Abstract
Development of novel carriers and optimization of their design parameters has led to significant advances in the field of targeted drug delivery. Since carrier shape has recently been recognized as an important design parameter for drug delivery, we sought to investigate how carrier shape influences their flow in the vasculature and their ability to target the diseased site. Idealized synthetic microvascular networks (SMNs) were used for this purpose since they closely mimic key physical aspects of real vasculature and at the same time offer practical advantages in terms of ease of use and direct observation of particle flow. The attachment propensities of surface functionalized spheres, elliptical/circular disks and rods with dimensions ranging from 1 µm to 20 µm were compared by flowing them through bifurcating SMNs. Particles of different geometries exhibited remarkably different adhesion propensities. Moreover, introduction of a bifurcation as opposed to the commonly used linear channel resulted in significantly different flow and adhesion behavior, which may have important implications in correlating these results to in vivo behavior. This study provides valuable information for design of carriers for targeted drug delivery.
Keywords: Vascular dynamics, SMN, non-spherical, shape, drug delivery, targeting
INTRODUCTION
Targeted drug delivery offers several advantages over conventional delivery methods including reduced side effects and lower drug doses [1]. Various proteins, peptides, aptamers and antibodies have been developed to target specific receptors up-regulated in various diseased states [2–5]. Optimization of the carrier design features is necessary to realize the true potential of these targeting agents. In spite of the numerous advancements in carrier design with respect to size [6] and surface chemistry [7, 8], the desired targeting efficiency still remains sub-optimal. Recent reports on the importance of carrier shape in various biological processes [9] have fueled interest in exploring and optimizing carrier geometry to increase the targeting efficiency.
Carrier shape has been shown to have a profound effect on their cellular internalization [10, 11] and circulation half life [12, 13] which directly affect the targeting ability of carriers. Studies have demonstrated that the shape of carriers travelling through the blood vessels plays an important role in their margination towards the vessel wall [14, 15]. For example, a neutrally buoyant spherical particle moving in proximity to a wall can drift laterally only in presence of an external force whereas non-spherical particles exhibit more complex motion with tumbling and rolling which can be exploited to control their margination dynamics without the need for lateral external forces. Studies have also reported on higher targeting efficiency of non-spherical particles compared to their spherical counterparts [13, 16]. Collectively, these studies clearly demonstrate the role of carrier shape in flow and motivate detailed studies of the effect of geometry in the transport of carriers through the vasculature and their attachment at the target site.
Direct observations of carrier flow in blood vessels, though of high relevance to drug delivery, are very challenging due to practical challenges associated with visualization of particles in blood vessels and isolating contributions from various confounding factors. This challenge can be addressed by utilizing synthetic systems that simulate fluid dynamics in the vasculature. The most common system in use for over a decade now is the parallel plate flow chamber which consists of a transparent apparatus that can be perfused to attain shear conditions observed in vivo [17]. The lower plate of the chamber may be coated by appropriate proteins or cultured with cells to mimic the vessel wall in vivo. These linear flow chambers have been used to study particle adhesion [18–20] as well as cellular adhesion [21] to the endothelium. However, the chamber size in these devices is substantially larger than the vessels in the microvasculature environment where most of the particle adhesion occurs. Recent developments using BioMEMS have led to the use of simple microfluidic devices for performing adhesion assays [22, 23]. However, these devices still do not accurately represent the in vivo vasculature, characterized by convolutions and bifurcations which render straight channels an oversimplification of the in vivo flow behavior [24, 25]. Recently, investigators have reported on development of synthetic microvascular networks (SMNs), a novel microfluidic platform for studying particle adhesion that overcomes the limitations associated with linear channels [26].
In the current study, idealized SMNs were employed to determine the adhesion profiles of particles of different shapes and sizes in a dynamic fluidic environment. The surfaces of the SMNs and the particles were modified using complementary biomolecules, Bovine Serum Albumin (BSA) and anti-BSA antibody respectively, to determine the effect of particle shape on targeted adhesion. Quantification of adhesion profiles revealed the importance of carrier shape in flow and adhesion and also illustrated the advantages of using SMNs.
MATERIALS AND METHODS
Materials
Polystyrene (PS) spheres were purchased from Polysciences, Warrington, PA. Poly vinyl alcohol (PVA), glycerol, bovine serum albumin, mineral oil and toluene were purchased from Sigma Aldrich, St. Louis, MO. The anti-BSA mAb was purchased from Fitzgerald Industries International, Concord, MA. PDMS was obtained from Dow Corning, Midland, MI, microbore tubing (Tygon) was purchased from Small parts, Miramar, FL and syringes were purchased from BD, Franklin Lakes, New Jersey.
Fabrication of bifurcating microchannels
Masters for fabricating the microchannels with a bifurcation angle of 90° and depth of 50µm were developed using standard photolithography process. Sylgard 184 PDMS was prepared according to manufacturer’s (Dow Corning, Midland, MI) instructions and poured over the developed masters in a 150mm petri dish, and degassed for 15 minutes. The polymer was then allowed to cure overnight in an oven at 65°C to create complementary microchannels in PDMS. Through holes, defining the inlets and outlets, were punched using a biopsy punch. The bonding surfaces of the PDMS and a pre-cleaned 1×3 inch glass slide were plasma treated (200mTorr, 18 W, 30 s) in a plasma generator (Harrick Scientific, Ithaca, NY). Tygon Microbore tubing with an outside diameter of 0.06 inch and inner diameter of 0.02 inch served as the connecting ports for the fabricated microfluidic device.
Fabrication of particles of different shapes
Particles of different shapes were fabricated using the film stretching method described by Champion et al [10]. Briefly, spherical PS particles (5×108) were embedded in a PVA film. Glycerol (0.75–2 %) was added as a plasticizer. The film was dried and stretched either in oil at high temperatures (above the glass transition temperature of PS) or in toluene at room temperature using custom made 1D and 2D stretchers. The extent of stretching (e = final film length after stretching / film length before stretching), film thickness, oil vs. solvent stretching and 1D or 2D stretching determined the shape of the particle. The particles were then extracted from the film by dissolving the film in 15% isopropanol. The particles were purified by washing them 10 times with 15% isopropanol (centrifugation and subsequent resuspension) to ensure all the PVA was removed.
Scanning electron microscopy
SEM micrographs of particles of different shapes and sizes were obtained. Sample preparation involved pipetting 10 µL of the particle suspension on the stub followed by vacuum drying and coating with palladium (Hummer 6.2 Sputtering System, Anatech Ltd., Union City, CA). Particles were imaged with the Sirion 400 SEM (FEI Company, Hillsboro, OR) at an acceleration voltage of 5 kV.
Coating with BSA
The inlet port of the microfluidic device was connected to a 1mL syringe filled with 0.1% sterile BSA solution mounted on a programmable syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA). BSA was introduced into the channel at a flow rate of 10 µL/min. After 15 minutes, the flow was stopped, the tubing’s were removed and the entire device was covered with parafilm and placed in a humidified chamber overnight at 4°C.
Coating of particles with anti-BSA monoclonal antibody
Particles at a concentration of 108/mL were washed twice in PBS and mixed with anti-BSA mAb at a concentration of 350 µg/mL in a total volume of 500 µL in a microcentrifuge tube. The tube was placed on a vortex mixer at room temperature for 2 hours at 500 rpm followed by an overnight mixing in a roto-mixer at 4°C. Particles were washed twice in PBS and resuspended at a final concentration of 5×105/mL for the binding experiments.
Binding of antibody coated beads to BSA coated channels
The device coated with BSA at 4°C was allowed to come to room temperature (~10 minutes) and placed on an inverted motorized stage (LEP Ltd, Hawthorne, NY) mounted on an epi-fluorescence microscope (TE 2000, Nikon Instruments Inc., Melville, NY). The device was centered with the junction of the microchannel in focus at 4× magnification. A 1 mL syringe was loaded with the antibody coated particles and the flow rate was set to shear rate of 500 sec−1 to prime the tubing. The outlets of the device were immersed in a centrifuge tube filled with water to maintain a constant pressure. The primed tubing was inserted into the inlet port with the shear rate dropped to 240 sec−1. At the end of three minutes, a fluorescent image was taken using a cooled CCD Camera (Retiga Exi, Qimaging, Canada). The shear rate was then lowered progressively to 120, 60, 30 and 15 sec−1 with each shear rate being held constant for 3 minutes and an image taken sequentially. An AOI of 200 µm was created at the junction to calculate the number of bound particles/cm2 using NIKON Elements (Nikon, Melville, NY).
RESULTS
Fabrication of particles
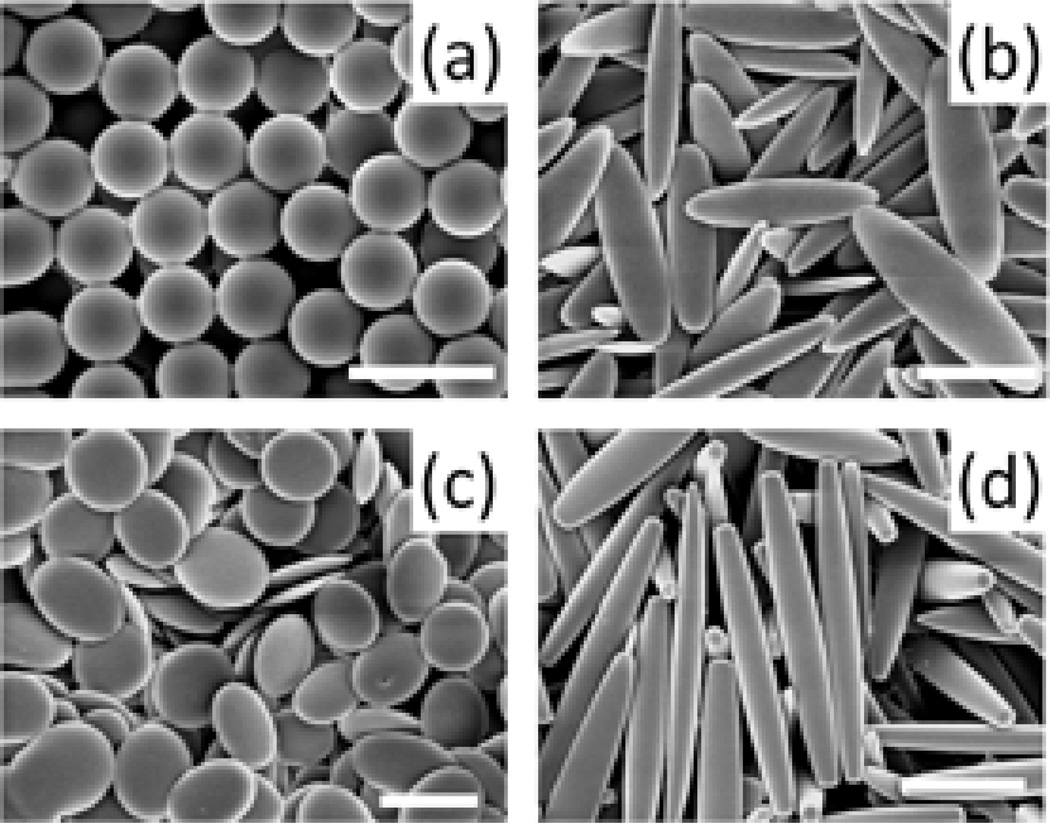
A library of particles of different shapes and sizes were used for the study. Spheres of three different sizes (1, 3, 6 µm) were used whereas particles of different shapes were fabricated by stretching the spheres thereby maintaining the volume constant and equal to that of the original sphere. Film stretching method described by Champion et al. was used to generate elliptical/circular disks, and rod-shaped particles [27] (Figure 1). In order to determine the effect of aspect ratio of particles on flow and adhesion, elliptical disks fabricated from 3 µm spheres were stretched to two different aspect ratios.
Figure 1.
Representative scanning electron micrographs of particles of different shapes used for the study. Spheres of three different sizes were used (1µm, 3 µm and 6 µm) whereas elliptical disks, circular disks and rods were fabricated by stretching these spheres using the film-stretching method, thereby resulting in carriers with 12 different geometries. (a) Spheres (b) Elliptical disks (c) Circular Disks (d) Rods. (scale bar 2 µm for (a), (b), (c) and 5 µm for (d))
Fabrication of SMNs
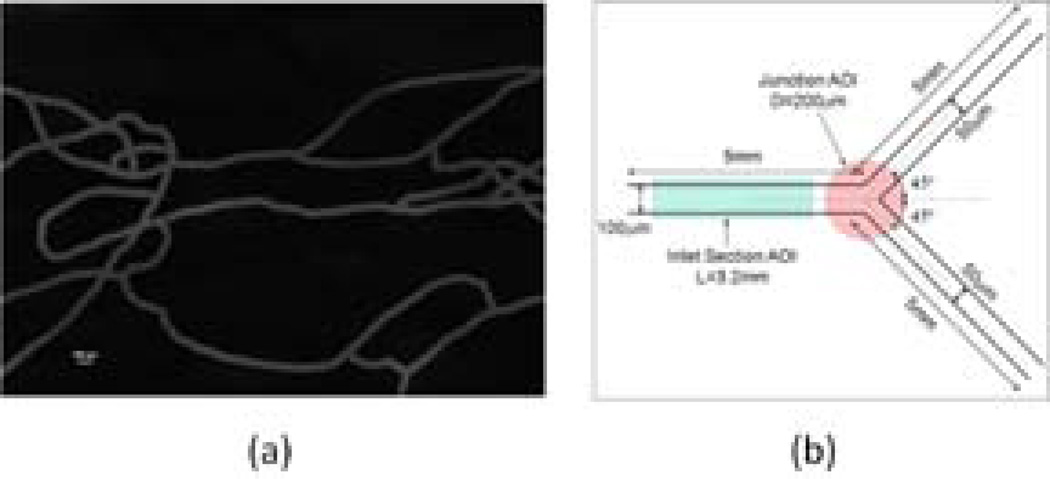
SMNs (Figure 2a) are physiological microchips that simulate the vascular microenvironment including scale, morphology, and fluidics. In this study, an idealized SMN comprising of a bifurcating microfluidic channel was prototyped using soft lithography techniques. The bifurcation was symmetric and the dimensions of the different channel sections are shown in Figure 2b.
Figure 2.
Synthetic microvascular networks (a) Image of a typical SMN generated from the hamster microvascular network perfused with FITC (scale bar 100 µm). (b) The symmetric bifurcating microchannel used for the study. The areas highlighted in red and green correspond to the sections of the microchannel used to quantify the number of particles attached in the inlet section and the junction region respectively.
Effect of geometry on receptor-mediated adhesion
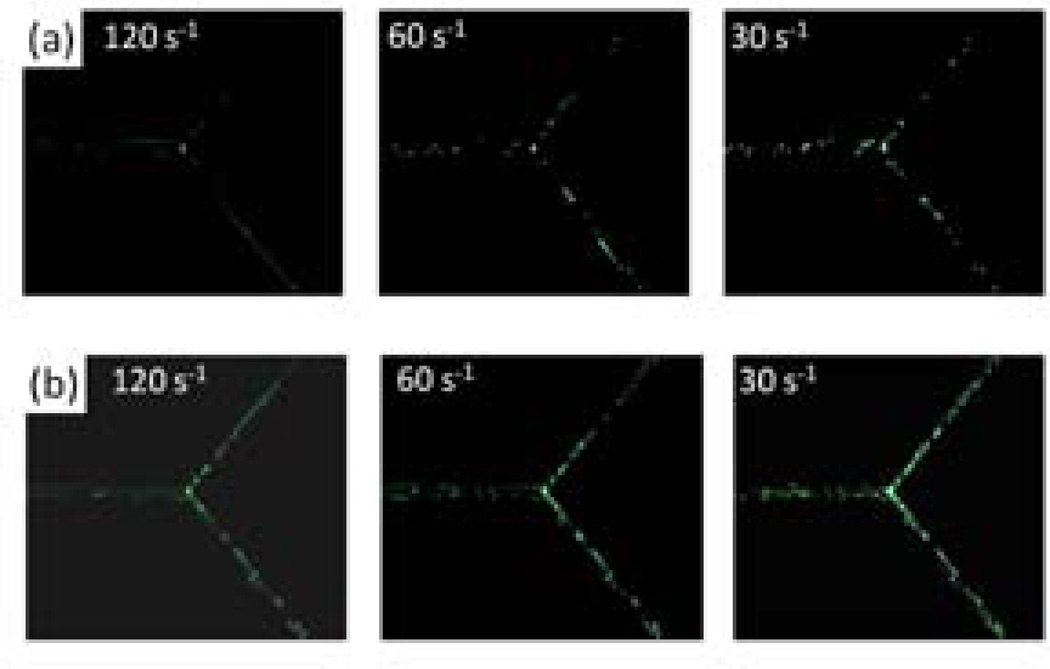
The SMNs were coated with bovine serum albumin (BSA) and particles of different geometry were coated with anti-BSA to facilitate receptor-mediated adhesion. Particles were flown through the bifurcating SMN at different shear rates ranging from 250 s−1 to 15 s−1 which are the typical physiological shear rates observed in the microcirculation [28]. Fluorescent images of the straight section and the junction were taken at the end of a fixed time interval (Figure 3) and the adhesion propensity was quantified in terms of the number of particles attached per unit area of the channel. The straight section and the region of the junction used for the analysis are highlighted in Figure 2b.
Figure 3.
Effect of carrier geometry on adhesion in the SMN at different shear rates. Images show adhesion of anti-BSA-coated articles to BSA-coated microchannels at various shear rates. (a) spheres (b) elliptical disks. The difference in the adhesion propensities between spheres and elliptical disks can be clearly observed especially at low shear rates.
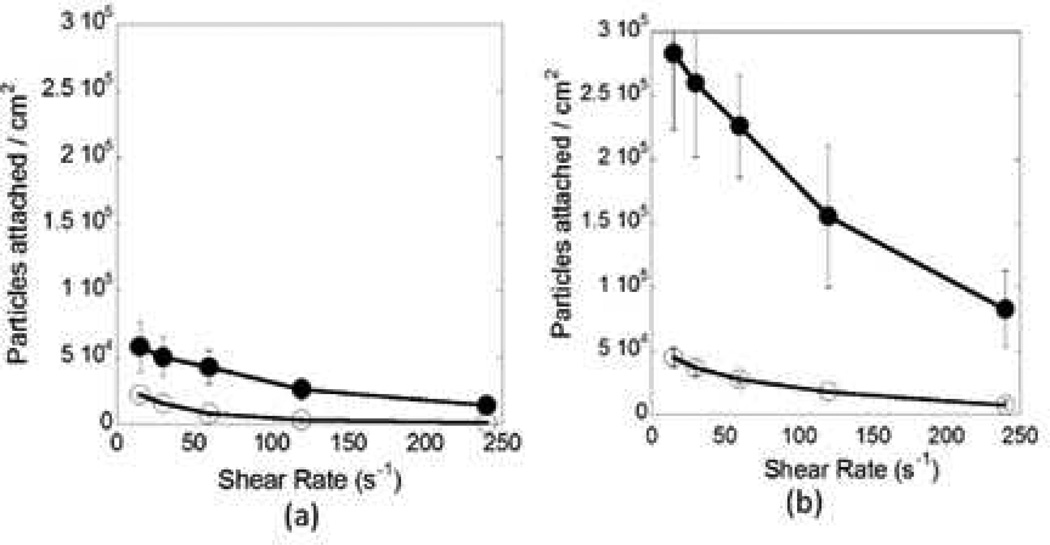
Typical relationships between particle adhesion and shape as well as shear rate are shown in Figures 4a (inlet section) and 4b (junction). These data correspond to 3 µm spheres (open circles) and elliptical disks made from these spheres (closed circles, aspect ratio of 6.5). Particle adhesion was significantly higher at the junctions compared to the straight section of the SMN. Similar trend was observed for particles of all shapes and sizes (Table 1). Adhesion in either region was dependent on particle geometry. For example, at low shear rates (15 s−1), high aspect ratio elliptical disks fabricated from 3 µm spheres showed ~6.5 fold higher attachment at the junction compared to spheres of the same volume (Figure 4b). The same ratio, at the inlet section was ~2.5 (Figure 4a). At higher shear rates (250 s−1), the total number of particles attached per unit area of the junction decreased, however, the difference between spheres and elliptical disks increased significantly (~11 fold at the junction and ~10 fold at the inlet section, Figures 4a,b). The attachment propensity of circular disks fabricated from 3 µm spheres was intermediate between the spheres and elliptical disks, indicating that elongation, rather than flatness, may be a dominant factor in adhesion. In order to further verify the importance of elongated shape of particles in adhesion, elliptical disks of two different aspect ratios stretched from 3 µm spheres were tested. A two-fold enhancement in adhesion was observed both at the inlet section and at the junction when the aspect ratio was increased from ~4.7 to ~6.5.
Figure 4.
Comparison of the adhesion propensity of 3 µm spheres and elliptical disks stretched from 3 µm spheres. (a) Number of particles attached per unit area at the inlet section of the microchannel as a function of shear rate. (b) Number of particles attached per unit area in the junction region of the microchannel as a function of shear rate (closed circles: elliptical disks, open circles: spheres).
Table 1.
The ratio of the number of particles attached at the junction to the inlet section for particles of all geometries at the lowest shear rate of 15 s−1. The ratio is always greater than one, which confirms that the attachment at the junction was always higher than the attachment at the inlet section for particles of all shapes and sizes.
| Adhesion at Junction / Adhesion at Inlet | ||||
|---|---|---|---|---|
| Shape | Sphere | ED | CD | Rods |
| Size | ||||
| 1 µm | 1.56 | 2.70 | 3.54 | 3.93 |
| 3 µm | 1.99 | 4.95 (AR 4.7) 4.86 (AR 6.5) |
1.96 | - |
| 6 µm | 6.69 | 2.61 | 11.62 | 7.85 |
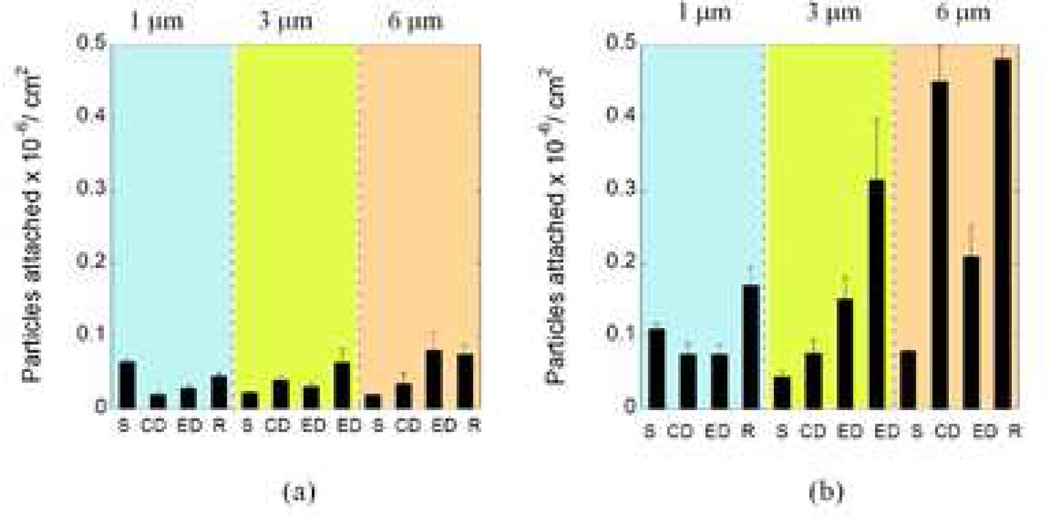
To assess the role of size in adhesion, experiments were conducted using particles of different shapes fabricated from 1 µm and 6 µm spheres. Since elongation of the particles seemed to have a stronger effect on attachment compared to flatness, high aspect ratio (AR ~9) rod-shaped particles were also fabricated from 1 µm and 6 µm spheres in addition to elliptical and circular disks. Although there were quantitative differences in the attachment tendency of these particles, the qualitative trends remained the same as 3 µm particles (Figure 5). Rod-shaped particles exhibited significantly higher attachment compared to any other particle shape. Moreover, with an increase in size, the difference between the adhesion profiles of spheres and other shapes was increased.
Figure 5.
Comparison of the adhesion propensity of carriers in terms of the number of particles attached per unit area for all shapes and sizes used for the study at the lowest shear rate of 15 s−1. (S= spheres, ED = Elliptical disks, CD = Circular Disk, R = Rods. For 3 µm particles, the two EDs correspond to low aspect ratio and high aspect ratio elliptical disks) (a) Particle attachment profile at the inlet section (b) Particle attachment profile at the junction.
DISCUSSION
Spheres, rods, and elliptical/circular disks together constitute representative shapes that provide insights into the role of particle shape in vascular dynamics. Rods provide information about the importance of elongation, circular disks help determine the importance of flatness whereas elliptical disks represent a combination of elongation and flatness. Moreover, variation of size for each shape sheds light on the interplay between size and shape in terms of particle attachment. Particles of ~1 µm size have been used in recent studies focused on targeted delivery to the endothelium and were shown to provide certain advantages compared to nano-scale particles typically used in drug delivery applications [13]. Three and 6 µm particles used in this study, though much larger than those generally used for drug delivery, are comparable to the size of circulating cells such as red blood cells and platelets whose vascular dynamics is also of significant interest.
A synthetic system that simulates the vasculature offers great advantage since it allows variation of a single design parameter to study the particle transport and adhesion dynamics. SMNs are microfluidic systems that capture the structure, fluid flow characteristics, and physiological behavior of biological microvascular networks and offer significant advantages over conventional parallel plate or straight channels since they better mimic the in vivo environment [26]. In addition to the advantages of SMNs mentioned above, reduced reagent consumption, low cost of manufacture, disposable nature, minimal contamination and suitability for cell culture make it a suitable system for simulating and predicting the interaction of particles with the vasculature. In comparison with linear flow chambers, SMNs clearly show both increased adhesion and a better ability to discriminate among different types of particles. Further experiments with more complex SMNs will provide further insight into the flow and adhesion properties of carriers and will better represent the in vivo behavior.
The adhesion of carriers to the channel wall depends on several parameters: (i) particle surface chemistry and texture (ii) the strength of interactions between the complementary biomolecules present on the surface of particles and the channel, (iii) the contact surface area between the particles and the channel wall, (iv) the margination of the carriers towards the channel wall during flow [29], (v) the settling velocity of particles, and (vi) hydrodynamic drag [14, 16]. Some aspects of the trends observed in this study can be explained based on the contribution of the above parameters. The surface chemistry of particles of all shapes and sizes was maintained constant by coating them with anti-BSA. All particles used in the study possess a smooth surface since the stretching process does not alter the surface texture. Previous studies have already shown that elongated particles exhibit higher margination tendency compared to spheres irrespective of size and density [29]. They also exhibit lower drag and higher contact surface area compared to spheres. Consequently, it is understandable that for all sizes, the rod shaped particles are likely to exhibit significantly higher adhesion compared to spheres.
The effect of shape was amplified at larger particle sizes, which could potentially be attributed to higher drag or higher settling velocity of larger particles compared to smaller ones. The adhesion propensity of spheres in the linear inlet section decreased with increasing particle size, which is in good qualitative agreement with previously reported findings [30]. This is largely due to the fact that larger particles experience stronger shear (detachment) forces. However, this is not the case for non-spherical particles, where particle flatness and elongation as well as orientation during particle-wall interactions become critical to particle arrest and firm adhesion. This trend of lower adhesion for larger sizes is not observed for adhesion at the junction, even for spherical particles. Regardless of size and shape, significantly higher adhesion was observed at the junction compared to the straight channel. This substantiates the use of synthetic microvascular networks in screening candidates for targeted delivery over the traditionally used straight microchannels.
Future studies should involve detailed modeling and simulation of the transport and adhesion of non-spherical particles in the microchannels which will help in predicting the targeting ability of carriers. In order to further mimic the vasculature, particle adhesion in more complex networks [26] needs to be investigated. Targeting efficiency can be studied by coating the channels with different proteins and testing the attachment of surface coated particles to a particular protein. Moreover, these networks can also be lined with endothelial cells to study particle interaction with surface receptors as well as particle uptake and transfection [25]. Evaluation of nanoscale (100–250 nm) particles will also be critical for drug delivery applications. Intravenously injected particles designed for targeting tumors are typically in this size range. These particles cannot be visualized at an individual level and hence analysis based on overall fluorescence will be necessary.
The flow and adhesion of particles of different geometries studied here using SMNs can have wide implications in the field of drug delivery. First, the trends showed in this study may prove to be instrumental in engineering carriers for specific functions required for drug delivery. For example, since rod-shaped particles show significantly higher adhesion compared to spherical particles, use of rod-shaped particles could be advantageous for targeted drug delivery to the endothelium. Moreover, the difference in the adhesion profiles at the junctions and the inlet sections can be utilized to specifically target vascular junctions for the treatment of certain diseases. For example, atherosclerosis is a geometrically focal disease which preferentially affects the outer edges of vessel bifurcations [28].
CONCLUSION
Particles of different geometries exhibited remarkably different adhesion profiles thereby proving the hypothesis that particle shape plays an important role in attachment to the target site. While the adhesion propensity of both elongated and flattened particles was higher than spheres, elongated particles consistently exhibited higher adhesion, the extent of which was dependent on the aspect ratio of the particles. The adhesion at the junction of a bifurcating network, with complex flow, was significantly increased from that in the linear section of the channel demonstrating the advantage of using SMNs over traditionally used straight channels. Further studies with more complex SMNs that simulate the vasculature more closely will elucidate the effect of particle shape on particle transport and adhesion to the vasculature for targeted drug delivery. Such studies will have important implications in designing carriers for targeted drug delivery.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from NIH (2R44HL076034-02) and NHLBI Program of Excellence in Nanotechnology (1UO1 HL080718) for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Langer R. Drug delivery and targeting. Nature (London) 1998;392(6679):5–10. [PubMed] [Google Scholar]
- 2.Sudimack J, Lee R. Targeted drug delivery via the folate receptor. Advanced drug delivery reviews. 2000;41(2):147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 3.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 4.Farokhzad O, Cheng J, Teply B, Sherifi I, Jon S, Kantoff P, Richie J, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. National Acad Sciences. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrama D, Reisfeld R, Becker J. Antibody targeted drugs as cancer therapeutics. Nature Reviews Drug Discovery. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 6.Kohane D. Microparticles and nanoparticles for drug delivery. Biotechnology and bioengineering. 2006;96(2):203–209. doi: 10.1002/bit.21301. [DOI] [PubMed] [Google Scholar]
- 7.Alexis F, Pridgen E, Molnar L, Farokhzad O. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghimi S, Hunter A, Murray J. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 9.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nature materials. 2009;8(1):15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi N, Mitragotri S. Designer biomaterials for nanomedicine. Advanced functional materials. 2009:1–13. [Google Scholar]
- 11.Champion J, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gratton S, Ropp P, Pohlhaus P, Luft J, Madden V, Napier M, DeSimone J. The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher D. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature nanotechnology. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular Delivery of Particulate Systems: Does Geometry Really Matter? Pharmaceutical Research. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 16.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Annals of biomedical engineering. 2005;33(2):179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 17.Park J, von Maltzahn G, Zhang L, Schwartz M, Ruoslahti E, Bhatia S, Sailor M. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging*. Adv. Mater. 2008;20(9):1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luscinskas F, Kansas G, Ding H, Pizcueta P, Schleiffenbaum B, Tedder T, Gimbrone M., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. Journal of Cell Biology. 1994;125(6):1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haun J, Hammer D. Quantifying nanoparticle adhesion mediated by specific molecular interactions. Langmuir: the ACS journal of surfaces and colloids. 2008;24(16):8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 20.Decuzzi P, Gentile F, Granaldi A, Curcio A, Causa F, Indolfi C, Netti P, Ferrari M. Flow chamber analysis of size effects in the adhesion of spherical particles. International Journal of Nanomedicine. 2007;2(4):689–696. [PMC free article] [PubMed] [Google Scholar]
- 21.Ham A, Goetz D, Klibanov A, Lawrence M. Microparticle adhesive dynamics and rolling mediated by selectin-specific antibodies under flow. Biotechnology and bioengineering. 2007;96(3):596–607. doi: 10.1002/bit.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucik D. Measurement of adhesion under flow conditions. In: Bonifacino Juan S., et al., editors. Current protocols in cell biology/editorial board. Unit 9.6. Chapter 9. 2003. [DOI] [PubMed] [Google Scholar]
- 23.Sarvepalli D, Schmidtke D, Nollert M. Design Considerations for a Microfluidic Device to Quantify the Platelet Adhesion to Collagen at Physiological Shear Rates. Annals of biomedical engineering. 2009;37(7):1331–1341. doi: 10.1007/s10439-009-9708-z. [DOI] [PubMed] [Google Scholar]
- 24.Chau L, Doran M, Cooper-White J. A novel multishear microdevice for studying cell mechanics. Lab on a Chip. 2009;9(13):1897–1902. doi: 10.1039/b823180j. [DOI] [PubMed] [Google Scholar]
- 25.Nicpon Marieb Elaine, Mallatt Jon, Wilhelm PB. Human anatomy, Pearson/Benjamin Cummings. 2004 [Google Scholar]
- 26.Rosano J, Tousi N, Scott R, Krynska B, Rizzo V, Prabhakarpandian B, Pant K, Sundaram S, Kiani M. A physiologically realistic in vitro model of microvascular networks. Biomedical microdevices. 2009;11(5):1–7. doi: 10.1007/s10544-009-9322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhakarpandian B, Pant K, Scott R, Patillo C, Irimia D, Kiani M, Sundaram S. Synthetic microvascular networks for quantitative analysis of particle adhesion. Biomedical microdevices. 2008;10(4):585–595. doi: 10.1007/s10544-008-9170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104(29):11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malek A, Alper S, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282(21):2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 30.Gentile F, Chiappini C, Fine D, Bhavane R, Peluccio M, Cheng M, Liu X, Ferrari M, Decuzzi P. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. Journal of Biomechanics. 2008;41(10):2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Patil V, Campbell C, Yun Y, Slack S, Goetz D. Particle diameter influences adhesion under flow. Biophysical Journal. 2001;80(4):1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]