery: Dedicated weight management program or RD and behavior counsel and structured activity Weekly for 8–12 weeks |
| Stage 4: tertiary care | |
| Consider: Medication Surgery Meal replacement Ongoing behavior change |
Delivery: Pediatric weight management center Multidisciplinary team Clinical or research protocol |
Abbreviations: MD, medical doctor; PA, physician’s assistant; PNP, pediatric nurse practitioner; RN, registered nurse; RD, registered dietician.
Not surprisingly, the evidence about effectiveness is more robust for the more intensive stages:
For stages 1 and 2, the science in this area is emerging. A large study of brief counseling to reduce multi-media use was not designed to improve weight, but the significant increase in the number of parents reporting media limitation a year later is a evidence that low-intensity intervention can change behavior, but effects on BMI were not assessed.192 Two controlled studies of low-intensity interventions aimed at improving weight status in younger children in primary-care settings showed no effect on BMI at 1 year.135,193 Several large studies are now underway to evaluate more intensive practice-based interventions, and include (1) motivational interviewing delivered by a either a primary-care provider or more intensively by dietitians,194,195 and (2) collaboration between a multidisciplinary team and primary-care provider with the use of web-based software.196
For stage 3, a Cochrane review concluded from an assessment of RCTs that ‘Family based, lifestyle interventions with a behavioral program aimed at changing diet and physical activity thinking patterns produce significant and clinically meaningful decrease in overweight in children and adolescents…in the short- and long-term’.197 Such programs need to be moderate-to-high intensity, generally weekly or more often for 3–4 months. Although programs like this could be offered through the health-care system, its current structure creates many barriers such as: insurance not always paying for obesity counseling, physicians and other health providers are usually not trained in counseling, and standard consultations are too short. An observation of primary-care providers documented that they spend only 70 s on average on diet, growth and activity counseling during well child visits, and the maximum was <2.5 min.198 Thus, feasibility and effectiveness of addressing the causes of obesity within medical homes needs to be addressed.
For stage 4, the most effective treatment is bariatric surgery. Reports of adolescent bariatric surgery to date demonstrate a BMI decrease of around 11–17 kg m−2.199 However, this highly aggressive approach is not widely available, although the physiologic selection criteria used by a current NIH consortium study, which includes BMI>40 kg m−2 and physical maturity, apply to several hundred thousand adolescents.17,200 Access, capacity, insurance and patient preference limit the usefulness of this approach. For medication, Orlistat, which reduces absorption of dietary fat, is the single weight-control medication studied in and approved for youth aged 12 years and older. Its benefit is modest and when accompanied by diet and activity counseling, orlistat has led to a placebo-subtracted BMI difference of −0.88 kg m−2.201 In contrast, a meal replacement program in severely obese adolescents showed no difference at 1 year when compared with conventional-restricted diet.202
Finally, more deliberate partnerships between health-care and community and school systems are the focus of three CDC-sponsored Childhood Obesity Demonstrations Projects. Each site has a different protocol, but all will engage primary health-care, schools, early childhood education sites, community centers, community health workers, as well as family within catchment areas that contain high populations of low income, racially and ethnically diverse children aged 2–12 years.203 The results of these studies will further delineate ways that health care can effectively combat the childhood obesity epidemic.
PHARMACOLOGICAL APPROACHES TO TREAT PEDIATRIC OBESITY
As behavioral interventions have shown relatively limited success among a substantial proportion of severely obese children and adolescents,139,142,204,205 there is interest in combining lifestyle modification with more intensive strategies to ameliorate pediatric obesity.206 Consequently, the use of pharmacologic agents is of considerable interest, although to date only orlistat, as mentioned earlier, holds Food and Drug Administration (FDA) approval to treat obesity among adolescents aged 12–16 years, and none are approved for children below age 12 years.
The classical anorexiants, such as phentermine,207 diethylpropion208 and mazindol,209–212 alter the release and reuptake of neurotransmitters (that is, norepinephrine, serotonin and dopamine) that impact on appetite.213 No weight loss medication with these mechanisms of action is currently approved for pediatric use and no available data support their long-term (>1 year) safety or efficacy in pediatric populations. Only small trials, using phentermine207,214 or diethylpropion,208,215 that lasted no more than 12 weeks, have been reported in a pediatric population.
Lorcaserin is a selective 5HT2C receptor agonist that acts primarily in the central nervous system to inhibit feeding behavior.216 Three phase III multicenter clinical trials217–219 found that lorcaserin decreased adult body weight modestly by about 3.2 kg more than placebo220 and improved comorbid conditions.218,221 No pediatric trials of lorcaserin have been reported. Bupropion222 is an antidepressant that inhibits pre-synaptic reuptake of both norepinephrine and dopamine.223 No pediatric RCTs of bupropion examining its effects on body weight have been published, although some short-term open-label studies suggest its use may be associated with small amounts of weight loss in adolescents.224,225
There are several older drugs for which pediatric trials exist that affect appetite primarily by increasing serotonergic release or inhibiting reuptake,226,227 including fluoxetine, chlorphentermine, fenfluramine and its stereoisomer, dexfenfluramine.228–233 None of these drugs are currently FDA-approved for weight loss and most have been removed from the US market.
Topiramate is a GABA-ergic anticonvulsant drug that was fortuitously found to cause weight loss in patients with epilepsy. When used in children for the treatment of epilepsy234 and migraine,235 its use was associated with 1–2 kg decrease in body weight versus placebo, a response confirmed in a limited number of open-label case series.236–239 Concerns over the impairment of cognitive function at dosages similar to those used to treat seizure disorders will likely limit its use as a stand-alone agent,240 and to date, no controlled trials restricted to obese children or adolescents have been reported. It is also important to note that there is concern that the risk for cleft lip with or without cleft palate is increased in children born to mothers who used topiramate during pregnancy.241,242
Phentermine/topiramate-extended release combines low-dose phentermine with a non-standard dose of the antiepileptic medication topiramate-extended release. Placebo-subtracted weight loss in adults243,244 at 1 year for 15 mg/92 mg day−1 is −8.9 kg (−8.3 to −9.4), but has not undergone any randomized pediatric studies.
Sibutramine was one of the best-studied weight loss medications in adolescents,245–254 and when combined with behavioral therapy for 6- to 12-month periods led to −2.9 to −3.6 kg m−2 decreases in BMI, but was never approved for use in children younger than 16 years.255,256
The other drugs investigated all focus on glucose metabolism and/or gut function. They include glucagon-like peptide 1, which activates central nervous system glucagon-like peptide 1 receptors to reduce food intake via glucose metabolism-dependent inhibition of central cAMP-activated protein kinase.257 A 12-week open-label crossover study of 12 extremely obese children has reported a treatment effect of −3.9 kg compared with behavioral intervention alone from exenatide,258 and a 3-month RCT259 in 26 severely obese adolescents found a similar effect (−3.26 kg placebo-subtracted weight loss). However, even after another 3 months of open-label treatment, BMI was reduced by only 4%. Studies documenting the long-term safety, tolerability and efficacy of glucagon-like peptide 1 analogs in children and adolescents are still needed.
Orlistat, the gastrointestinal lipase inhibitor, is approved by the FDA for management of obesity in adolescents 12–16 years of age.201,260–266 The largest study201 randomized 539 adolescents for 52 weeks to orlistat or placebo. There was an overall −0.55 kg m−2 decrease in BMI with orlistat versus a +0.31 kg m−2 increase with placebo after 52 weeks. The most common adverse events were oily stools (50%), oily spotting (29%) and oily evacuation (23%); 2% of the dropouts in the orlistat group were described as due to drug-related adverse effects.201 Although orlistat is the only FDA-approved treatment for obesity among adolescents under the age of 16 years, it appears to offer little prospect of benefit to those with severe obesity and to date, limited metabolic benefits from orlistat therapy among adolescents have been reported.201,262,267
Metformin is a biguanide that inhibits intestinal glucose absorption, reduces hepatic glucose production and increases insulin sensitivity in peripheral tissues.268,269 It is approved for the treatment of type 2 diabetes in adults and children over 10 years of age270 but not approved for treatment of obesity. Metformin has only modest effects on weight loss in children or adolescents.271–287
Octreotide is a somatostatin analog.288 Pediatric studies evaluating this drug for weight loss via subcutaneous injection in Prader–Willi syndrome or hypothalamic obesity suggest limited efficacy.289–292
Growth hormone stimulates lipolysis and decreases fat mass293–297 and stimulates an increase in lean body mass as observed in both adult and pediatric patients with Prader–Willi syndrome to whom it has been given.298–300 However, a review of clinical trials of recombinant growth hormone administration in patients with obesity, showed no better performance than a hypocaloric diet.301
Effective pharmacotherapy that reverses excessive adiposity and improves obesity-related comorbid conditions in pediatric patients remains elusive. Currently, the weight management impact of available drugs has been modest. Meta-analyses of trials for weight loss in pediatric samples have shown minimal effects, and in many cases no greater than the response to behavioral interventions.139 Even when combined with state-of-the-art behavioral interventions, existing pharmacotherapy among adolescents has only moderate efficacy.201,286,302 Current guidelines, however, include medication in their recommended approaches to treat obese adolescents.191,303
Unfortunately, the most efficacious medications for treating obesity have had to be withdrawn because of adverse events. However, because of the importance of the metabolic pathways involved in the regulation of energy balance, it is unlikely that any highly effective weight loss medication will be risk free. It remains to be demonstrated if the potential benefits from long-term use of anti-obesity medications for individual children will outweigh their risks.
BARIATRIC SURGERY FOR OBESITY AND RISK FACTOR REDUCTION IN PEDIATRICS
The group with the most compelling medical complications of obesity are the 4–6% with severe pediatric obesity (BMI ≥99th percentile or BMI ≥120% of the 95th percentile),304 in which lifestyle modification and behaviorally based interventions have little if any effect,139 despite being effective in moderately obese youth.264,305 In addition, for those who can lose some weight with lifestyle changes, maintenance of weight loss is challenging and recidivism is common.306
In adult patients, surgical interventions for weight loss (bariatric surgery) have generally been effective for inducing a sustained decrease in BMI, improving important health conditions and extending longevity beyond that which would be expected without the surgical intervention.307 Thus, it is logical to consider which and how such procedures may be used appropriately in adolescents.
A variety of procedures that anatomically alter the gastrointestinal tract have been used for weight loss. The procedures result in the restriction of stomach capacity, interference with meal progression or diversion of ingested contents from one region of the gastrointestinal tract to another.308 The most commonly used procedures for adolescents today include Roux-en-Y gastric bypass, adjustable gastric banding and the more recently introduced vertical sleeve gastrectomy.309 Adolescents treated with Roux-en-Y gastric bypass typically experience a significant 35–37% reduction in BMI by 1 to 2 years postoperatively.310,311
Although definitive studies are underway, many have shown that surgery in adolescents results in improvement or resolution of numerous comorbid conditions including sleep apnea,312 diabetes313 and improvement in quality of life.314,315 Perioperative and longer term risks must be acknowledged,309,316 and bariatric surgery among severely obese adolescents is a viable option,317,318 but further research is required to determine the long-term effectiveness of this approach.
TRANSLATION TO CLINICAL AND PUBLIC HEALTH PRACTICE
This review and symposium have taken a broad approach to the problem of pediatric obesity, and evidence presented on factors related to pediatric obesity including genetics, epigenetics, intrauterine and maternal health, as well as behavioral, pharmacological and surgical interventions. Based on these discussions, the following recommendations are made with respect to translating these findings to clinical and public health practice in order to overcome pediatric obesity:
It is never too soon to initiate preventive efforts, and given the evidence for inter-generational and intrauterine effects on pediatric obesity, these should begin before conception, continue during pregnancy and in the early post-partum period, and sustained across these developmental windows.
Promoting appropriate weight gain during pregnancy with regard to the IOM recommendations should be widely disseminated and their adoption promoted. Importantly, the implications of weight loss for obese pregnant women are not understood and should be discouraged until safety data are accumulated.
The many benefits of breastfeeding, including a potential preventive role for pediatric obesity, indicate that it should continue to be strongly promoted.
Behavioral and family-based interventions show effectiveness in lowering BMI of overweight and obese children and adolescents, especially when initiated at a young age. However, these interventions demonstrate lower efficacy in cases of severe obesity, and treatment effects decrease with advancing age. Therefore, efforts that focus on preventing normal weight and overweight children from becoming obese are key to the reduction of pediatric obesity prevalence.
Efficacious lifestyle-based interventions for pediatric obesity need to be translated and modifications documented and tested for effectiveness in real-world settings, including adapting the role of the health-care provider in identification, treatment and/or referral.
Efficacy and effectiveness of approaches to pediatric obesity using novel technologies are required. Interventions that rely on mHealth solutions and exergaming are promising strategies that appear to engage and encourage healthy behavior, but more research is required.
Although pharmacological approaches to treat pediatric obesity are being sought, there are currently no agents available that can be recommended for use in children. There may be specific subgroups of obese children who will particularly benefit from pharmacotherapy, but again, further research on identifying predictive factors for success is needed.
Early evidence indicates that bariatric surgery holds considerable promise as an effective and safe approach to treat severe obesity among adolescents who are at high risk of health complications and premature mortality because of their extreme levels of adiposity. Ongoing studies will further elucidate advantages and potential risks of use of surgery in youth.
In addition to these practice-based recommendations, further research is required on all aspects of obesity prevention and treatment explored in this review, particularly the basic biology into the regulation of energy balance, particularly energy intake and metabolism in early life. Furthermore, fundamental studies are needed to establish the driving factors determining the profile of adipose tissue distribution, how it becomes individualized, and potential opportunities to intervene on these processes. Research also needs to continue on the pre-pregnancy and in utero determinants of adiposity in infancy and throughout the growth period. All of the above recommendations require that the power of genetics, epigenetics and developmental biology are used to address these fundamentals questions, which have the potential, if resolved, to develop the tools to prevent or treat excess adiposity at critical stages of development.
Acknowledgments
SB is supported in part by 1U18 DP00336l7-01 Systems Approach to Obesity Prevention in Underserved Children in Texas, Centers for Disease Control and Prevention and by NIH/NIDDK 5U01DK 061718 Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). CB is funded, in part, by the John W Barton, Sr. Endowed Chair in Genetics and Nutrition. PMC is funded by NICHD HD22965-19 and the Clinical Research Unit of the CTSCUL1TR000439 from NCATS at Case Western Reserve University. THI is supported in part by grant UM1DK072493 from the National Institute of Diabetes and Digestive and Kidney Diseases. PTK is partially supported by the Marie Edana Corcoran Endowed Chair in Pediatric Obesity and Diabetes. DS-M is partly supported by National Center for Research Resources and the Office of Science Education of the National Institutes of Health through grant number R25 RR011113. HR has received research funding from Weight Watchers, International. LMR is supported by funding from the National Institutes of Health (U01DK094418, R01099175, R00HD0607662). AES is supported, in part, by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. MV is supported in part by Gravida: National Centre for Growth and Development and The Marsden Fund of the Royal Society of New Zealand. DW is supported in part, by the Scott Rudolph University Endowed Professor in Psychiatry, Medicine, Pediatrics and Psychology. Her other support includes NIH grantsK24MH070446-09; R01HD036904-01A1; T32HL007456-30; P30DK056341 NORC, and her research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. JAY is supported by the Intramural Research Program of NICHD, NIH and is a Commissioned Officer in the US Public Health Service, Department of Health and Human Services. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the US Public Health Service, the National Institutes of Health or the US Department of Health and Human Services.
Footnotes
CONFLICT OF INTEREST
CB is a scientific advisor to Weight Watchers, Pathway Genomics and Nike SPARQ. THI receives research funding from Ethicon Endosurgery. DW serves as an advisor to Shire and United Health Group. JAY has received orlistat and matching placebo from Roche and betahistine and matching placebo and grant support from Obecure for prior studies. The remaining authors declare no conflict of interest.
References
- 1.Lobstein T, Baur L, Uauy R for the International Obesity Task Force. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5:4–85. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 4.Williams J, Wake M, Hesketh K, Maher E, Waters E. Health-related quality of life of overweight and obese children. JAMA. 2005;293:70–76. doi: 10.1001/jama.293.1.70. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr. 2009;90:210–216. doi: 10.3945/ajcn.2009.27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198–e205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Craig WM, Boyce WF, Pickett W. Associations between overweight and obesity with bullying behaviors in school-aged children. Pediatrics. 2004;113:1187–1194. doi: 10.1542/peds.113.5.1187. [DOI] [PubMed] [Google Scholar]
- 8.Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics. 2013;131:e1–e9. doi: 10.1542/peds.2012-1106. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Dietz WH. Economic burden of obesity in youths aged 6 to 17 years: 1979–1999. Pediatrics. 2002;109:E81. doi: 10.1542/peds.109.5.e81. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 11.De Onis M, Garza C, Victoria CG, Bhan M, Norum KR. The WHO Multicentre Growth Reference Study (MGRS): rationale, planning, and implementation. Food Nutr Bull. 2004;25 :S3–S84. [Google Scholar]
- 12.De Onis M, Onyanga AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull WHO. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9:322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6:12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24:176–188. doi: 10.3109/09540261.2012.688195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD. Prevalence of obesity among children and adolescents: United States, trends 1963–65 through 2007–2008. NCHS Health E Stat. 2010 Available at: www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.htm.
- 21.Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6:342–360. doi: 10.3109/17477166.2011.605895. [DOI] [PubMed] [Google Scholar]
- 22.Pan L, Blanck HM, Sherry B, Dalenius K, Grummer-Stawn LM. Trends in the prevalence of extreme obesity among US preschool-aged children living in low-income families, 1998–2010. JAMA. 2012;308:2563–2565. doi: 10.1001/jama.2012.108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slatkin M. Epigenetic inheritance and the missing heritability problem. Genetics. 2009;182:845–850. doi: 10.1534/genetics.109.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beales PL. Obesity in single gene disorders. Prog Mol Biol Transl Sci. 2010;94:125–157. doi: 10.1016/B978-0-12-375003-7.00005-4. [DOI] [PubMed] [Google Scholar]
- 25.Beales PL, Farooqi IS, O’Rahilly S, editors. The Genetics of Obesity Syndromes. Oxford University Press; Oxford: 2009. [Google Scholar]
- 26.Ramachandrappa S, Farooqi IS. Genetic approaches to understanding human obesity. J Clin Invest. 2011;121:2080–2086. doi: 10.1172/JCI46044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanktree MB, Johansen CT, Joy TR, Hegele RA. A translational view of the genetics of lipodystrophy and ectopic fat deposition. Prog Mol Biol Transl Sci. 2010;94:159–196. doi: 10.1016/B978-0-12-375003-7.00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 29.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. J Epidemiol Community Health. 2001;55:873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr. 2010;91:1560–1567. doi: 10.3945/ajcn.2009.28838. [DOI] [PubMed] [Google Scholar]
- 31.Loos RJ. Genetic determinants of common obesity and their value in prediction. Best Pract Res Clin Endocrinol Metab. 2012;26:211–226. doi: 10.1016/j.beem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249 796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14:507–515. doi: 10.1038/nrg3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: genetic interactions create phantom heritability. Proc Natl Acad Sci USA. 2012;109:1193–1198. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visscher PM, Yang J, Goddard ME. A commentary on ‘common SNPs explain a large proportion of the heritability for human height’ by Yang et al. Twin Res Hum Genet. 2010;13:517–524. doi: 10.1375/twin.13.6.517. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloom JS, Ehrenreich IM, Loo WT, Lite TL, Kruglyak L. Finding the sources of missing heritability in a yeast cross. Nature. 2013;494:234–237. doi: 10.1038/nature11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 39.Hainer V, Stunkard AJ, Kunesova M, Parizkova J, Stich V, Allison DB. Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. Int J Obes Relat Metab Disord. 2000;24:1051–1057. doi: 10.1038/sj.ijo.0801358. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw KT, et al. Physical activity attenuates the genetic predisposition to obesity in 20 000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 2010;7:e1000332. doi: 10.1371/journal.pmed.1000332. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218 166 adults and 19 268 children. PLoS Med. 2011;8:e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606. doi: 10.1007/s00125-012-2505-5. [DOI] [PubMed] [Google Scholar]
- 44.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 46.Pearce LR, Atanassova N, Banton MC, Bottomley B, van der Klaauw AA, Revelli JP, et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155:765–777. doi: 10.1016/j.cell.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symonds ME. Brown adipose tissue growth and develoment. Scientifica (Cairo) 2013:305763. doi: 10.1155/2013/305763. [DOI] [PMC free article] [PubMed]
- 49.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 50.Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev. 2007;19:53–63. doi: 10.1071/rd06130. [DOI] [PubMed] [Google Scholar]
- 51.Cannon B, Connoley E, Obregon M-J, Nedergaard J. Perinatal activation of brown adipose tissue. In: Kunzel W, Jesen A, editors. The Endocrine Control of the Fetus. Springer Verlag; Berlin: 1988. pp. 306–320. [Google Scholar]
- 52.Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91:223–234. doi: 10.1002/path.1700910126. [DOI] [PubMed] [Google Scholar]
- 53.Robinson L, Ojha S, Symonds ME, Budge H. Body mass index as a determinant of brown adipose tissue function as measured by thermal imaging in healthy children. J Pediatr. 2013;164:318–322. e1. doi: 10.1016/j.jpeds.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Pope M, Budge H, Symonds ME. The developmental transition of ovine adipose tissue through early life. Acta Physiol. 2013;210:20–30. doi: 10.1111/apha.12053. [DOI] [PubMed] [Google Scholar]
- 55.Symonds ME, Sebert SP, Budge H. Nutritional regulation of fetal growth and implications for productive life in ruminants. Animal. 2010;4:1075–1083. doi: 10.1017/S1751731110000479. [DOI] [PubMed] [Google Scholar]
- 56.Symonds ME, Mendez MA, Meltzer HM, Koletzko B, Godfrey K, Forsyth S, et al. Early life nutritional programming of obesity: mother-child cohort studies. Ann Nutr Metab. 2013;62:137–145. doi: 10.1159/000345598. [DOI] [PubMed] [Google Scholar]
- 57.Griffith R, Lluberas R, Luhrman M. Long-term change in diet. Institute for Fiscal Studies; 2013. Gluttony in England? [Google Scholar]
- 58.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, et al. Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci USA. 2013;110:3387–3392. doi: 10.1073/pnas.1222643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs nonrecruited molecular signatures of brown, ‘brite,’ and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–E31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 61.Kozak LP, Koza RA. The genetics of brown adipose tissue. Prog Mol Biol Transl Sci. 2010;94:75–123. doi: 10.1016/B978-0-12-375003-7.00004-2. [DOI] [PubMed] [Google Scholar]
- 62.Symonds ME, Budge H, Frazier-Wood AC. Epigenetics and obesity: a relationship waiting to be explained. Hum Hered. 2013;75:90–97. doi: 10.1159/000352009. [DOI] [PubMed] [Google Scholar]
- 63.Volkmar M, Dedeurwaerder S, Cunha DA, Ndlovu MN, Defrance M, Deplus R, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31:1405–1426. doi: 10.1038/emboj.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8:679–688. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- 65.Bloor ID, Sebert SP, Saroha V, Gardner DS, Keisler DH, Budge H, et al. Sex differences in metabolic and adipose tissue responses to juvenile-onset obesity in sheep. Endocrinology. 2013;154:3622–3631. doi: 10.1210/en.2013-1207. [DOI] [PubMed] [Google Scholar]
- 66.Bloor ID, Sebert SP, Mahajan RP, Symonds ME. The influence of sex on early stage markers of kidney dysfunction in response to juvenile obesity. Hypertension. 2012;60:991–997. doi: 10.1161/HYPERTENSIONAHA.112.195412. [DOI] [PubMed] [Google Scholar]
- 67.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–1790. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Symonds ME, Henderson K, Elvidge L, Bosman C, Sharkey D, Perkins AC, et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr. 2012;161:892–898. doi: 10.1016/j.jpeds.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 69.Symonds ME, Pope M, Budge H. Adipose tissue development during early life: novel insights into energy balance from small and large mammals. Proc Nutr Soc. 2012;71:363–370. doi: 10.1017/S0029665112000584. [DOI] [PubMed] [Google Scholar]
- 70.Symonds ME, Budge H. How promising is thermal imaging in the quest to combat obesity? Imag Med. 2012;4:589–591. [Google Scholar]
- 71.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 72.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. In: Rasmussen KM, Yaktine AL, editors. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. National Academies Press; Washington, DC, USA: 2009. [PubMed] [Google Scholar]
- 73.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–433. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 74.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Catalano PM, Tyzbir ED, Allen SR, McBean JH, McAuliffe TL. Evaluation of fetal growth by estimation of neonatal body composition. Obstet Gynecol. 1992;79:46–50. [PubMed] [Google Scholar]
- 76.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012;97:3648–3654. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diab Care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 79.Catalano PM, Hauguel de Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 81.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Resi V, Basu S, Haghiac M, Presley L, Minium J, Kaufman B, et al. Molecular inflammation and adipose tissue matrix remodeling precede physiological adaptations to pregnancy. Am J Physiol Endocrinol Metab. 2012;303:E832–E840. doi: 10.1152/ajpendo.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gluckman PD, Hanson MA, Beedle AS, Spencer HG. Predictive adaptive responses in perspective. Trends Endocrinol Metab. 2008;19:109–110. doi: 10.1016/j.tem.2008.02.002. author reply 112. [DOI] [PubMed] [Google Scholar]
- 84.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 86.Gray C, Li M, Reynolds CM, Vickers MH. Pre-weaning growth hormone treatment reverses hypertension and endothelial dysfunction in adult male offspring of mothers undernourished during pregnancy. PLoS One. 2013;8:e53505. doi: 10.1371/journal.pone.0053505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds CM, Li M, Gray C, Vickers MH. Preweaning growth hormone treatment ameliorates adipose tissue insulin resistance and inflammation in adult male offspring following maternal undernutrition. Endocrinology. 2013;154:2676–2686. doi: 10.1210/en.2013-1146. [DOI] [PubMed] [Google Scholar]
- 88.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 89.Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx1 in the intrauterine growth retarded rat. Diabetologia. 2011;54:2606–2614. doi: 10.1007/s00125-011-2250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai S, Briggs D, Vickers MH. Increased systolic blood pressure in rat offspring following a maternal low-protein diet is normalized by maternal dietary choline supplementation. J Dev Origins Health Dis. 2012;3:1–8. doi: 10.1017/S2040174412000256. [DOI] [PubMed] [Google Scholar]
- 91.Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, et al. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 93.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, et al. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- 94.Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.150. e-pub ahead of print 16 August 2013. [DOI] [PMC free article] [PubMed]
- 95.Zambrano E, Martinez-Samayoa PM, Rodriguez-Gonzalez GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol. 2010;588:1791–1799. doi: 10.1113/jphysiol.2010.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 97.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention. Pregnancy Nutrition Surveillance 2011 Report. Department of Health and Human Services; Atlanta: 2012. [Google Scholar]
- 99.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 100.Sohlstrom A, Wahlund LO, Forsum E. Total body fat and its distribution during human reproduction as assessed by magnetic resonance imaging. Basic Life Sci. 1993;60:181–184. doi: 10.1007/978-1-4899-1268-8_41. [DOI] [PubMed] [Google Scholar]
- 101.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
- 102.Gilmore LA, Butte NF, Ravussin E, Han H, Redman LM. Energy intake, not an adaptation in energy expenditure, is a strong determinant of gestational weight gain (Abstract). Obesity; The 31st Annual Scientific Meeting of The Obesity Society; 2013. p. S72. [Google Scholar]
- 103.Kinnunen TI, Luoto R, Gissler M, Hemminki E. Pregnancy weight gain from 1960s to 2000 in Finland. Int J Obes Relat Metab Disord. 2003;27:1572–1577. doi: 10.1038/sj.ijo.0802471. [DOI] [PubMed] [Google Scholar]
- 104.Beyerlein A, Lack N, von Kries R. Within-population average ranges compared with Institute of Medicine recommendations for gestational weight gain. Obstet Gynecol. 2010;116:1111–1118. doi: 10.1097/AOG.0b013e3181f1ad8b. [DOI] [PubMed] [Google Scholar]
- 105.Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J. 2008;12:557–567. doi: 10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]
- 106.Ludwig DS, Rouse HL, Currie J. Pregnancy weight gain and childhood body weight: a within-family comparison. PLoS Med. 2013;10:e1001521. doi: 10.1371/journal.pmed.1001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 108.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91:1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142:1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Retnakaran R, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. Cmaj. 2012;184:1353–1360. doi: 10.1503/cmaj.111154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95:5365–5369. doi: 10.1210/jc.2010-0697. [DOI] [PubMed] [Google Scholar]
- 113.American Academy of Pediatrics Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 114.Horta BL, Bahl R, Martines JC, Victoria CG. [accessed 18 December 2013];Evidence for the long-term effects of breastfeeding: Systematic reviews and meta-analyses. http://www.who.intPublished2007.
- 115.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13:362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 117.Bogen DL, Hanusa BH, Whitaker RC. The effect of breast-feeding with and without formula use on the risk of obesity at 4 years of age. Obes Res. 2004;12:1527–1535. doi: 10.1038/oby.2004.190. [DOI] [PubMed] [Google Scholar]
- 118.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–1845. doi: 10.3945/ajcn.2008.27091. [DOI] [PubMed] [Google Scholar]
- 120.Oddy WH. Infant feeding and obesity risk in the child. Breastfeed Rev. 2012;20:7–12. [PubMed] [Google Scholar]
- 121.Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med. 2012;166:431–436. doi: 10.1001/archpediatrics.2011.1665. [DOI] [PubMed] [Google Scholar]
- 122.Li R, Fein SB, Grummer-Strawn LM. Association of breastfeeding intensity and bottle-emptying behaviors at early infancy with infants’ risk for excess weight at late infancy. Pediatrics. 2008;122:S77–S84. doi: 10.1542/peds.2008-1315j. [DOI] [PubMed] [Google Scholar]
- 123.Reynolds KD, Spruijt-Metz D. Translational research in childhood obesity prevention. Eval Health Prof. 2006;29:219–245. doi: 10.1177/0163278706287346. [DOI] [PubMed] [Google Scholar]
- 124.Rohrbach LA, Grana R, Sussman S, Valente TW. Type II translation: transporting prevention interventions from research to real-world settings. Eval Health Prof. 2006;29:302–333. doi: 10.1177/0163278706290408. [DOI] [PubMed] [Google Scholar]
- 125.Sussman S, Valente TW, Rohrbach LA, Skara S, Pentz MA. Translation in the health professions: converting science into action. Eval Health Prof. 2006;29:7–32. doi: 10.1177/0163278705284441. [DOI] [PubMed] [Google Scholar]
- 126.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 127.Pratt CA, Stevens J, Daniels S. Childhood obesity prevention and treatment: recommendations for future research. Am J Prev Med. 2008;35:249–252. doi: 10.1016/j.amepre.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sargent GM, Pilotto LS, Baur LA. Components of primary care interventions to treat childhood overweight and obesity: a systematic review of effect. Obes Rev. 2011;12:e219–e235. doi: 10.1111/j.1467-789X.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 129.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95:256–261. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- 131.Kolsgaard ML, Joner G, Brunborg C, Anderssen SA, Tonstad S, Andersen LF. Correction: ‘reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo adiposity intervention study—a hospital/public health nurse combined treatment’. BMC Pediatr. 2012;12:77. doi: 10.1186/1471-2431-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boutelle KN, Norman GJ, Rock CL, Rhee KE, Crow SJ. Guided self-help for the treatment of pediatric obesity. Pediatrics. 2013;131:e1435–e1442. doi: 10.1542/peds.2012-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of U.S. paediatric obesity primary care guidelines: two randomized trials. Pediatr Obes. 2012;7:28–38. doi: 10.1111/j.2047-6310.2011.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taveras EM, Gortmaker SL, Hohman KH, Horan CM, Kleinman KP, Mitchell K, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165:714–722. doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: how much weight change is necessary for normalization of weight status in children? JAMA Pediatr. 2013;167:21–26. doi: 10.1001/jamapediatrics.2013.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilfley DE, Kass AE, Kolko RP. Counseling and behavior change in pediatric obesity. Pediatr Clin North Am. 2011;58:1403–1424. x. doi: 10.1016/j.pcl.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93:4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 140.Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130:e1647–e1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- 141.Jelalian E, Lloyd-Richardson EE, Mehlenbeck RS, Hart CN, Flynn-O’Brien K, Kaplan J, et al. Behavioral weight control treatment with supervised exercise or peer-enhanced adventure for overweight adolescents. J Pediatr. 2010;157:923–928. e921. doi: 10.1016/j.jpeds.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124:1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 144.Tsiros MD, Sinn N, Coates AM, Howe PR, Buckley JD. Treatment of adolescent overweight and obesity. Eur J Pediatr. 2008;167:9–16. doi: 10.1007/s00431-007-0575-z. [DOI] [PubMed] [Google Scholar]
- 145.Snethen JA, Broome ME, Cashin SE. Effective weight loss for overweight children: a meta-analysis of intervention studies. J Pediatr Nurs. 2006;21:45–56. doi: 10.1016/j.pedn.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 146.Stitzel KF. Position of the American Dietetic Association: the roles of registered dietitians and dietetic technicians, registered in health promotion and disease prevention. J Am Diet Assoc. 2006;106:1875–1884. doi: 10.1016/j.jada.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 147.U S. Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125:361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 148.Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- 149.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 150.Wilfley DE, Van Buren DJ, Theim KR, Stein RI, Saelens BE, Ezzet F, et al. The use of biosimulation in the design of a novel multilevel weight loss maintenance program for overweight children. Obesity (Silver Spring) 2010;18 :S91–S98. doi: 10.1038/oby.2009.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80:1086–1096. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boutelle KN, Cafri G, Crow SJ. Parent predictors of child weight change in family based behavioral obesity treatment. Obesity (Silver Spring) 2012;20:1539–1543. doi: 10.1038/oby.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salvy SJ, Bowker JC, Germeroth L, Barkley J. Influence of peers and friends on overweight/obese youths’ physical activity. Exerc Sport Sci Rev. 2012;40:127–132. doi: 10.1097/JES.0b013e31825af07b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Epstein LH, Raja S, Daniel TO, Paluch RA, Wilfley DE, Saelens BE, et al. The built environment moderates effects of family-based childhood obesity treatment over 2 years. Ann Behav Med. 2012;44:248–258. doi: 10.1007/s12160-012-9383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med. 2013;45:228–236. doi: 10.1016/j.amepre.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Patrick K, Griswold WG, Raab F, Intille SS. Health and the mobile phone. Am J Prev Med. 2008;35:177–181. doi: 10.1016/j.amepre.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hekler EB, Klasnja P, Traver V, Hendriks M. Realizing effective behavioral management of health: the metamorphosis of behavioral science methods. IEEE Pulse. 2013;4:29–34. doi: 10.1109/MPUL.2013.2271681. [DOI] [PubMed] [Google Scholar]
- 158.Nilsen WJ, Pavel M. Moving behavioral theories into the 21st century: technological advancements for improving quality of life. IEEE Pulse. 2013;4:25–28. doi: 10.1109/MPUL.2013.2271682. [DOI] [PubMed] [Google Scholar]
- 159.Saranummi N, Spruijt-Metz D, Intille SS, Korhone I, Nilsen WJ, Pavel M. Moving the science of behavior change into the 21st century: novel solutions to prevent disease and promote health. IEEE Pulse. 2013;4:22–24. doi: 10.1109/MPUL.2013.2271680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Petersen JE, Shunturov V, Janda K, Platt G, Weinberger K. Dormitory residents reduce electricity consumption when exposed to real-time visual feedback and incentives. Int J Sustainability Higher Educ. 2007;8:16–33. [Google Scholar]
- 161.Kehrer P, Kelly K, Heffernan N. Does immediate feedback while doing homework improve learning?. The Twenty-Sixth International FLAIRS Conference; 22–24 May 2013; St. Pete Beach, FL, USA. 2013. [Google Scholar]
- 162.Peck SD, Raleigh DM, Stehle Werner JL. Improved class preparation and learning through immediate feedback in group testing for undergraduate nursing students. Nursing Edu Perspect. 2013;34:400–404. doi: 10.5480/11-507. [DOI] [PubMed] [Google Scholar]
- 163.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5:185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care. 2009;32:813–815. doi: 10.2337/dc08-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shapiro JR, Bauer S, Hamer RM, Kordy H, Ward D, Bulik CM. Use of text messaging for monitoring sugar-sweetened beverages, physical activity, and screen time in children: a pilot study. J Nutr Educ Behav. 2008;40:385–391. doi: 10.1016/j.jneb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Woolford SJ, Clark SJ, Strecher VJ, Resnicow K. Tailored mobile phone text messages as an adjunct to obesity treatment for adolescents. J Telemed Telecare. 2010;16:458–461. doi: 10.1258/jtt.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Niet J, Timman R, Bauer S, van den Akker E, de Klerk C, Kordy H, et al. Short message service reduces dropout in childhood obesity treatment: a randomized controlled trial. Health Psychol. 2012;31:797–805. doi: 10.1037/a0027498. [DOI] [PubMed] [Google Scholar]
- 168.O’Reilly GA, Spruijt-Metz D. Current mHealth technologies for physical activity assessment and promotion. Am J Prev Med. 2013;45:501–507. doi: 10.1016/j.amepre.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Emken BA, Li M, Thatte G, Lee S, Annavaram M, Mitra U, et al. Recognition of physical activities in overweight Hispanic youth using KNOWME Networks. J Phys Act Health. 2012;9:432–441. doi: 10.1123/jpah.9.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li M, Rozgica V, Thatte G, Lee S, Emken A, Annavaram M, et al. Multimodal physical activity recognition by fusing temporal and cepstral information. IEEE Trans Neural Syst Rehabil Eng. 2010;18:369–380. doi: 10.1109/TNSRE.2010.2053217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mitra U, Emken BA, Sangwon L. KNOWME: a case study in wireless body area sensor network design. Communications Magazine. 2012;50:116–125. [Google Scholar]
- 172.Tate EB, Spruijt-Metz D, O’Reilly G. MHealth approaches to child obesity prevention: successes, unique challenges, and next directions. Transl Behav Med. 2013;3:406–415. doi: 10.1007/s13142-013-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Toscos T, Faber A, An S, Gandhi MP. Chick clique: persuasive technology to motivate teenage girls to exercise. Proceedings of the 24th Annual ACM Conference on Human Factors in Computing Systems; New York, USA: ACM; 2006. pp. 1873–1878. [Google Scholar]
- 174.Hope Lab. . [accessed 3 January 2014]; http://www.hopelab.org/innovative-solutions/gditty/
- 175.Staiano AE, Calvert SL. Exergames for physical education courses: physical, social, and cognitive benefits. Child Dev Perspect. 2011;5:93–98. doi: 10.1111/j.1750-8606.2011.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barnett A, Cerin E, Baranowski T. Active video games for youth: a systematic review. J Phys Act Health. 2011;8:724–737. doi: 10.1123/jpah.8.5.724. [DOI] [PubMed] [Google Scholar]
- 177.Biddiss E, Irwin J. Active video games to promote physical activity in children and youth: a systematic review. Arch Pediatr Adolesc Med. 2010;164:664–672. doi: 10.1001/archpediatrics.2010.104. [DOI] [PubMed] [Google Scholar]
- 178.Peng W, Lin JH, Crouse J. Is playing exergames really exercising? A meta-analysis of energy expenditure in active video games. Cyberpsychol Behav Soc Netw. 2011;14:681–688. doi: 10.1089/cyber.2010.0578. [DOI] [PubMed] [Google Scholar]
- 179.Staiano AE, Abraham AA, Calvert SL. Adolescent exergame play for weight loss and psychosocial improvement: a controlled physical activity intervention. Obesity (Silver Spring) 2013;21:598–601. doi: 10.1038/oby.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Staiano AE, Calvert SL. Wii tennis play for low-income African American adolescents’ energy expenditure. Cyberpsychology. 2011;5:4. pii. [PMC free article] [PubMed] [Google Scholar]
- 181.Christison A, Khan HA. Exergaming for health: a community-based pediatric weight management program using active video gaming. Clin Pediatr (Phila) 2012;51:382–388. doi: 10.1177/0009922811429480. [DOI] [PubMed] [Google Scholar]
- 182.Chinapaw MJ, Proper KI, Brug J, van Mechelen W, Singh AS. Relationship between young peoples’ sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obes Rev. 2011;12:e621–e632. doi: 10.1111/j.1467-789X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 183.LeBlanc AG, Chaput JP, McFarlane A, Colley RC, Thivel D, Biddle SJ, et al. Active video games and health indicators in children and youth: a systematic review. PLoS One. 2013;8:e65351. doi: 10.1371/journal.pone.0065351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wagener TL, Fedele DA, Mignogna MR, Hester CN, Gillaspy SR. Psychological effects of dance-based group exergaming in obese adolescents. Pediatr Obes. 2012;7:e68–e74. doi: 10.1111/j.2047-6310.2012.00065.x. [DOI] [PubMed] [Google Scholar]
- 185.Gao Z, Hannan P, Xiang P, Stodden DF, Valdez VE. Video game-based exercise, Latino children’s physical health, and academic achievement. Am J Prev Med. 2013;44:S240–S246. doi: 10.1016/j.amepre.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 186.Baranowski T, Abdelsamad D, Baranowski J, O’Connor TM, Thompson D, Barnett A, et al. Impact of an active video game on healthy children’s physical activity. Pediatrics. 2012;129:e636–e642. doi: 10.1542/peds.2011-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Madsen KA, Yen S, Wlasiuk L, Newman TB, Lustig R. Feasibility of a dance videogame to promote weight loss among overweight children and adolescents. Arch Pediatr Adolesc Med. 2007;161:105–107. doi: 10.1001/archpedi.161.1.105-c. [DOI] [PubMed] [Google Scholar]
- 188.Maloney AE, Bethea TC, Kelsey KS, Marks JT, Paez S, Rosenberg AM, et al. A pilot of a video game (DDR) to promote physical activity and decrease sedentary screen time. Obesity (Silver Spring) 2008;16:2074–2080. doi: 10.1038/oby.2008.295. [DOI] [PubMed] [Google Scholar]
- 189.Maddison R, Foley L, Ni Mhurchu C, Jiang Y, Jull A, Prapavessis H, et al. Effects of active video games on body composition: a randomized controlled trial. Am J Clin Nutr. 2011;94:156–163. doi: 10.3945/ajcn.110.009142. [DOI] [PubMed] [Google Scholar]
- 190.Murphy EC, Carson L, Neal W, Baylis C, Donley D, Yeater R. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int J Pediatr Obes. 2009;4:205–214. doi: 10.3109/17477160902846187. [DOI] [PubMed] [Google Scholar]
- 191.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 192.Barkin SL, Finch SA, Ip EH, Scheindlin B, Craig JA, Steffes J, et al. Is office-based counseling about media use, timeouts, and firearm storage effective? Results from a cluster-randomized, controlled trial. Pediatrics. 2008;122:e15–e25. doi: 10.1542/peds.2007-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wake M, Baur LA, Gerner B, Gibbons K, Gold L, Gunn J, et al. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ. 2009;339:b3308. doi: 10.1136/bmj.b3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Resnicow K, McMaster F, Woolford S, Slora E, Bocian A, Harris D, et al. Study design and baseline description of the BMI2 trial: reducing paediatric obesity in primary care practices. Pediatr Obes. 2012;7:3–15. doi: 10.1111/j.2047-6310.2011.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- 196.Christie D, Hudson L, Mathiot A, Cole TJ, Karlsen S, Kessel A, et al. Assessing the efficacy of the Healthy Eating and Lifestyle Programme (HELP) compared with enhanced standard care of the obese adolescent in the community: study protocol for a randomized controlled trial. Trials. 2011;12:242. doi: 10.1186/1745-6215-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011;(12):CD001871. doi: 10.1002/14651858.CD001871.pub3. [DOI] [PubMed] [Google Scholar]
- 198.Martin LA, Ariza AJ, Thomson JS, Binns HJ. Seconds for care: evaluation of five health supervision visit topics using a new method. J Pediatr. 2008;153:706–711. 711 e701–702. doi: 10.1016/j.jpeds.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 199.Black JA, White B, Viner RM, Simmons RK. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev. 2013;14:634–644. doi: 10.1111/obr.12037. [DOI] [PubMed] [Google Scholar]
- 200.Howden LM, Meyer JA. 2010 Census Briefs. United States Census Bureau; 2011. Age and sex composition: 2010. [Google Scholar]
- 201.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–2883. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 202.Berkowitz RI, Wadden TA, Gehrman CA, Bishop-Gilyard CT, Moore RH, Womble LG, et al. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring) 2011;19:1193–1199. doi: 10.1038/oby.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dooyema CA, Belay B, Foltz JL, Williams N, Blanck HM. The childhood obesity research demonstration project: a comprehensive community approach to reduce childhood obesity. Child Obes. 2013;9:454–459. doi: 10.1089/chi.2013.0060. [DOI] [PubMed] [Google Scholar]
- 204.Fowler-Brown A, Kahwati LC. Prevention and treatment of overweight in children and adolescents. Am Fam Physician. 2004;69:2591–2598. [PubMed] [Google Scholar]
- 205.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009:CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 206.Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 207.von Spranger J. Phentermine resinate in obesity. Clinical trial of Mirapront in adipose chldren. Munch Med Wochenschr. 1965;38:1833–1834. [PubMed] [Google Scholar]
- 208.Andelman MB, Jones C, Nathan S. Treatment of obesity in underprivileged adolescents. Comparison of diethylpropion hydrochloride with placebo in a double-blind study. Clin Pediatr (Phila) 1967;6:327–330. doi: 10.1177/000992286700600607. [DOI] [PubMed] [Google Scholar]
- 209.Dolecek R. Endocrine studies with mazindol in obese patients. Pharmatherapeutica. 1980;2:309–316. [PubMed] [Google Scholar]
- 210.Golebiowska M, Chlebna-Sokol D, Kobierska I, Konopinska A, Malek M, Mastalska A, et al. Clinical evaluation of Teronac (mazindol) in the treatment of obesity in children. Part II. Anorectic properties and side effects (author’s transl) Przegl Lek. 1981;38:355–358. [PubMed] [Google Scholar]
- 211.Golebiowska M, Chlebna-Sokol D, Mastalska A, Zwaigzne-Raczynska J. The clinical evaluation of teronac (Mazindol) in the treatment of children with obesity. Part I. Effect of the drug on somatic patterns and exercise capacity (author’s transl) Przegl Lek. 1981;38:311–314. [PubMed] [Google Scholar]
- 212.Komorowski JM, Zwaigzne-Raczynska J, Owczarczyk I, Golebiowska M, Zarzycki J. Effect of mazindol (teronac) on various hormonal indicators in children with simple obesity. Pediatr Pol. 1982;57:241–246. [PubMed] [Google Scholar]
- 213.Samanin R, Garattini S. Neurochemical mechanism of action of anorectic drugs. Pharmacol Toxicol. 1993;73:63–68. doi: 10.1111/j.1600-0773.1993.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 214.Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Arch Dis Child. 1966;41:309–312. doi: 10.1136/adc.41.217.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stewart DA, Bailey JD, Patell H. Tenuate dospan as an appetitie suppressant in the treatment of obese children. Appl Ther. 1970;12:34–36. [PubMed] [Google Scholar]
- 216.Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, et al. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab. 2011;96:837–845. doi: 10.1210/jc.2010-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 218.O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 219.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 220.Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev. 2013;14:383–392. doi: 10.1111/obr.12015. [DOI] [PubMed] [Google Scholar]
- 221.Eisai Inc. BELVIQ tablets, for oral use. 2013 [Google Scholar]
- 222.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 223.Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32:822–834. doi: 10.1038/sj.npp.1301155. [DOI] [PubMed] [Google Scholar]
- 224.Becker EA, Shafer A, Anderson R. Weight changes in teens on psychotropic medication combinations at Austin State Hospital. Tex Med. 2005;101:62–70. [PubMed] [Google Scholar]
- 225.Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ. Open trial of bupropion SR in adolescent major depression. J Child Adolesc Psychiatr Nurs. 2003;16:123–130. doi: 10.1111/j.1744-6171.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 226.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- 227.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 228.Bacon GE, Lowrey GH. A clinical trial of fenfluramine in obese children. Curr Ther Res Clin Exp. 1967;9:626–630. [PubMed] [Google Scholar]
- 229.Goldstein DJ, Rampey AH, Jr, Enas GG, Potvin JH, Fludzinski LA, Levine LR. Fluoxetine: a randomized clinical trial in the treatment of obesity. Int J Obes Relat Metab Disord. 1994;18:129–135. [PubMed] [Google Scholar]
- 230.Malecka-Tendera E, Koehler B, Muchacka M, Wazowski R, Trzciakowska A. Efficacy and safety of dexfenfluramine treatment in obese adolescents. Pediatr Pol. 1996;71:431–436. [PubMed] [Google Scholar]
- 231.Pedrinola F, Cavaliere H, Lima N, Medeiros-Neto G. Is DL-fenfluramine a potentially helpful drug therapy in overweight adolescent subjects? Obes Res. 1994;2:1–4. doi: 10.1002/j.1550-8528.1994.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 232.Pedrinola F, Sztejnsznajd C, Lima N, Halpern A, Medeiros-Neto G. The addition of dexfenfluramine to fluoxetine in the treatment of obesity: a randomized clinical trial. Obes Res. 1996;4:549–554. doi: 10.1002/j.1550-8528.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 233.Rauh JL, Lipp R. Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double-blind study of 30 teen-agers. Clin Pediatr (Phila) 1968;7:138–140. doi: 10.1177/000992286800700305. [DOI] [PubMed] [Google Scholar]
- 234.Glauser TA, Dlugos DJ, Dodson WE, Grinspan A, Wang S, Wu SC. Topiramate monotherapy in newly diagnosed epilepsy in children and adolescents. J Child Neurol. 2007;22:693–699. doi: 10.1177/0883073807303997. [DOI] [PubMed] [Google Scholar]
- 235.Ferraro D, Di Trapani G. Topiramate in the prevention of pediatric migraine: literature review. J Headache Pain. 2008;9:147–150. doi: 10.1007/s10194-008-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Canitano R. Clinical experience with Topiramate to counteract neuroleptic induced weight gain in 10 individuals with autistic spectrum disorders. Brain Dev. 2005;27:228–232. doi: 10.1016/j.braindev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 237.Carter GT, Yudkowsky MP, Han JJ, McCrory MA. Topiramate for weight reduction in Duchenne muscular dystrophy. Muscle Nerve. 2005;31:788–789. doi: 10.1002/mus.20321. [DOI] [PubMed] [Google Scholar]
- 238.Lessig MC, Shapira NA, Murphy TK. Topiramate for reversing atypical anti-psychotic weight gain. J Am Acad Child Adolesc Psychiatry. 2001;40:1364. doi: 10.1097/00004583-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 239.Pavuluri MN, Janicak PG, Carbray J. Topiramate plus risperidone for controlling weight gain and symptoms in preschool mania. J Child Adolesc Psychopharmacol. 2002;12:271–273. doi: 10.1089/104454602760386978. [DOI] [PubMed] [Google Scholar]
- 240.Nathan PJ, O’Neill BV, Napolitano A, Bullmore ET. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther. 2011;17:490–505. doi: 10.1111/j.1755-5949.2010.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fountain NB. A pregnant pause to consider teratogenicity of topiramate. Epilepsy Curr. 2009;9:36–38. doi: 10.1111/j.1535-7511.2008.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Roberts MD. US Food and Drug Administration Endocrinologic and Metabolic Drugs Advisory Committee Clinical Briefing Document February 22, 2012. VIVUS, Inc; 2012. [Google Scholar]
- 243.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 245.Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145:81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. [DOI] [PubMed] [Google Scholar]
- 246.Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- 247.Budd GM, Hayman LL, Crump E, Pollydore C, Hawley KD, Cronquist JL, et al. Weight loss in obese African American and Caucasian adolescents: secondary analysis of a randomized clinical trial of behavioral therapy plus sibutramine. J Cardiovasc Nurs. 2007;22:288–296. doi: 10.1097/01.JCN.0000278959.75253.1d. [DOI] [PubMed] [Google Scholar]
- 248.Daniels SR, Long B, Crow S, Styne D, Sothern M, Vargas-Rodriguez I, et al. Cardiovascular effects of sibutramine in the treatment of obese adolescents: results of a randomized, double-blind, placebo-controlled study. Pediatrics. 2007;120:e147–e157. doi: 10.1542/peds.2006-2137. [DOI] [PubMed] [Google Scholar]
- 249.Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab. 2007;92:4101–4106. doi: 10.1210/jc.2007-0826. [DOI] [PubMed] [Google Scholar]
- 250.Garcia-Morales LM, Berber A, Macias-Lara CC, Lucio-Ortiz C, Del-Rio-Navarro BE, Dorantes-Alvarez LM. Use of sibutramine in obese mexican adolescents: a 6-month, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther. 2006;28:770–782. doi: 10.1016/j.clinthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 251.Godoy-Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L, et al. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2005;90:1460–1465. doi: 10.1210/jc.2004-0263. [DOI] [PubMed] [Google Scholar]
- 252.Reisler G, Tauber T, Afriat R, Bortnik O, Goldman M. Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J. 2006;8:30–32. [PubMed] [Google Scholar]
- 253.Van Mil EG, Westerterp KR, Kester AD, Delemarre-van de Waal HA, Gerver WJ, Saris WH. The effect of sibutramine on energy expenditure and body composition in obese adolescents. J Clin Endocrinol Metab. 2007;92:1409–1414. doi: 10.1210/jc.2006-0264. [DOI] [PubMed] [Google Scholar]
- 254.Violante-Ortiz R, Del-Rio-Navarro BE, Lara-Esqueda A, Perez P, Fanghanel G, Madero A, et al. Use of sibutramine in obese Hispanic adolescents. Adv Ther. 2005;22:642–649. doi: 10.1007/BF02849958. [DOI] [PubMed] [Google Scholar]
- 255.Dunican KC, Desilets AR, Montalbano JK. Pharmacotherapeutic options for overweight adolescents. Ann Pharmacother. 2007;41:1445–1455. doi: 10.1345/aph.1K022. [DOI] [PubMed] [Google Scholar]
- 256.Wald AB, Uli NK. Pharmacotherapy in pediatric obesity: current agents and future directions. Rev Endocr Metab Disord. 2009;10:205–214. doi: 10.1007/s11154-009-9111-y. [DOI] [PubMed] [Google Scholar]
- 257.Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab. 2013;304:E677–E685. doi: 10.1152/ajpendo.00446.2012. [DOI] [PubMed] [Google Scholar]
- 258.Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012;20:364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Maahs D, de Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18–28. doi: 10.4158/EP.12.1.18. [DOI] [PubMed] [Google Scholar]
- 261.Mathis LL. [accessed 05 November 2013];Orlistat Update. 2010 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM205380.pdf.
- 262.McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE, et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab. 2004;17:307–319. doi: 10.1515/jpem.2004.17.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS, et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res. 2002;10:642–650. doi: 10.1038/oby.2002.87. [DOI] [PubMed] [Google Scholar]
- 264.Norgren S, Danielsson P, Jurold R, Lotborn M, Marcus C. Orlistat treatment in obese prepubertal children: a pilot study. Acta Paediatr. 2003;92:666–670. doi: 10.1080/08035250310002353. [DOI] [PubMed] [Google Scholar]
- 265.Ozkan B, Bereket A, Turan S, Keskin S. Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr. 2004;163:738–741. doi: 10.1007/s00431-004-1534-6. [DOI] [PubMed] [Google Scholar]
- 266.Zhi J, Moore R, Kanitra L. The effect of short-term (21-day) orlistat treatment on the physiologic balance of six selected macrominerals and microminerals in obese adolescents. J Am Coll Nutr. 2003;22:357–362. doi: 10.1080/07315724.2003.10719318. [DOI] [PubMed] [Google Scholar]
- 267.McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22:814–822. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- 268.Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 269.Mehnert H. Metformin, the rebirth of a biguanide: mechanism of action and place in the prevention and treatment of insulin resistance. Exp Clin Endocrinol Diabetes. 2001;109:S259–S264. doi: 10.1055/s-2001-18587. [DOI] [PubMed] [Google Scholar]
- 270.Bestermann W, Houston MC, Basile J, Egan B, Ferrario CM, Lackland D, et al. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci. 2005;329:292–305. doi: 10.1097/00000441-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 271.Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L, et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18:761–768. doi: 10.1515/jpem.2005.18.8.761. [DOI] [PubMed] [Google Scholar]
- 272.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 273.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyper-insulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–348. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 274.Bjorkhem-Bergman L, Asplund AB, Lindh JD. Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol. 2011;25:299–305. doi: 10.1177/0269881109353461. [DOI] [PubMed] [Google Scholar]
- 275.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–246. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 276.Clarson CL, Mahmud FH, Baker JE, Clark HE, McKay WM, Schauteet VD, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine. 2009;36:141–146. doi: 10.1007/s12020-009-9196-9. [DOI] [PubMed] [Google Scholar]
- 277.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 278.Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM, et al. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes Relat Metab Disord. 2007;31:15–22. doi: 10.1038/sj.ijo.0803453. [DOI] [PubMed] [Google Scholar]
- 279.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ibanez L, de Zegher F. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89:1592–1597. doi: 10.1210/jc.2003-031281. [DOI] [PubMed] [Google Scholar]
- 281.Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–2079. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- 282.Legro RS. Impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women: do we need a new drug? J Clin Endocrinol Metab. 2008;93:4218–4220. doi: 10.1210/jc.2008-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mastorakos G, Koliopoulos C, Deligeoroglou E, Diamanti-Kandarakis E, Creatsas G. Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril. 2006;85:420–427. doi: 10.1016/j.fertnstert.2005.07.1306. [DOI] [PubMed] [Google Scholar]
- 284.Rezvanian H, Hashemipour M, Kelishadi R, Tavakoli N, Poursafa P. A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity. World J Pediatr. 2010;6:317–322. doi: 10.1007/s12519-010-0232-x. [DOI] [PubMed] [Google Scholar]
- 285.Wiegand S, l’Allemand D, Hubel H, Krude H, Burmann M, Martus P, et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur J Endocrinol. 2010;163:585–592. doi: 10.1530/EJE-10-0570. [DOI] [PubMed] [Google Scholar]
- 286.Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164:116–123. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–485. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gambineri A, Patton L, De Iasio R, Cantelli B, Cognini GE, Filicori M, et al. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3854–3862. doi: 10.1210/jc.2004-2490. [DOI] [PubMed] [Google Scholar]
- 289.De Waele K, Ishkanian SL, Bogarin R, Miranda CA, Ghatei MA, Bloom SR, et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur J Endocrinol. 2008;159:381–388. doi: 10.1530/EJE-08-0462. [DOI] [PubMed] [Google Scholar]
- 290.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 291.Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 292.Lustig RH, Rose SR, Burghen GA, Velasquez-Mieyer P, Broome DC, Smith K, et al. Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J Pediatr. 1999;135:162–168. doi: 10.1016/s0022-3476(99)70017-x. [DOI] [PubMed] [Google Scholar]
- 293.Dietz J, Schwartz J. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism. 1991;40:800–806. doi: 10.1016/0026-0495(91)90006-i. [DOI] [PubMed] [Google Scholar]
- 294.Eden Engstrom B, Burman P, Holdstock C, Karlsson FA. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J Clin Endocrinol Metab. 2003;88:5193–5198. doi: 10.1210/jc.2003-030713. [DOI] [PubMed] [Google Scholar]
- 295.Gregory JW, Greene SA, Jung RT, Scrimgeour CM, Rennie MJ. Changes in body composition and energy expenditure after six weeks’ growth hormone treatment. Arch Dis Child. 1991;66:598–602. doi: 10.1136/adc.66.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hoos MB, Westerterp KR, Gerver WJ. Short-term effects of growth hormone on body composition as a predictor of growth. J Clin Endocrinol Metab. 2003;88:2569–2572. doi: 10.1210/jc.2002-021633. [DOI] [PubMed] [Google Scholar]
- 297.Snel YE, Doerga ME, Brummer RJ, Zelissen PM, Zonderland ML, Koppeschaar HP. Resting metabolic rate, body composition and related hormonal parameters in growth hormone-deficient adults before and after growth hormone replacement therapy. Eur J Endocrinol. 1995;133:445–450. doi: 10.1530/eje.0.1330445. [DOI] [PubMed] [Google Scholar]
- 298.Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87:1581–1585. doi: 10.1210/jcem.87.4.8414. [DOI] [PubMed] [Google Scholar]
- 299.Hoybye C, Hilding A, Jacobsson H, Thoren M. Growth hormone treatment improves body composition in adults with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2003;58:653–661. doi: 10.1046/j.1365-2265.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 300.Myers SE, Davis A, Whitman BY, Santiago JV, Landt M. Leptin concentrations in Prader-Willi syndrome before and after growth hormone replacement. Clin Endocrinol (Oxf) 2000;52:101–105. doi: 10.1046/j.1365-2265.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 301.Shadid S, Jensen MD. Effects of growth hormone administration in human obesity. Obes Res. 2003;11:170–175. doi: 10.1038/oby.2003.27. [DOI] [PubMed] [Google Scholar]
- 302.Seo DC, Sa J. A meta-analysis of obesity interventions among U.S. minority children. J Adolesc Health. 2010;46:309–323. doi: 10.1016/j.jadohealth.2009.11.202. [DOI] [PubMed] [Google Scholar]
- 303.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011:S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 305.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolescent Med. 2012;166:1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 306.Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127:402–410. doi: 10.1542/peds.2010-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes Relat Metab Disord. 2008;32:S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 308.Inge TH, Zeller MH, Lawson ML, Daniels SR. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr. 2005;147:10–19. doi: 10.1016/j.jpeds.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 309.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Study. JAMA Pediatr. 2014;168:47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Inge TH, Jenkins TM, Zeller M, Dolan L, Daniels SR, Garcia VF, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156:103–108. e101. doi: 10.1016/j.jpeds.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Olbers T, Gronowitz E, Werling M, Marlid S, Flodmark CE, Peltonen M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS) Int J Obes Relat Metab Disord. 2012;36:1388–1395. doi: 10.1038/ijo.2012.160. [DOI] [PubMed] [Google Scholar]
- 312.Kalra M, Mannaa M, Fitz K, Kumar S, Chakraborty R, Sheng X, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obes Surg. 2008;18:675–679. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 313.Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–222. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 314.Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity (Silver Spring) 2009;17:985–990. doi: 10.1038/oby.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7:727–732. doi: 10.1016/j.soard.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Miyano G, Jenkins TM, Xanthakos SA, Garcia VF, Inge TH. Perioperative outcome of laparoscopic Roux-en-Y gastric bypass: a children’s hospital experience. J Pediatr Surg. 2013;48:2092–2098. doi: 10.1016/j.jpedsurg.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Michalsky M, Kramer RE, Fullmer MA, Polfuss M, Porter R, Ward-Begnoche W, et al. Developing criteria for pediatric/adolescent bariatric surgery programs. Pediatrics. 2011;128:S65–S70. doi: 10.1542/peds.2011-0480F. [DOI] [PubMed] [Google Scholar]
- 318.Pratt JS, Lenders CM, Dionne EA, Hoppin AG, Hsu GL, Inge TH, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009;17:901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]