Abstract
As we approach the end of two decades of leptin research, the comparative biology of leptin is just beginning. We now have several leptin orthologs described from nearly every major clade among vertebrates, and are moving beyond gene descriptions to functional studies. Even at this early stage, it is clear that non-mammals display clear functional similarities and differences with their better-studied mammalian counterparts. This review assesses what we know about leptin function in mammals and non-mammals, and gives examples of how these data can inform leptin biology in humans.
Introduction
Nine years have passed since the first leptin sequence was identified in a non-mammalian vertebrate (Takifugu rubripes; Kurokawa et al., 2005). Due largely to advances in genomic technology, leptin and leptin receptor genes now have been cloned from all the major vertebrate classes, with the possible exception of Aves (Figure 1). Although non-mammal leptin studies still comprise ~ 1% of all leptin studies (Web of Science returns ~29,000 leptin studies (non-reviews or editorials); <300 of those focus on non-mammals), there is now a sufficient body of literature to approach the question of whether leptin function is conserved among vertebrates (Figure 2). Comparative study of leptins has the goal of not only solving comparative questions, but also clarifying our understanding of leptin function in mammals (by uncovering the origin of leptin function). Thus in this review we describe recent advances of leptin biology in both comparative and biomedical contexts.
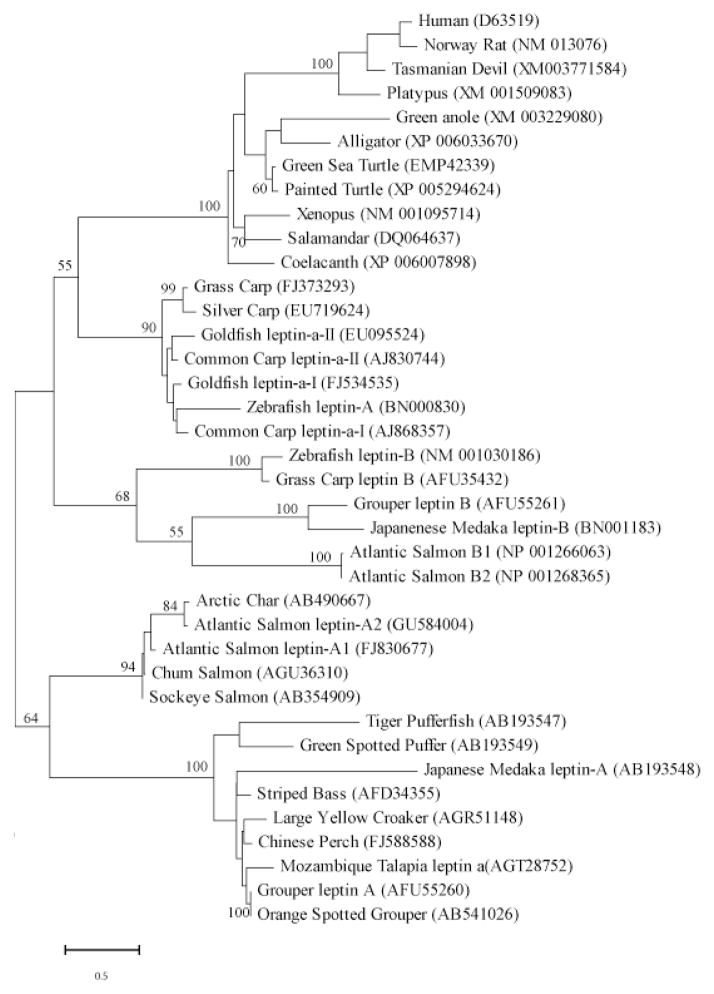
Figure 1. Phylogenetic tree of 29 boney fish and representative tetrapod leptins.
The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al., 1992) as conducted in MEGA5 (Tamura et al., 2011). Numbers at nodes represent percentage of 100 bootstrap replicates. Notes with no number indicate bootstrap support of less than 50%. Inferred leptin amino acid sequences were manually aligned in MEGA5 informed by protein structural homologies. GenBank accession numbers in parentheses represent protein accessions.
Figure 2. Diversity of organisms in which leptin physiology has been studied from 1990s on-ward.
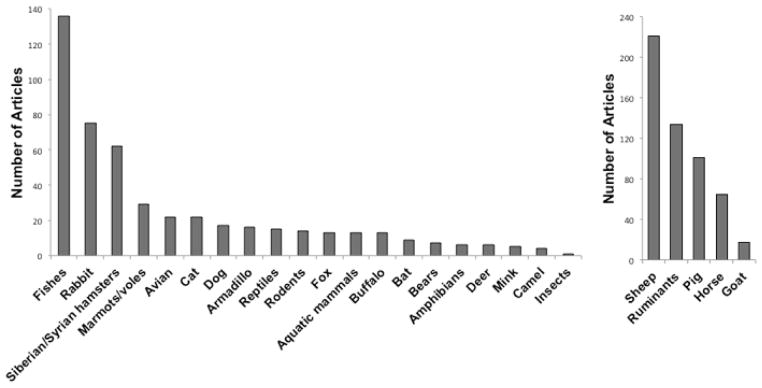
We resolved the number of published studies on leptin in non-model organisms and agricultural organisms resulting from searches conducted in Web of Science. More than 15,000 articles involving biomedical model organisms (rat, mouse, human, non-human primate). When searching for articles involving leptin in non-model and agricultural organisms, each article that was listed using various search terms was inspected to verify the primary organism(s) studied. We did not include zebrafish or Xenopus in biomedical models even though they are genetic and developmental model organisms, nor did we include chicken or fishes reared in fisheries or chicken as agricultural organisms because these organisms better reflect phylogenetic diversity of leptin in this context. For non-model organisms, we included studies that used both homologous and heterologous leptins or leptin probes in our lists.
Leptin’s Tertiary Structure is Conserved among Vertebrates
One aspect that both aided and hindered leptin research in non-mammalian systems was its structure. Early studies assumed conserved primary structure between mammalian and fish leptins, and thus interpreted fish immunoreactivity with anti-mouse leptin antibodies as evidence of leptin expression (e.g. Johnson et al., 2000). However, traditional redundant-primer strategies were unsuccessful at amplifying a fish leptin for over a decade. Kurokawa’s insight of searching for leptin in a conserved pattern of genes, or gene synteny, finally resulted in a true non-mammal leptin gene from a fish genome (Kurokawa et al., 2005). With this breakthrough, the nonintuitive idea that tertiary structure was conserved even though primary sequence was not gained support with each new leptin clone (Crespi and Denver, 2006; Denver et al., 2011; Gorissen et al., 2009; He et al., 2013; Prokop et al., 2012). Leptin orthologues are now described for all major classes of vertebrates, (with the exception of birds (Figure 1)), and all are predicted to have a very similar, class I helical cytokine tertiary structure. It is important to note, however, that crystal structure has been determined for only a single leptin, and that structure was for a leptin modified to enhance crystal formation (human e-100 leptin; Zhang et al., 1997). All non-mammal leptin structures (and most mammal leptin structures) are inferred by modeling algorithms; an empirical determination of a non-mammal leptin structure is needed to validate the computationally derived models.
Basal Vertebrates Express Multiple Leptin Orthologs
All mammals (e.g. Ball et al., 2013; Clarke et al., 2001; Comuzzie et al., 1997; Zhang et al., 1994) and amphibians (Boswell et al., 2006; Crespi and Denver, 2006) express a single ortholog of leptin (lep). Two lep orthologues are present in the green anole (lizard) genome, but only one may be expressed (Denver et al., 2011). The presence of multiple orthologs within a genome is generally attributed to genome and/or gene duplication (Gorissen et al., 2009; Kurokawa and Murashita, 2009; Ronnestad et al., 2010). Fish leptins are, by far, the best-studied among non-mammal leptins (Figure 2). Initially our group proposed that all fishes express two lep paralogs (reviewed by Copeland et al., 2011), with the possible exception of Fugu rubripes (Kurokawa et al., 2005). Now, more recent work indicates that some advanced fishes (including Fugu and other Percomorphs) lost the second lep B ortholog (striped bass Morone saxatilis and stickleback Gasterosteus aculeatus; Won et al., 2012, and chinese perch Siniperca chuatsi; He et al., 2013). The scenario of vertebrates expressing a single paralog after the Percomorph split (advanced by Won et al., 2012) is contradicted by two leptin paralogs in more-derived groups (orange-spotted grouper Epinephelus coioides (Zhang et al., 2013) and green anole (Denver et al., 2011)).
Leptin orthologs now are identified for many different vertebrate taxa separated by considerable evolutionary time (Figure 1). Given the caveat that the number of orthologs per species is often revised (up) as each genome is analyzed (e.g. initial estimates in salmonids did not recognize a leptin B; Angotzi et al., 2013), we can state that at least some advanced fish taxa express a single leptin ortholog (e.g. Takifugu, given that its genome was among the first to be completed and the extent to which it is annotated) and some advanced fish taxa express two leptins (grouper; Zhang et al., 2013). Adding to the confusion, the two leptins present in some fish lineages (carp, goldfish, and salmon) should not be considered homologous to the A and B leptins that appear to be the result of a deep divergence in bony fishes. Rather these are the result of much more recent duplications of leptin A in these lineages, hence the labels leptin a-1 (A1) and a-II (A2). Hereafter, A and B will refer to the presumed deep orthologs of leptin as represented by the two copies of leptins in zebrafish and Japanese Medaka. Similarly the green anole paralogs are the result of a recent gene duplication and thus not homologous to both A and B leptins of fish (Denver et al., 2011).
Are the ‘A’ and ‘B’ leptin paralogs functionally distinct? Although the tissue distribution pattern of A and B are discrete (suggesting functional differences, (Angotzi et al., 2013; Gorissen et al., 2009), a great majority of fish studies exclusively focus on leptin A (Dalman et al., 2013; Frøiland et al., 2010; Huising et al., 2006; Liu et al., 2012). To date, functional study of leptin B has been limited to expression studies (responds to food restriction- Gorissen et al., 2009; increases during early development- Angotzi et al., 2013), and all manipulations using species-specific recombinant leptins have used leptin A (Dalman et al., 2013; Liu et al., 2012; Lu et al., 2012; Murashita et al., 2011, 2008).
It may be that leptin B contributes little to leptin function. Relative qPCR cannot determine copy number among different mRNAs (e.g. leptin A vs. leptin B), only relative differences within a given mRNA (Ball et al., 2013). Thus studies using relative qPCR would not identify if a gene were expressed at very low mRNA levels overall, just relative differences among treatments. Using absolute qPCR, we estimate that leptin B copy number is at least 10x lower than leptin A in zebrafish (Ball and Londraville, unpublished). Similarly, leptin 2 in Anolis cannot be amplified by RT-PCR (Boorse and Libbon, J.V., 2010), although it is undetermined whether lep 2 in Anolis is homologous to leptin B in fish. Finally, the binding energy of leptin interacting with its receptor (in silico simulations) is an order of magnitude higher for A vs. B in both zebrafish and Medaka (Prokop et al., 2012).
Given that all tetrapods express a single ortholog of leptin (n.b.-Aves may not express any leptin ortholog), and many more ancestral vertebrates express two or more leptins, which of the ancestral orthologs is the homolog to tetrapod leptin (particularly mammalian leptin)? Gorissen et al. (2009) argued for leptin B based on its exon structure and gene synteny. However, recent analyses clearly indicate that the synteny associated with mammalian leptin is parsed between A and B paralogs in ancestral vertebrates (with rbm28 associating with lep A and snd1, lrrc4, and impdh genes associating with lep B; Denver et al., 2011; Won et al., 2012). Further, some phylogenetic analyses of leptin sequences favor A over B as the mammalian homolog, but with weak bootstrap support (He et al., 2013; Zhang et al., 2013). Our phylogeny cannot resolve which leptin ortholog is most similar to tetrapod leptin, and other approaches (Neighbor Joining, Maximum Likelihood, Maximum Parsimony, multiple assumptions of sequence evolution) return essentially the same tree. Even inclusion of the recently identified coelacanth leptin sequence (Figure 1) has yielded little resolution, as this sequence has very clear affinities to tetrapods rather than fish. Clearly, more leptin genes among more diverse taxa, and more functional work on the physiology of leptin B is needed to determine the origin of the tetrapod leptins.
The leptin/leptin receptor complex
LepRb is the only isoform that can activate Jak/STAT signaling through its intracellular domain (reviewed in Denver et al., 2011). A vast majority of the work done on non-mammal leptin receptors concentrates on the long-form of the leptin receptor (lepRb), although multiple leptin receptors are expressed throughout vertebrates (Cao et al., 2011; Ronnestad et al., 2010). Recent simulation (Prokop et al., 2012), crystal structure of the leptin receptor (Carpenter et al., 2012) and cryo-electron microscopy imaging (Mancour et al., 2012) studies identify the extracellular cytokine homology region (CHR2 domain) as the site of leptin/leptin receptor binding. Two lepRb molecules cross over each other, each binding a separate leptin molecule to form an “X” quaternary structure (Mancour et al., 2012). Mutations that disrupt this quaternary structure eliminate leptin signaling (Peelman et al., 2006). Prokop et al. (2012) did a comparative analysis of leptin and leptin receptor interaction across vertebrates. They identified several instances of potential coevolution between receptor and ligand within the CHR2 domain. In macaque, platypus, green anole, Xenopus, and guinea pig, amino acid substitutions (relative to human) in the binding site of the ligand were accompanied by compensatory changes in the receptor. The authors hypothesize that these substitutions served to stabilize the interaction and maintain predicted binding energy across taxa.
Given that the binding site for leptin to its receptor is well established, and that it is conserved across diverse taxa (Hammond et al., 2012; Prokop et al., 2012; Ronnestad et al., 2010) it can now be used as another character to evaluate new leptin clones. In the Prokop et al. (2012) in-silico evaluation of 35 taxa, only bird leptins did not form stable complexes with their homologous receptors. Leptin receptors are represented in many bird genomes (chicken CGNC:49091; mallard NW_004677703.1; zebra finch ENSTGUG00000010030.1; turkey NC_015020.1; rock pigeon LOC102098873; saker falcon LOC102049003; peregrine falcon 101921754; collared flycatcher NC_021680.1; medium ground finch LOC102041765; white-throated sparrow NW_005081684.1). However, the ligand that binds to these receptors has been notoriously difficult to find, even in the face of considerable effort. The original reports of chicken leptin (Taouis et al., 1998) cannot be independently verified (Friedman-Einat et al., 1999; Pitel et al., 2000; Sharp et al., 2008). In addition, many of the genes expected to be found in synteny with leptin are missing from current builds of the chicken genome, and from chicken EST libraries; in sum there are considerable and varied data that suggest that chickens do not express leptin (Pitel et al., 2010). Is leptin missing from all bird genomes? The zebra finch genome project has identified a partial leptin transcript (XM_004175791) that retains features of leptin primary structure consistent with predicted phylogenetic distance for birds (unlike the reported chicken leptin; Prokop et al. unpublished results). Binding analyses, expression studies, and functional data will be needed before we know if a true bird leptin has been cloned, and how (if) it differs from other vertebrate leptins.
Leptin functional diversity
Given the diverse life histories, physiologies, and ecologies of the organisms with leptin clones, the functional contexts in which leptin is being studied has greatly expanded, and will continue to expand with new transcriptomes and genomes sequenced each year. Thus far, the overwhelming majority (47%) of studies conducted in non-model organisms have focused on leptin’s function as an adipostat and anorexigen, followed by reproductive function (16%; Figure 3). This trend mirrors the research focus using biomedical models during the first years of leptin research. However, there is a disproportionately large community of researchers investigating leptin-immune system interactions in non-model organisms (12.6%) relative to biomedical models (1.7%), which highlights particular interests in this field of leptin research (see recent reviews by Carlton et al., 2012; French et al., 2011). In addition, because scientists studying these non-model organisms are following their own historical species-specific research interests, we see different foci of leptin research within taxa. For example, we see studies investigating leptin’s role in thermogenesis in the high-elevation Plateau pika (Yang et al., 2011) or vole (Chen et al., 2012), seasonality in Siberian hamsters and bears (Adam and Mercer, 2004; Demas, 2004; Gardi et al., 2011), and in embryonic or larval development in zebrafish (Liu et al., 2012, 2010) and Xenopus (Crespi and Denver, 2006). In the following sections, we highlight what we currently know about leptin function across vertebrates, and we integrate these insights with recent findings within the biomedical literature to provide a prospectus of exciting, future research directions in this field.
Figure 3. Diversity of leptin functions studied in biomedical model organisms, agricultural species, and non-model organisms.

(see definition of categories in Figure 1).
Articles retrieved by Web of Science searches were saved to EndNote and categorized by their various general animal names as represented in the figures of the papers. For the non-model and agricultural organisms the articles were reviewed individually and organized into their specific research area. We included studies investigating the role of leptin in the timing of puberty in the Life History Transitions category. Due to the large numbers of biomedical studies, they were sorted into categories based on keywords associated with the article.
Leptin as an anorexigen and adipostat
Administration of exogenous leptin (either homologous or heterologous) reduces food intake in mammals, birds, amphibians, lizards, and fishes (Table 1A); as such leptin’s function as an anorexigen may be the only function that is shared among vertebrates (without qualifying statements). These studies were some of the first done in a comparative context, as this question was arguably the foremost in a comparative scientist’s mind (what motivates organisms to feed). In many cases these studies were done before homologous recombinant protein was available (e.g. Londraville and Duvall, 2002), although non-homologous leptins are still used in feeding studies (Table 1A). Given the fact that leptin primary structure is highly variable, many researchers rightly argued for caution in interpreting studies that use heterologous leptins (Denver et al., 2011; Murashita et al., 2008). One irony is that the artifactual chicken leptin has been used in feeding studies (Lõhmus et al., 2006) even though it does not form a stable complex with the chicken leptin receptor (Prokop et al., 2012); most leptin administration studies in birds use mammalian leptin (Cerasale et al., 2011; Song et al., 2009; Yang and Denbow, 2007). However, with recent structural studies indicating the leptin/leptin receptor binding interface is highly conserved (Prokop et al., 2012), and empirical studies that have shown leptin derived from diverse species can activate mammalian leptin receptors (Crespi and Denver, 2006), perhaps the ‘older’ studies should not be dismissed. Certainly homologous proteins, and species-specific antibodies should be used whenever possible, especially given that recombinant leptins from many vertebrates are now available.
Table 1A.
Effects of Leptin Manipulation on Food intake in Non-Mammalian Species
| Species | Leptin Source | Mode | Effects | Reference |
|---|---|---|---|---|
| Xenopus | Xenopus | ICV |
 food intake food intake limb development limb development |
Crespi and Denver, 2006 |
| Fence lizard | Mouse | pellet |
 food intake food intake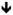 activity activity metabolic rate metabolic rate |
Niewiarowski et al., 2000 |
| Chicken | Human | ICV |
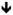 food intake food intake |
Kuo et al., 2005 |
| Goldfish | Mouse | ICV, IP |
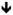 food intake food intake |
Volkoff et al., 2003 |
| Goldfish | Human | ICV, IP | ≈ food intake (8 hrs.)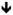 weight gain weight gain |
de Pedro et al., 2006 |
| Green sunfish | Mouse | IP |
 FABP FABP≈ total lipid stores |
Londraville and Duvall, 2002 |
| Rainbow trout | Human | ICV |
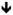 food intake food intake glucose sensing glucose sensing |
Aguilar et al., 2010 |
| Rainbow trout | Rainbow trout | IP |
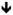 food intake (8 hrs.) food intake (8 hrs.) |
Murashita et al., 2008 |
| Rainbow trout | Rainbow trout | IP |
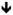 growth growth |
Murashita et al., 2011 |
| Grass carp | Grass carp | IP |
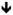 food intake (short term) food intake (short term) |
Li et al., 2010 |
| Zebrafish | Zebrafish | Morpholino knockdown |
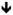 metabolic rate metabolic rate |
Dalman et al., 2013 |
ICV – intracerebroventricular injection; IP = intraperitoneal injection, pellet=slow release implant, morpholino knockdown= morpholino oligonucleotide knockdown of leptin A.
The second element of leptin dogma known widely to the lay public is its role as an adipostat. In early models leptin was thought to faithfully report total lipid stores to the CNS, such that changes in stores can be sensed quickly and physiology adjusted to survive a starvation event (Ahima and Flier, 2000). However, regressions between BMI (body mass index) and leptin titer in humans contain significant scatter, such that individuals at a given BMI can vary in their leptin titer by an order of magnitude (Cnop et al., 2002). In hibernating woodchucks, leptin titer and total body mass are out of phase (Concannon et al., 2001). A majority of fish studies report an increase in leptin mRNA or circulating protein with fasting (Table 1B) and a rapid decrease upon refeeding (Fuentes et al., 2013), which does not support the adipostat model. Recently, a leptin receptor mutant was generated in medaka (Chisada et al., 2014) and these leptin receptor −/− fish have increased appetite and growth rate. Unexpectedly, these mutants do not have increased fat stores in liver or skeletal muscle, but do develop a visceral fat body as adults (controls do not). The authors did not report the response of leptin to the knockout, but it is curious that the one tissue that increases fat stores does not express leptin in fish. Clearly leptin does not faithfully report energy stores in fishes, and as such should not be considered an ‘adipostat’; other species need further study. Fuentes et al. (2013) argue that increased circulating leptin during fasting activates energy liberating pathways (through AMPK), while inhibiting anabolic pathways (through TOR).
Table 1B.
Leptin Response to Treatment
| Species | Treatment | Assay | Δ leptin | Reference |
|---|---|---|---|---|
| Common carp | Fasting | qPCR | ≈ 6d, 6 wks | Huising et al., 2006 |
| Zebrafish | Fasting | qPCR | ≈ leptin A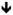 leptin B leptin B |
Gorissen et al., 2009 |
| Rainbow trout | Fasting | Salmon RIA |

|
Kling et al., 2009 |
| Atlantic salmon | Fasting | qPCR |

|
Trombley et al., 2012 |
| Fine flounder | Fasting | Salmon RIA |

|
Fuentes et al., 2012 |
| Fine flounder | Refeeding | Salmon RIA |
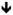
|
Fuentes et al., 2013 |
| Arctic charr | Seasonal cycle | qPCR |
 (during fasting months) (during fasting months) |
Frøiland et al., 2010 |
| Common carp | Hypoxia | qPCR |

|
Bernier et al., 2012 |
| Zebrafish | Hypoxia | qPCR |

|
Chu et al., 2010 |
Leptin as a pleiotropic stress-responsive hormone
As described above, leptin has anorexigenic effects via its actions in the central nervous system in all vertebrate groups in which it has been tested, which strongly supports an ancient origin of this function. While this action has been primarily studied within the context of the regulation of meal size and frequency in biomedical models, leptin’s anorexigenic effects may be associated with adaptive responses to other physiological stressors. For example, hypoxia increases leptin mRNA expression in zebrafish (Chu et al., 2010) and carp (Bernier et al., 2012), as it does in the neonatal rat (Bruder et al., 2005), the mammalian placenta (Grosfeld et al., 2001), and in adipocytes of obese humans (Trayhurn, 2013). Functionally, this increase in leptin message is associated with the anorexia, but it also protects gill tissues or neurons from apoptotic effects of hypoxia (see Gavello et al., 2012; Shin et al., 2009). Similarly, increases in circulating leptin associated with sickness behavior have been proposed to induce anorexia (i.e. cachexia), but also stimulate pro-inflammatory immune responses and wound healing associated with illness or injury (Carlton et al., 2012). Leptin also has been proposed to be a cold stress protein, as it is expressed in brown adipose tissue and induces thermogenesis in cold-adapted mammals (Yang et al., 2011). These examples demonstrate that leptin has adaptive pleiotropic actions geared toward either the protection or recovery from these challenges as well as the alteration of feeding behavior.
In addition, interactions between leptin and glucocorticoids or corticotropin-releasing factor (CRF) have been established in mammals, which suggests that there may be direct links between the hypothalamo-pituitary-adrenal/interrenal axes (Roubos et al., 2012). These interactions have been implicated in the homeostatic controls of food intake (Ahima et al., 2000) but also been associated with stress responses. For example, leptin treatments blunt stress responses in mice (Glasow and Bornstein, 2000; Heiman et al., 1997), suggesting that leptin could mediate condition-dependent responses. Leptin also attenuated activation of the neuroendocrine stress axis in the common carp by blunting CRF-induced adrenocorticotropic (ACTH) hormone at the level of the pituitary, but it did not affect ACTH sensitivity of the interrenal gland (Gorissen et al. 2012). However, the interactions between leptin and both glucocorticoids and CRF are context-dependent and can vary with developmental stage (Ahima et al., 2000; Spencer, 2013). Surely, additional studies in novel contexts across all vertebrate species will yield a much greater mechanistic understanding of leptin’s role as a stress-responsive hormone.
Leptin as an immunomodulator
Given that leptin is an adipokine and its receptor is a class I helical cytokine receptor, it is not surprising that leptin signaling is involved in multiple arms of the immune system. While leptin has been studied primarily as an energy balance factor that also affects the immune system, many cytokines, such as interleukins and chemokines, are primarily studied as immune factors with effects on energy balance (Huising et al., 2006). Indeed, in mammals leptin has effects on innate and acquired immune responses (Dardeno et al., 2010; Lam and Lu, 2007). Leptin, itself proinflammatory, stimulates the secretion of other proinflammatory cytokines in the innate immune system such as IL-1, IL-6 and TNF-α (Loffreda et al., 1998), and increases phagocytic activity of macrophages (Mancuso et al., 2002). In relation to the acquired immune system, leptin treatment enhances proliferation and suppresses apoptosis of T-cells (Lord et al., 1998; Papathanassoglou et al., 2006). Leptin also enhances wound healing in rodents (Frank et al., 2000; Slavkovsky et al., 2011).
Leptin also has enhancing interactions with the immune system in different vertebrate classes (e.g. Dalman et al., 2013; Mariano et al., 2013 in fishes; Lõhmus et al., 2004 in birds; French et al., 2011 in lizards; Crespi et al., 2012; Hicks-Courant, M.L. and Crespi, E.J., 2006 in amphibians), but recent studies emphasize the role leptin plays in energetic tradeoffs between the immune system and other physiological systems (see Demas, 2004). For example, in lizards leptin treatment also accelerates wound healing (using murine leptin, French et al., 2011a), but enhancement only occurred in food-restricted conditions during the reproductive season when wounds typically heal more slowly. Leptin actions in this context suggests that leptin mediates resource-dependent trade-offs between reproduction and immunity (French et al., 2011). Changes in leptin levels also appear to mediate changes in immune function with seasonal changes in resource availability. In Siberian hamsters, stored fat, leptin levels, and humoral immune responses are reduced during the short-days of winter, but leptin treatment can restore humoral immunity along with increasing food intake and cortisol levels (Drazen et al., 2001). Although much more research needs to be conducted in this area, these studies suggest that leptin is an evolutionarily conserved immunomodulator that regulates immune responsiveness within the context of the life history stage, nutritional state, and energetic demands of the animal.
Leptin as a growth factor during early development
Another exciting area of research that is emerging is on the role of leptin as a potential morphogen and growth factor during embryonic and larval development. Studies of leptin function in zebrafish and Xenopus, both primary models of early developmental processes in vertebrates, have shown a role for leptin. In zebrafish, leptin receptor is expressed first in the notochord area, but also in the gut, muscle, inner ear and hindbrain of early-staged larvae (Liu et al., 2010). Morpholino knockdown showed that leptin signaling is important for proper formation of the inner ear and dorsal brain, as well as retina and overall body size and curvature (Liu et al., 2012). In salmonids, the story is more complicated because of differential mRNA expression of leptin paralogs across development and in different tissues (Angotzi et al., 2013): Leptin A1 is highly expressed in embryos, and its expression increases until hatching, whereas Leptin B1 is the dominant leptin expressed in the brain and B2 is expressed in the gill through subsequent developmental stages. Leptin may also affect bone formation in zebrafish, as leptin knockdown dramatically reduces calcified bone (Figure 4; Liu and Londraville unpublished data). The field is very unsettled as to the effect of leptin on bone in mammals. There are proponents of central leptin control of bone growth exclusively through a hypothalamic relay, and the effects of increased leptin signaling as inhibitory to bone formation (Wei and Ducy, 2010), and other groups strongly advocating for the control of bone growth through peripheral leptin, and the effects of increased leptin signaling as stimulatory to bone formation (Turner et al., 2013). Again, a comparative approach can only contribute to solving this conundrum.
Figure 4. Effect of leptin knockdown on bone mineralization in zebrafish.
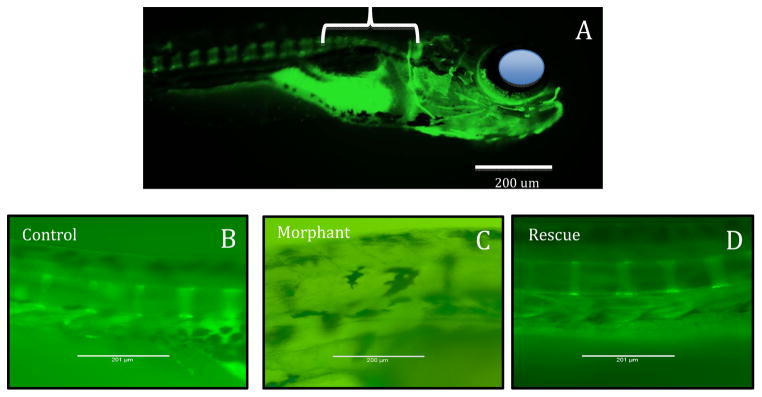
A) Fourteen day post-fertilization (DPF) zebrafish stained with calcein, which stains mineralized bone (Du et al., 2001). Eye is depicted by blue circle for orientation, bracket indicates area magnified in B-D. Mineralized bone stains bright green (there is also a bright signal from ingested stain because the embryos are stained live). B) Control fish 9 DPF showing the first vertebrae to mineralize immediately caudal to the cranium C) 9 DPF fish treated with anti-leptin A morpholino oligonucleotide lepMO1 as in Liu et al., (2012). No mineralization is evident. D) 9DPF fish coinjected with anti-leptin A morpholino oligonucleotide lepMO1 and recombinant zebrafish leptin A as in Liu et al., (2012). Bone mineralization is rescued.
Studies of leptin function in Xenopus have resulted in additional roles of leptin during early development not described in mammalian species. Leptin administration also accelerated limb development in amphibian tadpoles in vivo and in culture, supporting the idea that leptin acts on leptin receptors (which are expressed in the developing limb) to stimulate proliferation (Crespi and Denver, 2006). Leptin also enhances lung development in Xenopus tadpoles (Torday et al., 2009), and an up-regulation in leptin mRNA expression at the site of tail excision links paracrine actions of leptin to regenerative processes in Xenopus tadpoles (Love et al., 2011). These studies have only scratched the surface of describing both paracrine and endocrine roles of leptin in early development.
Future directions in leptin biomedical research
Leptin was the first identified hormone produced by adipose tissue in mammals and established fat as an endocrine organ (Zhang et al., 1994). The research that followed during the last 20 years shows that leptin orchestrates a complex physiological system that maintains homeostatic control of body weight, informing the brain about the status of the body’s energy reserves (in the form of fat tissue), and at the same time regulates food intake and energy expenditure (Friedman, 2011). The leptin message is of greatest importance since it informs the central nervous system whether energy reserves are sufficient to maintain important functions such as reproduction and immune homeostasis (Friedman, 2011). Regardless of the actual amount of body fat stores, the leptin signal is essential to maintain glucose homeostasis, food intake, immune function, and reproduction. This is clearly observed in leptin- or leptin receptor-deficient rodents and humans, which are obese and have multiple neuroendocrine and immune alterations (e.g. lipodystrophy syndromes; Mantzoros, 2012).
Leptin resistance
Obese individuals show high serum levels of leptin (R V Considine et al., 1996; Maffei et al., 1995), and leptin treatment has little effect on weight loss in humans (Fogteloo et al., 2003; Heymsfield et al., 1999). Understanding how leptin resistance or tolerance arises is one of the most important areas of biomedical research related not only to leptin biology, but most importantly, to the pathophysiology of obesity. Current findings support the notion that during obesity, leptin action can be impaired at the signaling level; inhibitor proteins (SOCS3, PTP1B, SHP2), which normally act as negative feedback regulators, may be over-activated during obesity and thus contribute to leptin resistance (Dardeno et al., 2010). Leptin transport across the blood brain barrier is also impaired, due to reduced transport activity and saturation of transporters by high leptin levels (Burguera et al., 2000; Vilà et al., 1998). The most recently proposed mechanisms of leptin resistance include: endoplasmic reticulum (ER) stress (Ozcan et al., 2009), decreased mitochondria-ER contacts (Schneeberger et al., 2013), and mitochondrial dysfunction in the hypothalamus (Kleinridders et al., 2013). Additionally, leptin resistance may be selective, in that some leptin signaling pathways are diminished while others are overactivated, leading (for example) to increased blood pressure and hypertension in obesity (Mark, 2013). A deeper understanding of the intricate mechanisms leading to selective leptin resistance and its interaction with glucose homeostasis and insulin signaling (Dardeno et al., 2010; Kim et al., 2000) will lead to the development of new therapies against obesity, diabetes, and insulin resistance syndromes.
Although it has received relatively little attention, the leptin receptor overlapping transcript (LEPROT) may be a key controller of leptin signaling, and an opportunity for comparative approaches to contribute to solving the problem of leptin resistance. In humans and other mammals, LEPROT is a product of the leptin receptor gene via an alternate start site; its mRNA is spliced from four exons not expressed in leptin receptors. Thus LEPROT shares no sequence identity with leptin receptors but is under control of the leptin receptor promoter (Bailleul et al., 1997). Work from the labs of Ralf Jockers and Yves Rouille, suggests that LEPROT (renamed endospanin) is a powerful regulator of leptin signaling (Couturier et al., 2007; Seron et al., 2011). LEPROT codes for a small (131 amino acid), 4-transmembrane spanning protein that localizes to endosomes and the trans-Golgi (Seron et al., 2011). LEPROT does not regulate total expression of the leptin receptor, but rather how many copies of leptin receptor are functional at the cell surface. Immunolabeling experiments suggest that LEPROT negatively regulates the post-translational Golgi processing of leptin receptor, such that when LEPROT expression is high, surface expression of the receptor is low. Further, knockout mice for LEPROT express maximal leptin receptors at the cell surface and are resistant to diet-induced obesity (Couturier et al., 2007). Jeon et al. found evidence of a similar mechanism in humans; they documented a negative correlation between exon 2 (present in LEPROT) copy number and leptin receptor expression, and positive correlation between exon 2 and LEPROT expression (Jeon et al., 2010). LEPROT control of leptin receptor expression may be specific to neuronal (vs. peripheral) leptin receptors (Satoh et al., 2009), and may also regulate growth hormone receptors through a similar mechanism (Touvier et al., 2009).
Unlike leptin or its receptor, LEPROT primary structure is highly conserved among vertebrates (86% identical between zebrafish and humans, vs. 22% for leptin; Londraville and Niewiarowski, 2010), and is structurally and functionally related to yeast VPS55p, a membrane-trafficking protein. Such sequence conservation across distant taxa usually means that the gene is essential. Interestingly, both Medaka and zebrafish LEPROT genes are not under control of the leptin receptor promoter, but instead are separate genes on separate chromosomes (Kurokawa and Murashita, 2009). Is the control of leptin signaling via LEPROT a mammalian innovation, and is control by a common promoter necessary for LEPROT to regulate leptin signaling? Is LEPROT tied to leptin resistance? A comparative approach can test this question.
Cancer and stem cell biology
In addition to its prominent role in metabolic homeostasis, leptin is implicated in cancer development and progression; studies show a positive association between leptin serum levels and breast and thyroid cancer (Akinci et al., 2009; Niu et al., 2013). Proposed mechanisms include: increased estrogen production and decreased estrogen receptor degradation, upregulated expression of VEGF and VEGFR (Dutta et al., 2012), stimulation of cyclin D1 expression, and induction of cancer stem cell survival and pluripotency (Zheng et al., 2013, 2012) in breast cancer and colorectal cancer (Bartucci et al., 2010), and increased expression of leptin receptor leading to higher levels of the anti-apoptotic protein XIAP (Cheng et al., 2010) in thyroid cancer. Leptin receptor antagonists are being developed and could be useful tools for cancer therapeutics (Otvos and Surmacz, 2011).
Another exciting area for present and future leptin biomedical research is stem cell biology, not only in the context of cancer as mentioned previously, but also in maintenance of the hematopoietic stem cell pool in which bone marrow leptin-receptor-expressing perivascular stromal cells play an important role (Ding et al., 2012); in addition, leptin regulates mesenchymal progenitor cell differentiation (Scheller et al., 2010) and promotes mobilization of bone marrow progenitors and their differentiation into vascular cells (Schroeter et al., 2012). Leptin also seems to play an important role in neurogenesis; it induces fetal hypothalamic stem/progenitor cell differentiation into neurons in vitro (Desai et al., 2011), increases neurogenesis in the hippocampus of adult mice (Garza et al., 2008), stimulates neural stem cell proliferation, neurogenesis, gliogenesis, and angiogenesis after stroke (Avraham et al., 2011), and attenuates neurodegeneration via increased neural progenitor proliferation in rodent models of Alzheimer’s disease (Pérez-González et al., 2011). Interestingly, neuronal leptin resistance has been suggested in Alzheimer’s disease (Bonda et al., 2014), raising the question of whether leptin resistance in obesity and other pathologies may lead to previously unrecognized neurological alterations.
Leptin as a therapeutic drug
Soon after the long-expected cloning of the ob gene in 1994 (Zhang et al., 1994), weight loss effects of leptin were evaluated in obese patients with and without congenital leptin deficiency. Treatment with recombinant methionyl human leptin proved to be a life-changing intervention for leptin-deficient patients, since it not only reduced body weight dramatically but also normalized most neuroendocrine and immune abnormalities observed in such patients (Farooqi et al., 2002, 1999). Leptin has also been used in partial leptin-deficiency states including lipodystrophy (congenital or related to highly active retroviral therapy for HIV) and hypothalamic amenorrhea. Lipodystrophy is characterized by drastically reduced subcutaneous adipose tissue, dyslipidemia, insulin resistance, hyperglycemia, and hepatic steatosis. Treatment of these patients with physiological doses of leptin reduces central fat mass, improves insulin and glucose levels, and increases insulin sensitivity (Chong et al., 2010; Lee et al., 2006). Hypothalamic amenorrhea is due to dysfunction of the hypothalamic-pituitary-gonadal axis, resulting in the absence of ovulatory menses, and it occurs secondary to stress, excessive exercise, and low food intake. Leptin treatment results in the recovery of menstrual cycles and normalization of neuroendocrine abnormalities in women with this condition (Chou et al., 2011; Welt et al., 2004).
In contrast to its clear therapeutic effects on leptin-deficient states, clinical trials using recombinant leptin in most obese subjects showed little or no weight-reduction (Fogteloo et al., 2003; Heymsfield, 1999), a result not completely unexpected knowing that, rather than a lack of leptin, most obese patients have elevated levels of serum leptin and are presumably tolerant or resistant to its effects (Considine et al., 1996; Maffei et al., 1995). Nonetheless, more recently, leptin regained promise as a therapeutic factor against obesity-related metabolic alterations. Combination therapy with metreleptin (human recombinant leptin) and pramlintide (an amylin analog) in overweight and obese subjects showed significantly greater weight loss effects than monotherapy-treated groups (Ravussin et al., 2009). Also, leptin treatment after weight loss prevents declines in energy expenditure, muscle work efficiency, and thyroid hormone levels that normally occur to compensate for reduced energy stores. Thus, leptin therapy is suggested to help maintain reduced body weight (Rosenbaum et al., 2005, 2002). Another new area for leptin therapeutics is its potential for achieving better glucose control with lower insulin dosage in type 1 diabetes patients; a clinical trial evaluating these effects is currently ongoing and is based on previous studies showing improved glucose metabolism in type 1 diabetes in rodents after combinatorial treatment with insulin and leptin. Several other clinical trials are assessing the safety and efficacy of metreleptin treatment on lipodystrophy, hypothalamic amenorrhea, obesity, and diabetes (Chou and Perry, 2013).
How comparative models may inform biomedical research
Given the predominant effect of leptin on food intake, energy homeostasis, reproduction, immune function and development, comparative models represent excellent tools to understand leptin’s function, particularly in species showing extreme phenotypes of metabolic plasticity, such as hibernators, marine mammals, and birds.
Hibernation is an evolutionary adaptation allowing survival in conditions of prolonged low ambient temperature and scarce food. Mammalian hibernators increase their food intake and fat storage in the active season; however, food intake peaks and starts declining months before fat storage reaches its maximum, right before the start of the hibernation period. This apparent discrepancy is explained by a drastic reduction in metabolic rate favoring fat accumulation. In most hibernators, leptin levels correlate positively with adipose tissue mass (Concannon et al., 2001). Serum leptin is lowest at the end of hibernation in spring and starts rising during the summer along with fat reserves, reaching its maximum level around the time when food intake declines, suggesting that leptin is one of the anorexigenic drivers. Leptin levels and fat stores stay high during autumn until both start declining in winter (Florant and Healy, 2012; Florant et al., 2004). Several relevant issues regarding leptin function may be studied using this model of dramatic circannual changes of body mass and food intake; for example, is leptin involved in establishing the set point for peak body mass? Also, leptin levels and fat stores remain high during autumn, together with reduced feeding behavior; if leptin is responsible for this anorexigenic state, what mechanisms prevent leptin resistance in this particular context of obesity? The Tups laboratory (Tups, 2009; Tups et al., 2012) showed that in the siberian hamster (Phodopus sungorus), seasonal changes in leptin sensitivity may involve fluctuations in hypothalamic STAT3 activity, resulting from leptin receptor binding to the inhibitor SOCS3 and to its competitive negative modulators: SHP2/GRB2. A relevant question for biomedical research is, what is the mediator of the photoperiod-induced reversibility of leptin resistance?
Finally, what are the molecular underpinnings of why obese non-hibernating rodents and humans develop leptin resistance and metabolic disease, whereas hibernating mammals stay healthy? For instance, despite presenting obesity, hypercholesterolemia, reduced blood flow and inactivity, brown and black bears are resistant to atherosclerosis (Arinell et al., 2012) and immobility-induced bone loss (Seger et al., 2011), respectively. Another feature of hibernators is their ability to excessively reduce body temperature, along with changes in blood pressure and flow during short cycles of hibernation (torpor) and arousal. Such drastic alterations in non-hibernating mammals are deleterious for most organs, as seen in cases of heart attack and stroke. Therefore, understanding the mechanisms mediating organ protection in hibernators could lead to development of treatments against ischemia-reperfusion injury as well as improved preservation of organs for transplants. Recently identified mechanisms that distinguish hibernation ischemia-reperfusion are increased fatty acid metabolism (Xu et al., 2013) and cytoskeletal reorganization (Hindle and Martin, 2013).
Marine mammals and diving birds represent opportunities for how comparative leptin study may inform human health. Marine birds and mammals maintain large lipid reserves without the morbid obesity syndrome seen in humans, and these large lipid reserves express very high copy numbers of leptin transcripts (Ball et al., 2013). Is there some aspect of leptin signaling that allows these ‘obese’ vertebrates to avoid leptin resistance? Marine mammal leptins evolved more rapidly than those of terrestrial mammals (Hammond et al., 2012), but the structural changes do not map where leptin interacts with its receptor (Hammond et al., 2012; Prokop et al., 2012). Hammond et al. (2012) postulate that the structural changes to seal leptins result in a leptin that is a more effective lung surfactant, which is critical to reinflating the lungs after deep dives. If so, the seal leptin could serve as a model for modifying human leptin as a treatment for newborn humans with pulmonary challenges.
Another area of research that will greatly influence biomedical studies is the examination of leptin actions in early developmental processes. Recent findings showing that leptin has roles in early morphogenesis and organogenesis in zebrafish and Xenopus (see references above) are likely the first of many studies to reveal roles for leptin during this developmental window. Hoggard et al. (2000) showed that leptin mRNA and protein are expressed in early fetal development of mouse, but little has been resolved regarding the function of leptin at these stages. Leptin may act as a growth factor during embryonic development, much like IGF and related peptides; but then take on the role of a nutritional modulator of the timing of early developmental processes and thus, adjust the timing of early life history transitions according to available resources (similar to leptin’s association with the timing of puberty, Cheung et al., 1997). This hypothesis is intriguing because adipose tissue is not present at these early developmental stages and the source of leptin as a hormone could be from the liver or placenta, or leptin can have effects through paracrine pathways, or both. Leptin regulation of development rate can happen in unexpected ways. For example, in the Indian short-nosed bat, the increase in leptin levels during gestation inhibits progesterone secretion, thereby delaying development rates (Banerjee et al., 2010). Whether leptin-progesterone interactions are important in humans is not yet resolved, but taking a comparative approach to investigating the many roles leptin may be playing in the fetus during early development, or in the placenta, is likely to generate novel hypotheses that can be applied to humans (Zhao et al., 2003).
Comparative endocrinology of leptin: research prospectus
The field of comparative endocrinology of leptin is in its infancy, and this review only highlights several of the exciting research directions currently undertaken by this growing community. The sequencing of leptins across diverse vertebrate taxa have given some insight into the evolution of this gene, and as shown here, the duplication of leptin genes in fishes has increased diversity of leptin, and potentially its function in these lineages. There are many novel contexts of leptin function that have been described, especially in the field of environmental endocrinology, but it is still too early to make definitive conclusions about how leptin function has evolved in vertebrates or even more broadly within the animal kingdom, as a presumptive leptin homologue in Drosophila recently has been described (Unpaired 2 gene). While initial experiments have demonstrated the anorexigenic effects of leptin across vertebrates, the physiological roles of leptin in the regulation of energy balance and food intake has not yet been resolved in most species; nor can we make clear distinctions between the roles of leptin in endoderms vs. ectotherms at this time. However, the necessity to generate species-specific molecular tools to examine leptin’s role in non-model organisms, given its tremendous amount of diversity in nucleic acid and amino acid sequence across vertebrates, continues to prevent the pace of research to keep up with the growing interest in working with leptins in non-model organisms. Furthermore, the association between leptin and obesity has been extremely stimulatory to the field, but is also a hurdle to overcome when trying to understand its ‘holistic’ function from an organismal and/or evolution point of view. It is clear that those working on leptin in non-model organisms need to steer clear of biases of leptin function as described in rodents and humans, but the more leptin is studied in novel phylogenetic groups and in animals with diverse life histories, the greater our understanding of the pleiotropic nature of this unique, adipokine hormone.
Highlights.
Leptin diversity within and among vertebrates is reviewed with a new phylogeny of leptins
Leptin universally reduces appetite among all vertebrates tested, but does not reflect fat stores in fish
Leptin is a stress-responsive hormone in multiple contexts and is an important immunomodulator throughout vertebrates
Several comparative models offer opportunity for studying human disease
Acknowledgments
We thank the participants of the Comparative Endocrinology of Leptins and other Adipokines Symposium at the 2013 NASCE annual meeting, who inspired this review. The authors were supported by NIH 1R15DK079282-01A1 and 2 R15 DK079282-02 to RLL and QL, NSF IOS-0818212 and NIH 1R15HD060009-01 to EJC, and Papiit-UNAM IA200113 and Conacyt 164423 and 174984 to YM. Stephen McNulty aided with bone staining (Fig. 4) and Dorothy Pless edited part of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam CL, Mercer JG. Appetite regulation and seasonality: implications for obesity. Proc Nutr Soc. 2004;63:413–419. doi: 10.1079/pns2004367. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Akinci M, Kosova F, Cetin B, Aslan S, Ari Z, Cetin A. Leptin levels in thyroid cancer. Asian J Surg Asian Surg Assoc. 2009;32:216–223. doi: 10.1016/S1015-9584(09)60397-3. [DOI] [PubMed] [Google Scholar]
- Angotzi AR, Stefansson SO, Nilsen TO, Rathore RM, Rønnestad I. Molecular cloning and genomic characterization of novel leptin-like genes in salmonids provide new insight into the evolution of the leptin gene family. Gen Comp Endocrinol. 2013;187:48–59. doi: 10.1016/j.ygcen.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Arinell K, Sahdo B, Evans AL, Arnemo JM, Baandrup U, Fröbert O. Brown bears (Ursus arctos) seem resistant to atherosclerosis despite highly elevated plasma lipids during hibernation and active state. Clin Transl Sci. 2012;5:269–272. doi: 10.1111/j.1752-8062.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Davidi N, Lassri V, Vorobiev L, Kabesa M, Dayan M, Chernoguz D, Berry E, Leker RR. Leptin induces neuroprotection neurogenesis and angiogenesis after stroke. Curr Neurovasc Res. 2011;8:313–322. doi: 10.2174/156720211798120954. [DOI] [PubMed] [Google Scholar]
- Bailleul B, Akerblom I, Strosberg AD. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Res. 1997;25:2752–8. doi: 10.1093/nar/25.14.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HC, Holmes RK, Londraville RL, Thewissen JGM, Duff RJ. Leptin in whales: validation and measurement of mRNA expression by absolute quantitative real-time PCR. PloS One. 2013;8:e54277. doi: 10.1371/journal.pone.0054277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Meenakumari KJ, Krishna A. Role of leptin in delayed embryonic development in the Indian short-nosed fruit bat, Cynopterus sphinx. Gen Comp Endocrinol. 2010;168:36–45. doi: 10.1016/j.ygcen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823–833. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Gorissen M, Flik G. Differential effects of chronic hypoxia and feed restriction on the expression of leptin and its receptor, food intake regulation and the endocrine stress response in common carp. J Exp Biol. 2012;215:2273–2282. doi: 10.1242/jeb.066183. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, Jicha G, Casadesus G, Smith MA, Zhu X, Lee H-G. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128:162–172. doi: 10.1111/jnc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorse GC, Libbon JV. Genomic characterization of two leptin genes and a leptin receptor gene in the Green Anole, Anolis carolinensis. Integr Comp Biol. 2010;50:E207. [Google Scholar]
- Boswell T, Dunn IC, Wilson PW, Joseph N, Burt DW, Sharp PJ. Identification of a non-mammalian leptin-like gene: characterization and expression in the tiger salamander (Ambystoma tigrinum) Gen Comp Endocrinol. 2006;146:157–166. doi: 10.1016/j.ygcen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bruder ED, Jacobson L, Raff H. Plasma leptin and ghrelin in the neonatal rat: interaction of dexamethasone and hypoxia. J Endocrinol. 2005;185:477–484. doi: 10.1677/joe.1.06159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000;49:1219–1223. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- Cao Y-B, Xue JL, Wu L-Y, Jiang W, Hu P-N, Zhu J. The detection of 3 leptin receptor isoforms in crucian carp gill and the influence of fasting and hypoxia on their expression. Domest Anim Endocrinol. 2011;41:74–80. doi: 10.1016/j.domaniend.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Carlton ED, Demas GE, French SS. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm Behav. 2012;62:272–279. doi: 10.1016/j.yhbeh.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Carpenter B, Hemsworth GR, Wu Z, Maamra M, Strasburger CJ, Ross RJ, Artymiuk PJ. Structure of the human obesity receptor leptin-binding domain reveals the mechanism of leptin antagonism by a monoclonal antibody. Struct Lond Engl. 2012;20:487–497. doi: 10.1016/j.str.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Cerasale DJ, Zajac DM, Guglielmo CG. Behavioral and physiological effects of photoperiod-induced migratory state and leptin on a migratory bird, Zonotrichia albicollis: I. Anorectic effects of leptin administration. Gen Comp Endocrinol. 2011;174:276–286. doi: 10.1016/j.ygcen.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Chen JF, Zhong WQ, Wang DH. Seasonal changes in body mass, energy intake and thermogenesis in Maximowiczi’s voles (Microtus maximowiczii) from the inner Mongolian grassland. J Comp Physiol [B] 2012;182:275–285. doi: 10.1007/s00360-011-0608-9. [DOI] [PubMed] [Google Scholar]
- Cheng S-P, Yin P-H, Chang Y-C, Lee C-H, Huang S-Y, Chi C-W. Differential roles of leptin in regulating cell migration in thyroid cancer cells. Oncol Rep. 2010;23:1721–1727. doi: 10.3892/or_00000817. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Chisada S, Kurokawa T, Murashita K, Rønnestad I, Taniguchi Y, Toyoda A, Sakaki Y, Takeda S, Yoshiura Y. Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen Comp Endocrinol. 2014;195:9–20. [PubMed] [Google Scholar]
- Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- Chou K, Perry CM. Metreleptin: first global approval. Drugs. 2013;73:989–997. doi: 10.1007/s40265-013-0074-7. [DOI] [PubMed] [Google Scholar]
- Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DLH, Li VWT, Yu RMK. Leptin: clue to poor appetite in oxygen-starved fish. Mol Cell Endocrinol. 2010;319:143–146. doi: 10.1016/j.mce.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Henry B, Iqbal J, Goding JW. Leptin and the regulation of food intake and the neuroendocrine axis in sheep. Clin Exp Pharmacol Physiol. 2001;28:106–7. doi: 10.1046/j.1440-1681.2001.03410.x. [DOI] [PubMed] [Google Scholar]
- Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, Wang F, Hull RL, Boyko EJ, Retzlaff BM, Walden CE, Knopp RH, Kahn SE. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Hixson JE, Almasy L, Braxton MD, Michael MC, Dyer TD, Stern MP, MacCluer JW, Blangero J. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 1997;15:273–276. doi: 10.1038/ng0397-273. [DOI] [PubMed] [Google Scholar]
- Concannon P, Levac K, Rawson R, Tennant B, Bensadoun A. Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am J Physiol Regul Integr Comp Physiol. 2001;281:R951–959. doi: 10.1152/ajpregu.2001.281.3.R951. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Copeland DL, Duff RJ, Liu Q, Prokop J, Londraville RL. Leptin in teleost fishes: an argument for comparative study. Front Physiol. 2011;2:26. doi: 10.3389/fphys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier C, Sarkis C, Seron K, Belouzard S, Chen P, Lenain A, Corset L, Dam J, Vauthier V, Dubart A, Mallet J, Froguel P, Rouille Y, Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proc Natl Acad Sci U A. 2007;104:19476–81. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc Natl Acad Sci U A. 2006;103:10092–7. doi: 10.1073/pnas.0507519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Fites JS, Rollins-Smith LA. Leptin enhances proliferation of amphibian lymphocytes. Integr Comp Biol. 2012;52:E37–E37. [Google Scholar]
- Dalman MR, Liu Q, King MD, Bagatto B, Londraville RL. Leptin expression affects metabolic rate in zebrafish embryos (D. rerio) Front Physiol. 2013;4:160. doi: 10.3389/fphys.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman Mark R, Mustafa A, Liu Q, Londraville RL. Leptin knockdown reduces innate immune function in zebrafish. Faseb J. 2013:27. [Google Scholar]
- Dardeno TA, Chou SH, Moon H-S, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Bonett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrin. 2011;94:21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen DL, Demas GE, Nelson RJ. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–2775. doi: 10.1210/endo.142.7.8271. [DOI] [PubMed] [Google Scholar]
- Du SJ, Frenkel V, Kindschi G, Zohar Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev Biol. 2001;238:239–246. doi: 10.1006/dbio.2001.0390. [DOI] [PubMed] [Google Scholar]
- Dutta D, Ghosh S, Pandit K, Mukhopadhyay P, Chowdhury S. Leptin and cancer: Pathogenesis and modulation. Indian J Endocrinol Metab. 2012;16:S596–600. doi: 10.4103/2230-8210.105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Healy JE. The regulation of food intake in mammalian hibernators: a review. J Comp Physiol [B] 2012;182:451–467. doi: 10.1007/s00360-011-0630-y. [DOI] [PubMed] [Google Scholar]
- Florant GL, Porst H, Peiffer A, Hudachek SF, Pittman C, Summers SA, Rajala MW, Scherer PE. Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris) J Comp Physiol [B] 2004;174:633–639. doi: 10.1007/s00360-004-0454-0. [DOI] [PubMed] [Google Scholar]
- Fogteloo AJ, Pijl H, Frölich M, McCamish M, Meinders AE. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab. 2003;16:109–114. [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SS, Dearing MD, Demas GE. Leptin as a physiological mediator of energetic trade-offs in ecoimmunology: implications for disease. Integr Comp Biol. 2011a;51:505–513. doi: 10.1093/icb/icr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. Leptin and the regulation of body weigh. Keio J Med. 2011;60:1–9. doi: 10.2302/kjm.60.1. [DOI] [PubMed] [Google Scholar]
- Friedman-Einat M, Boswell T, Horev G, Girishvarma G, Dunn IC, Talbot RT, Sharp PJ. The chicken leptin gene: has it been cloned? Gen. Comp Endocrinol. 1999;115:354–63. doi: 10.1006/gcen.1999.7322. [DOI] [PubMed] [Google Scholar]
- Frøiland E, Murashita K, Jørgensen EH, Kurokawa T. Leptin and ghrelin in anadromous Arctic charr: cloning and change in expressions during a seasonal feeding cycle. Gen Comp Endocrinol. 2010;165:136–43. doi: 10.1016/j.ygcen.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Fuentes EN, Safian D, Einarsdottir IE, Valdés JA, Elorza AA, Molina A, Björnsson BT. Nutritional status modulates plasma leptin, AMPK and TOR activation, and mitochondrial biogenesis: Implications for cell metabolism and growth in skeletal muscle of the fine flounder. Gen Comp Endocrinol. 2013;186:172–180. doi: 10.1016/j.ygcen.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Gardi J, Nelson OL, Robbins CT, Szentirmai E, Kapás L, Krueger JM. Energy homeostasis regulatory peptides in hibernating grizzly bears. Gen Comp Endocrinol. 2011;172:181–183. doi: 10.1016/j.ygcen.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu X-Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavello D, Rojo-Ruiz J, Marcantoni A, Franchino C, Carbone E, Carabelli V. Leptin counteracts the hypoxia-induced inhibition of spontaneously firing hippocampal neurons: a microelectrode array study. PloS One. 2012;7:e41530. doi: 10.1371/journal.pone.0041530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasow A, Bornstein SR. Leptin and the adrenal gland. Eur J Clin Invest. 2000;30(Suppl 3):39–45. doi: 10.1046/j.1365-2362.2000.0300s3039.x. [DOI] [PubMed] [Google Scholar]
- Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol. 2009;201:329–39. doi: 10.1677/JOE-09-0034. [DOI] [PubMed] [Google Scholar]
- Gorissen M, Bernier NJ, Manuel R, de Gelder S, Metz JR, Huising MO, Flik G. Recombinant human leptin attenuates stress axis activity in common carp (Cyprinus carpio L.) Gen Comp Endocrinol. 2012;178:75–81. doi: 10.1016/j.ygcen.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Grosfeld A, Turban S, André J, Cauzac M, Challier JC, Hauguel-de Mouzon S, Guerre-Millo M. Transcriptional effect of hypoxia on placental leptin. FEBS Lett. 2001;502:122–126. doi: 10.1016/s0014-5793(01)02673-4. [DOI] [PubMed] [Google Scholar]
- Hammond JA, Hauton C, Bennett KA, Hall AJ. Phocid seal leptin: tertiary structure and hydrophobic receptor binding site preservation during distinct leptin gene evolution. PloS One. 2012;7:e35395. doi: 10.1371/journal.pone.0035395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liang X-F, Li L, Huang W, Shen D, Tao Y-X. Gene structure and expression of leptin in Chinese perch. Gen Comp Endocrinol. 2013;194C:183–188. doi: 10.1016/j.ygcen.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Heymsfield Leptin injections cause weight loss in obese and normal-weight subjects. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hicks-Courant ML, CRJ, Crespi EJ. Leptin enhances growth and development under disease exposure in Xenopus laevis tadpoles. Integr Comp Biol. 2006;46:E60. [Google Scholar]
- Hindle AG, Martin SL. Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PloS One. 2013;8:e71627. doi: 10.1371/journal.pone.0071627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Lea RG, Trayhurn P, Mercer JG. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br J Nutr. 2000;83:317–326. doi: 10.1017/s0007114500000398. [DOI] [PubMed] [Google Scholar]
- Huising MO, Geven EJ, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FA, Verburg-van Kemenade BM, Flik G. Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology. 2006;147:5786–97. doi: 10.1210/en.2006-0824. [DOI] [PubMed] [Google Scholar]
- Jeon JP, Shim SM, Nam HY, Ryu GM, Hong EJ, Kim HL, Han BG. Copy number variation at leptin receptor gene locus associated with metabolic traits and the risk of type 2 diabetes mellitus. BMC Genomics. 2010;11:426. doi: 10.1186/1471-2164-11-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM, Johnson TM, Londraville RL. Evidence for leptin expression in fishes. J Exp Zool. 2000;286:718–24. doi: 10.1002/(sici)1097-010x(20000601)286:7<718::aid-jez6>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci CABIOS. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–2339. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- Kleinridders A, Lauritzen HPMM, Ussar S, Christensen JH, Mori MA, Bross P, Kahn CR. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J Clin Invest. 2013 doi: 10.1172/JCI67615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa T, Murashita K. Genomic characterization of multiple leptin genes and a leptin receptor gene in the Japanese medaka, Oryzias latipes. Gen Comp Endocrinol. 2009;161:229–37. doi: 10.1016/j.ygcen.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Uji S, Suzuki T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides. 2005;26:745–50. doi: 10.1016/j.peptides.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Lam QLK, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen Y, Copeland D, Ball H, Duff RJ, Rockich B, Londraville RL. Expression of leptin receptor gene in developing and adult zebrafish. Gen Comp Endocrinol. 2010;166:346–55. doi: 10.1016/j.ygcen.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Dalman M, Chen Y, Akhter M, Brahmandam S, Patel J, Thakkar M, Gregory A, Phelps D, Riley C, Londraville RL. Knockdown of leptin A expression dramatically alters zebrafish development. Gen Comp Endocrinol. 2012;178:562–572. doi: 10.1016/j.ygcen.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Nobel PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB. 1998;12:57–65. [PubMed] [Google Scholar]
- Lõhmus M, Olin M, Sundström LF, Troedsson MHT, Molitor TW, El Halawani M. Leptin increases T-cell immune response in birds. Gen Comp Endocrinol. 2004;139:245–250. doi: 10.1016/j.ygcen.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Lõhmus M, Sundström LF, Silverin B. Chronic administration of leptin in Asian Blue Quail. J Exp Zoolog A Comp Exp Biol. 2006;305:13–22. doi: 10.1002/jez.a.240. [DOI] [PubMed] [Google Scholar]
- Londraville RL, Duvall CS. Murine leptin injections increase intracellular fatty acid-binding protein in green sunfish (Lepomis cyanellus) Gen Comp Endocrinol. 2002;129:56–62. doi: 10.1016/s0016-6480(02)00510-5. [DOI] [PubMed] [Google Scholar]
- Londraville RL, Niewiarowski PH. Leptin signaling systems in reptiles and amphibians. In: Paolucci M, editor. Leptin in Non-Mammalian Vertebrates. Transworld Research Network; Kerala, India: 2010. [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Love NR, Chen Y, Bonev B, Gilchrist MJ, Fairclough L, Lea R, Mohun TJ, Paredes R, Zeef LAH, Amaya E. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev Biol. 2011;11:70. doi: 10.1186/1471-213X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R-H, Liang X-F, Wang M, Zhou Y, Bai X-L, He Y. The role of leptin in lipid metabolism in fatty degenerated hepatocytes of the grass carp Ctenopharyngodon idellus. Fish Physiol Biochem. 2012;38:1759–1774. doi: 10.1007/s10695-012-9673-6. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Mancour LV, Daghestani HN, Dutta S, Westfield GH, Schilling J, Oleskie AN, Herbstman JF, Chou SZ, Skiniotis G. Ligand-induced architecture of the leptin receptor signaling complex. Mol Cell. 2012;48:655–661. doi: 10.1016/j.molcel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol Baltim Md 1950. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS. Leptin in relation to the lipodystrophy-associated metabolic syndrome. Diabetes Metab J. 2012;36:181–189. doi: 10.4093/dmj.2012.36.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano G, Stilo R, Terrazzano G, Coccia E, Vito P, Varricchio E, Paolucci M. Effects of recombinant trout leptin in superoxide production and NF-κB/MAPK phosphorylation in blood leukocytes. Peptides. 2013;48:59–69. doi: 10.1016/j.peptides.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashita K, Jordal AE, Nilsen TO, Stefansson SO, Kurokawa T, Bjornsson BT, Moen AG, Ronnestad I. Leptin reduces Atlantic salmon growth through the central pro-opiomelanocortin pathway. Comp Biochem Physiol Mol Integr Physiol. 2011;158:79–86. doi: 10.1016/j.cbpa.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Murashita K, Uji S, Yamamoto T, Ronnestad I, Kurokawa T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol B Biochem Mol Biol. 2008;150:377–84. doi: 10.1016/j.cbpb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: A meta-analysis. PloS One. 2013;8:e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L, Jr, Surmacz E. Targeting the leptin receptor: a potential new mode of treatment for breast cancer. Expert Rev Anticancer Ther. 2011;11:1147–1150. doi: 10.1586/era.11.109. [DOI] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol Baltim Md 1950. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- Peelman F, Couturier C, Dam J, Zabeau L, Tavernier J, Jockers R. Techniques: new pharmacological perspectives for the leptin receptor. Trends Pharmacol Sci. 2006;27:218–225. doi: 10.1016/j.tips.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Pérez-González R, Antequera D, Vargas T, Spuch C, Bolós M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis JAD. 2011;24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- Pitel F, Faraut T, Bruneau G, Monget P. Is there a leptin gene in the chicken genome? Lessons from phylogenetics, bioinformatics and genomics. Gen Comp Endocrinol. 2010;167:1–5. doi: 10.1016/j.ygcen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Pitel F, Monbrun C, Gellin J, Vignal A. The chicken LEP (OB) gene has not been mapped. Anim Genet. 2000;31:281. doi: 10.1046/j.1365-2052.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- Prokop JW, Duff RJ, Ball HC, Copeland DL, Londraville RL. Leptin and leptin receptor: Analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides. 2012;38:326–336. doi: 10.1016/j.peptides.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obes Silver Spring Md. 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnestad I, Nilsen TO, Murashita K, Angotzi AR, Gamst Moen AG, Stefansson SO, Kling P, Thrandur Bjornsson B, Kurokawa T. Leptin and leptin receptor genes in Atlantic salmon: Cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen Comp Endocrinol. 2010;168:55–70. doi: 10.1016/j.ygcen.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- Roubos EW, Dahmen M, Kozicz T, Xu L. Leptin and the hypothalamo-pituitary-adrenal stress axis. Gen Comp Endocrinol. 2012;177:28–36. doi: 10.1016/j.ygcen.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Satoh T, Yoshino S, Katano A, Ishizuka T, Tomaru T, Shibusawa N, Hashimoto K, Yamada M, Mori M. Isolation of a novel leptin receptor gene promoter preferentially functioning in neuronal cells. Biochem Biophys Res Commun. 2009;389:673–7. doi: 10.1016/j.bbrc.2009.09.056. [DOI] [PubMed] [Google Scholar]
- Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodríguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter MR, Stein S, Heida N-M, Leifheit-Nestler M, Cheng I-F, Gogiraju R, Christiansen H, Maier LS, Shah AM, Hasenfuss G, Konstantinides S, Schäfer K. Leptin promotes the mobilization of vascular progenitor cells and neovascularization by NOX2-mediated activation of MMP9. Cardiovasc Res. 2012;93:170–180. doi: 10.1093/cvr/cvr275. [DOI] [PubMed] [Google Scholar]
- Seger RL, Cross RA, Rosen CJ, Causey RC, Gundberg CM, Carpenter TO, Chen TC, Halteman WA, Holick MF, Jakubas WJ, Keisler DH, Seger RM, Servello FA. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus) Bone. 2011;49:1205–1212. doi: 10.1016/j.bone.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Seron K, Couturier C, Belouzard S, Bacart J, Monte D, Corset L, Bocquet O, Dam J, Vauthier V, Lecoeur C, Bailleul B, Hoflack B, Froguel P, Jockers R, Rouille Y. Endospanins regulate a postinternalization step of the leptin receptor endocytic pathway. J Biol Chem. 2011;286:17968–81. doi: 10.1074/jbc.M111.224857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Waddington D, Boswell T. Chicken leptin. Gen Comp Endocrinol. 2008;158:2–4. doi: 10.1016/j.ygcen.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Shin E-J, Schram K, Zheng X-L, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol. 2009;221:490–497. doi: 10.1002/jcp.21883. [DOI] [PubMed] [Google Scholar]
- Slavkovsky R, Kohlerova R, Tkacova V, Jiroutova A, Tahmazoglu B, Velebny V, Rezačová M, Sobotka L, Kanta J. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2011;19:515–525. doi: 10.1111/j.1524-475X.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- Song Y, Wang Chonggang, Wang Cheng, Lv L, Chen Y, Zuo Z. Exogenous leptin promotes the recovery of regressed ovary in fasted ducks. Anim Reprod Sci. 2009;110:306–318. doi: 10.1016/j.anireprosci.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Spencer SJ. Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Front Neurosci. 2013;7:109. doi: 10.3389/fnins.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taouis M, Chen JW, Daviaud C, Dupont J, Derouet M, Simon J. Cloning the chicken leptin gene. Gene. 1998;208:239–242. doi: 10.1016/s0378-1119(97)00670-7. [DOI] [PubMed] [Google Scholar]
- Torday JS, Ihida-Stansbury K, Rehan VK. Leptin stimulates Xenopus lung development: evolution in a dish. Evol Dev. 2009;11:219–224. doi: 10.1111/j.1525-142X.2009.00321.x. [DOI] [PubMed] [Google Scholar]
- Touvier T, Conte-Auriol F, Briand O, Cudejko C, Paumelle R, Caron S, Bauge E, Rouille Y, Salles JP, Staels B, Bailleul B. LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice. J Clin Invest. 2009;119:3830–8. doi: 10.1172/JCI34997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- Tups A. Physiological models of leptin resistance. J Neuroendocrinol. 2009;21:961–971. doi: 10.1111/j.1365-2826.2009.01916.x. [DOI] [PubMed] [Google Scholar]
- Tups A, Stöhr S, Helwig M, Barrett P, Krol E, Schachtner J, Mercer JG, Klingenspor M. Seasonal leptin resistance is associated with impaired signalling via JAK2-STAT3 but not ERK, possibly mediated by reduced hypothalamic GRB2 protein. J Comp Physiol [B] 2012;182:553–567. doi: 10.1007/s00360-011-0637-4. [DOI] [PubMed] [Google Scholar]
- Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà R, Adán C, Rafecas I, Fernández-López JA, Remesar X, Alemany M. Plasma leptin turnover rates in lean and obese Zucker rats. Endocrinology. 1998;139:4466–4469. doi: 10.1210/endo.139.11.6296. [DOI] [PubMed] [Google Scholar]
- Wei J, Ducy P. Co-dependence of bone and energy metabolisms. Arch Biochem Biophys. 2010;503:35–40. doi: 10.1016/j.abb.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- Won ET, Baltzegar DA, Picha ME, Borski RJ. Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): control of feeding and regulation by nutritional state. Gen Comp Endocrinol. 2012;178:98–107. doi: 10.1016/j.ygcen.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shao C, Fedorov VB, Goropashnaya AV, Barnes BM, Yan J. Molecular signatures of mammalian hibernation: comparisons with alternative phenotypes. BMC Genomics. 2013;14:567. doi: 10.1186/1471-2164-14-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bromage TG, Zhao Q, Xu BH, Gao WL, Tian HF, Tang HJ, Liu DW, Zhao XQ. Functional evolution of leptin of Ochotona curzoniae in adaptive thermogenesis driven by cold environmental stress. PloS One. 2011;6:e19833. doi: 10.1371/journal.pone.0019833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Denbow DM. Interaction of leptin and nitric oxide on food intake in broilers and Leghorns. Physiol Behav. 2007;92:651–657. doi: 10.1016/j.physbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- Zhang Huixian, Chen H, Zhang Y, Li S, Lu D, Zhang Haifa, Meng Z, Liu X, Lin H. Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides) Gen Comp Endocrinol. 2013;181:295–305. doi: 10.1016/j.ygcen.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and it human homologue. Nature. 1994;372:425–431. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kunz TH, Tumba N, Schulz LC, Li C, Reeves M, Widmaier EP. Comparative analysis of expression and secretion of placental leptin in mammals. Am J Physiol Regul Integr Comp Physiol. 2003;285:R438–446. doi: 10.1152/ajpregu.00776.2002. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Hursting SD, Reizes O. Leptin regulates cyclin D1 in luminal epithelial cells of mouse MMTV-Wnt-1 mammary tumors. J Cancer Res Clin Oncol. 2012;138:1607–1612. doi: 10.1007/s00432-012-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]