Abstract
Design of carriers for effective delivery and targeting of drugs to cellular and sub-cellular compartments is an unmet need in medicine. Here, we report pure drug nanoparticles comprising camptothecin (CPT), trastuzumab (TTZ) and doxorubicin (DOX) to enable cell-specific interactions, subcellular accumulation and growth inhibition of breast cancer cells. CPT is formulated in the form of nanorods which are coated with TTZ. DOX is encapsulated in the TTZ corona around the CPT nanoparticle. Our results show that TTZ/DOX-coated CPT nanorods exhibit cell-specific internalization in BT-474 breast cancer cells, after which TTZ is recycled to the plasma membrane leaving CPT nanorods in the perinuclear region and delivering DOX into the nucleus of the cells. The effects of CPT-TTZ-DOX nanoparticles on growth inhibition are synergistic (combination index = 0.17±0.03) showing 10-10,000 fold lower inhibitory concentrations (IC50) compared to those of individual drugs. The design of antibody-targeted pure drug nanoparticles offers a promising design strategy to facilitate intracellular delivery and therapeutic efficiency of anticancer drugs.
Keywords: Camptothecin, herceptin, doxorubicin, nanoparticle, shape, synergistic, morphology
Formulating drugs to bind to desired cellular and sub-cellular targets is a necessary step in the design of anti-cancer therapies. Towards this goal, nanocarriers of polymers,1 gold,2 iron,3 carbon nanotubes,4 quantum dots,5 micelles,6 and liposomes,7 among others, have been developed and have been targeted to various cells using antibodies,1,2,8,9 aptamers10–13 and peptides.7,14,15 Interactions of drug nanocarriers with the cell membrane and their intracellular localization depend on the nanocarrier’s size, shape and surface chemistry. The size of nanocarriers has been shown to impact the extent and the mechanism of cellular internalization.16 Particle shape has been shown to impact the extent as well as specificity of internalization.17–20 Following internalization, drug nanocarriers are transported progressively to the endosomes, recycling endosomes, acidic lysosomes, mitochondria or nucleus, depending on the drug’s physico-chemical properties.16,19,21,22 During the intracellular transport, it is often desirable to protect drugs from the degradation in the acidic lysosomes. Drug nanocarriers with folate,23,24 transferrin2 or other monoclonal antibodies9 have been shown to release drugs in early endosomes, thereby minimizing potential drug loss due to acidification. These targeted drug nanocarriers have been shown to be more effective than free drugs both in vitro and in vivo.24–26
In this study, we explore sub-cellular distribution and therapeutic efficiencies of pure drug nanorods prepared using a combination of a hydrophobic anti-cancer drug camptothecin (CPT), a monoclonal antibody, trastuzumab (TTZ), and a water-soluble anti-cancer drug, doxorubicin (DOX). CPT is a topoisomerase I (topoI) enzyme inhibitor that targets the cleavable DNA-topoI complex (topoIcc).27–29 CPT stacks between the DNA and topoIcc, prevents their dissociation, and induces replication/transcription-mediated DNA damage.27,28 It selectively sensitizes cancer cells compared to normal cells by exhibiting S-phase cytotoxicity and G2-M cell cycle arrest. However, the major limitations of CPT in clinical applications include its chemical instability in the lactone ring form, which gets converted to toxic carboxylate form at physiological pH, inability to penetrate cell membrane and drug efflux by p-glycoproteins. TTZ is an FDA-approved humanized monoclonal therapeutic antibody for the treatment of breast, colon and gastric cancers.30–34 However, its individual treatment shows a low response of 12–34%.31,33,35 Combination of TTZ with other anticancer drugs such as capecitabine, docetaxel, DOX, gemcitabine, paclitaxel, platines, vinorelbine, and other therapeutic antibodies, e.g., pertuzumab has shown improved therapeutic effects.35–39 DOX interferes with nucleic acid synthesis by intercalating between DNA base pairs in fast growing cancer cells and the DNA topoisomerase II (topoII) enzyme by inhibiting the relaxation of super-coiled DNA during transcription.40 DOX is a first-line chemotherapeutic agent against many types of cancers including breast, lung, ovarian and uterine cancers.41 However, its severe cardiotoxicity and other side effects have limited its use with a maximum tolerated dose of 550 mg/m2.42 We sought to address limitations of CPT, TTZ and DOX by using their combinations in a synergistic manner.
RESULTS
Synthesis and characterization of CPT-TTZ-DOX nanoparticles
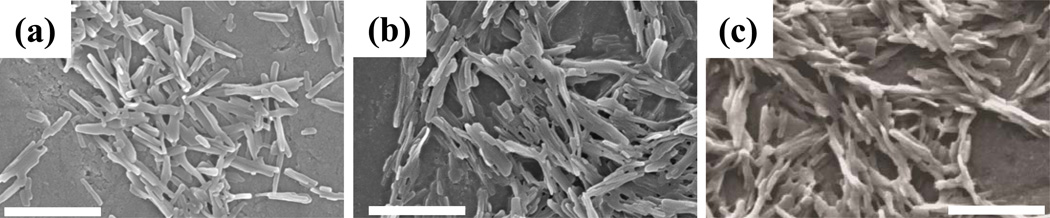
Rod-shaped CPT nanoparticles were prepared using solvent-diffusion method and the surfaces of the nanoparticles were coated with TTZ by physical adsorption. DOX was incorporated into CPT-TTZ nanoparticles by co-incubation. The SEM images of CPT nanorods, CPT-TTZ nanorods, and CPT-TTZ-DOX nanorods are shown in Fig. 1. The dimensions of these rods are 509.5±202.6 × 52.7±18nm, 511±156.9 × 56.7±14.7nm and 634.5±146.9 × 100.8±14.8nm, respectively. The w/w ratio of CPT:TTZ and CPT:DOX are 6 ± 0.1 and 0.38 ± 0.07, respectively (SI Table I). CPT-TTZ and CPT-TTZ-DOX particles possessed slightly negative surface zeta potentials (SI Table I). CPT nanorods themselves do not aggregate in PBS or even in medium, consistent with their strong negative zeta potential (SI Table I). The SEM images show appearance of aggregation due to drying of samples on the SEM stub.
Figure 1.
Scanning electron microscopy (SEM) showing the morphology and size of CPT, CPT-TTZ and CPT-TTZ-DOX nanorods. Scale bar = 500nm
Incubation of CPT nanorods in PBS for 72 h at physiological or low pH did not cause an appreciate change in size or shape (SI Fig. 1). This is consistent with low solubility of CPT in water (negligible in water and ~3µg/ml in 0.1M acetate buffer at pH 543,44). Both TTZ and DOX were released from the CPT surface. Desorption of TTZ from CPT particles has been previously characterized;20 only 12% TTZ desorbed after 2 and 22% in 24 h, when the particles were incubated with 10% fetal bovine serum (FBS) containing PBS buffer (pH 7.4) at 37°C. The amount of DOX release from CPT-TTZ-DOX nanoparticles was investigated in the same media (SI Fig. 2). About 25%, 45% and 55% of the loaded DOX were released after 30 min, 1 h and 2 h, respectively. This profile agrees with those reported previously.45
Intracellular uptake of CPT-TTZ
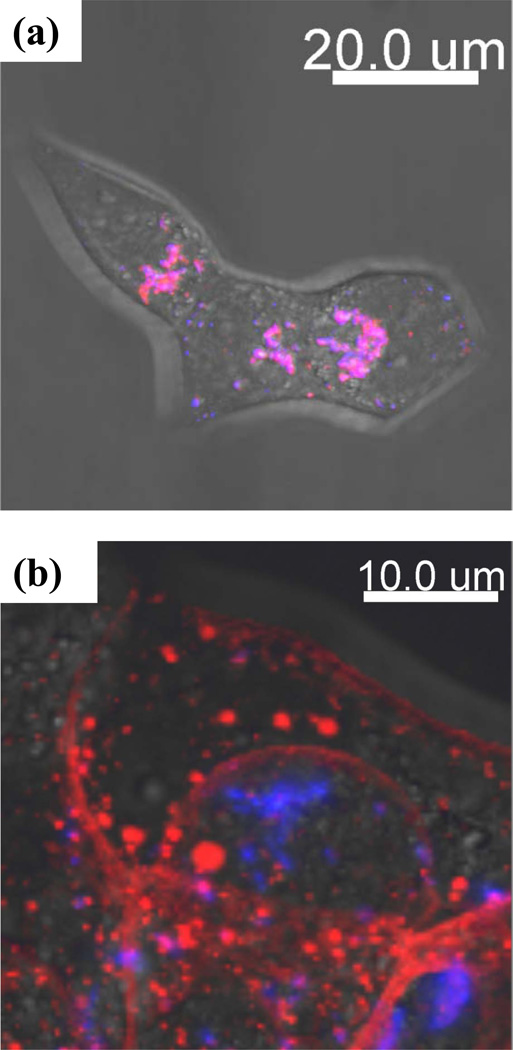
We first investigated intracellular delivery of Alexa 594-conjugated CPT-TTZ in BT-474 cells using confocal microscopy. CPT, because of its inherent fluorescence property, was highly fluorescent in the blue region upon excitation using a 405 nm UV laser (for details see SI Text 1). After 2 h incubation, CPT-TTZ accumulated in the cytoplasm showing high colocalization between Alexa 594-TTZ (red) and CPT nanorods (blue) inside the cells (Fig. 2a and SI Fig. 3). CPT nanorods without TTZ did not penetrate the cell membrane to a detectable extent (SI Fig. 4 (a)). To confirm that CPT-TTZ uptake occurs via specific interactions, cells were pre-incubated with excess of TTZ before exposure to CPT-TTZ. Indeed, no intracellular Alexa 594-TTZ or CPT signals were detected after blocking the receptor binding sites for TTZ (SI Fig. 4(b)).
Figure 2.
Intracellular localization of CPT (blue) and Alexa 594 conjugated TTZ (red) in BT-474 live cells after (a) 2 h and (b) 24 h incubation. BT-474 cells were treated with CPT-TTZ nanoparticles for 2 h, then (a) subsequently prepared for live cell imaging, or (b) incubated in fresh medium for 24 h at 37°C and imaged in live cells. The blue, red and DIC images are overlaid using Imaris. Magenta areas correspond to colocalization between CPT and TTZ.
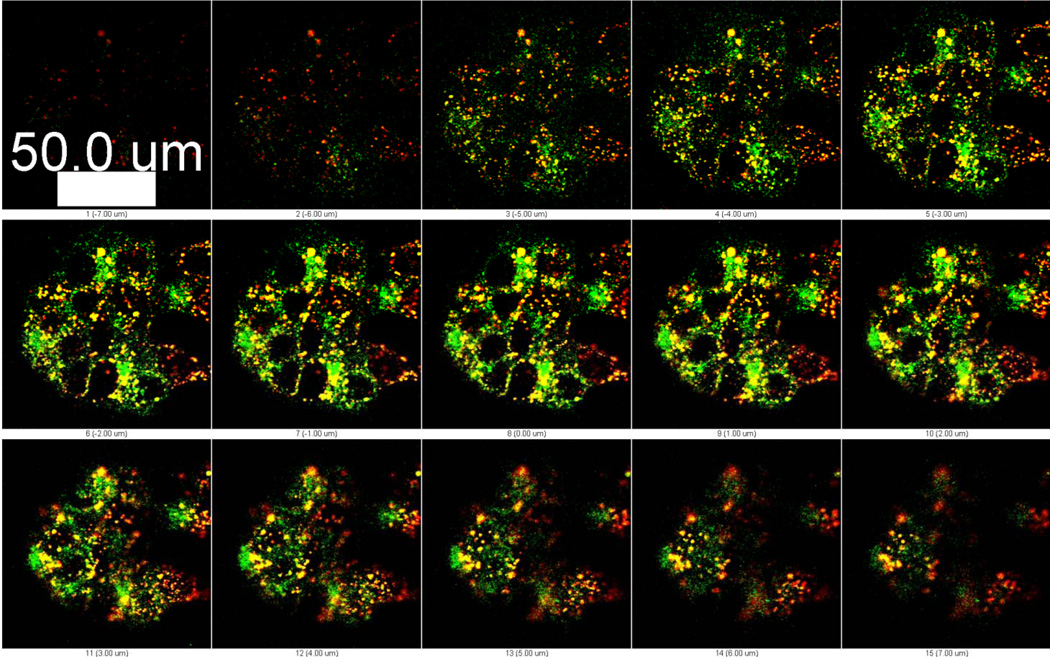
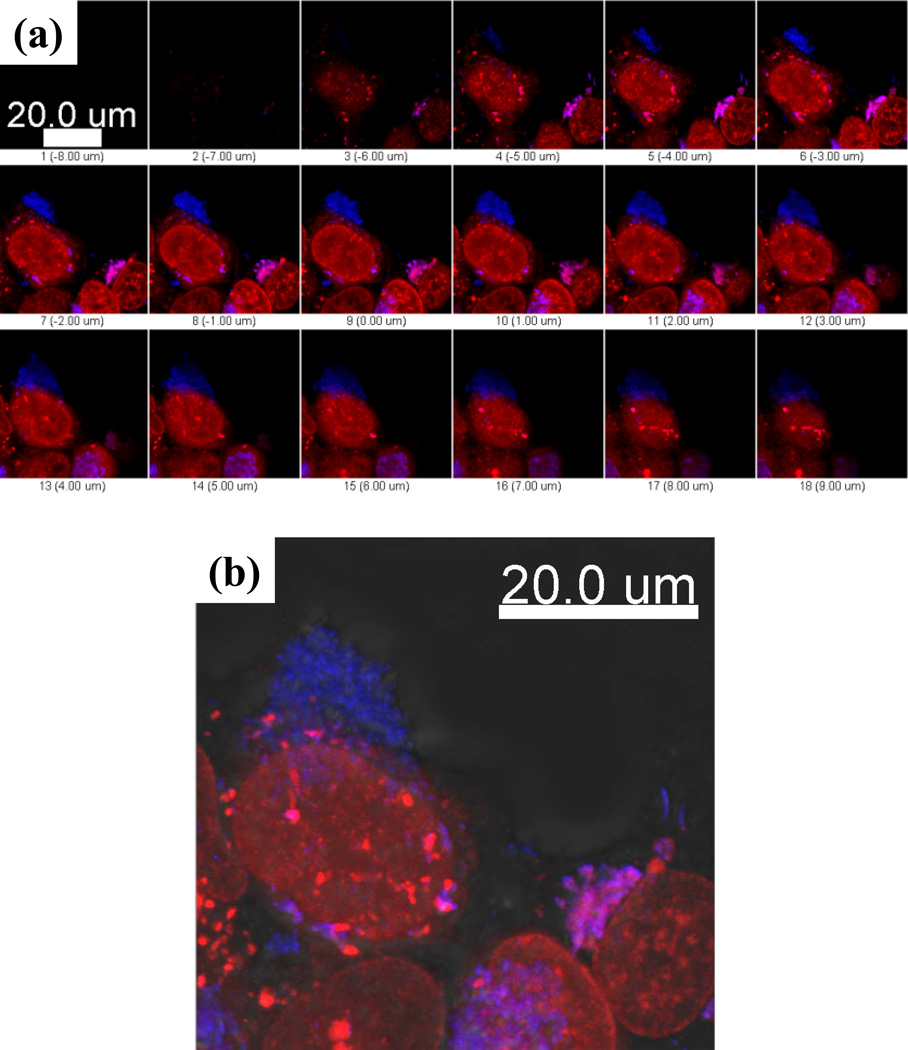
Upon internalization, Alexa 594-TTZ and CPT co-localized up until 2 h (R=0.73, Pearson’s correlation coefficient, Rr = 0.7) (Table I and SI Text 2). The extent of co-localization decreased over 24 h, indicating dissociation of TTZ from CPT over prolonged periods (Fig. 2b, Table I). It is likely that CPT-TTZ nanorod-containing early endosomes fuse to form sorting endosomes, where TTZ dissociates from CPT nanorods followed by recycling back to the plasma membrane. Indeed, experiments performed using Alexa 488-conjugated transferrin, a known endosomal recycling marker, indicated strong association of CPT-TTZ with sorting endosomes (Fig. 3, Table II and SI Text 3).
Table I.
Quantitative colocalization analysis of the confocal microscopic images of Alexa 594 conjugated TTZ (red) and CPT (blue). The coloclization coefficients were calculated using ImageJ’s intensity correlation analysis plugin and Imaris software. The Pearson’s correlation coefficient, Rr represents a correlation (positive, negative or zero) between red (TTZ) and blue (CPT) signals. The overlap coefficient, R demonstrates percentage colocalization between TTZ and CPT. m1 and m2 are colocalization coefficients of TTZ and CPT, respectively.
| Incubation time (h) |
Pearson’s correlation coefficient, Rr |
Overlap coefficient R |
Colocalization coefficients m1 and m2 |
|---|---|---|---|
| 2 | 0.7 ± 0.15 | 0.73 ± 0.03 | 0.63 ± 0.18 0.99 ± 0.02 |
| 24 | 0.2 ± 0.02 | 0.44 ± 0.15 | 0.58 ± 0.04 0.62 ±0.15 |
Figure 3.
Intracellular colocalization of surface-bound Alexa 594 TTZ (red) with the recycling endosome marker, transferrin (green). CPT- Alexa 594 TTZ nanoparticles were incubated with BT-474 cells for 2 h at 37°C and removed. Transferrin was added to the cells in fresh medium and incubated for an hour at 37°C. Cells were washed using PBS and reincubated in fresh medium for confocal microscopy. Z stacks at every 1µm cell section are shown. Greater colocalization (yellow) occurs in the middle sections of the cells than the top or bottom sections.
Table II.
Colocalization coefficients were calculated to estimate the colocaliztaion between TTZ (red) with recycling endosomal marker, transferrin (green), and CPT (blue) with transferrin (green).
| Channels | Pearson’s correlation coefficient Rr |
Overlap coefficient R |
Colocalization coefficients m1 and m2 |
|---|---|---|---|
|
Red : Green (TTZ : transferrin) |
0.44 ± 0.17 | 0.77 ± 0.13 | 0.72 ± 0.04 0.84 ± 0.08 |
|
Green : Blue Transferrin: CPT) |
0.13 ± 0.03 | 0.24 ± 0.11 | 0.52 ± 0.04 0.3 ± 0.06 |
TTZ recycling is also evident from red fluorescence of Alexa 594 at the plasma membrane (Fig. 2b). A continuous, high concentration of red signals was detected along the cell membrane of BT-474 cells indicating the predominant localization of TTZ at the cell surface. In contrast, free TTZ (stained with Alexa 594) itself was not recycled back to the plasma membrane even after 24 h when the cells were co-incubated with CPT-DMSO and TTZ solution in PBS, simultaneously (SI Fig. 4c). Free TTZ was internalized by the cells and remained inside the cells indicating no comparable recycling. CPT-DMSO precipitated outside the cells due to insolubility in water, and could not be internalized by the cells (SI Fig. 4c). Taken together, these data suggest that the overall properties of CPT-TTZ, including size and shape, play a key role in determining the intracellular distribution of the drugs.
The average fluorescence intensity of Alexa 594-TTZ per cell in BT-474 cells did not change between 2 and 24 h (SI Fig. 5), suggesting that only a small fraction of internalized TTZ is degraded and a majority is recycled back to the plasma membrane. To eliminate the possibility that Alexa 594 dye dissociated from TTZ conjugation and retained at the cell surface, we incubated BT-474 cells with the equal concentration of Alexa 594 conjugated anti-human IgG coated CPT nanorods and imaged cellular distribution of Alexa 594-IgG. Intracellular distribution of IgG was different than that observed for TTZ (SI Fig. 6). IgG was localized in clusters inside the cell rather spreading in the cytoplasm and plasma membrane. In addition, the total fluorescence intensity of Alexa 594-IgG was 8.7 times lower than that measured for Alexa 594-TTZ. Overall, these results demonstrate that TTZ enhances CPT uptake and TTZ itself recycles back to the plasma membrane with no significant amount of degradation.
Sub-cellular localization of CPT nanorods
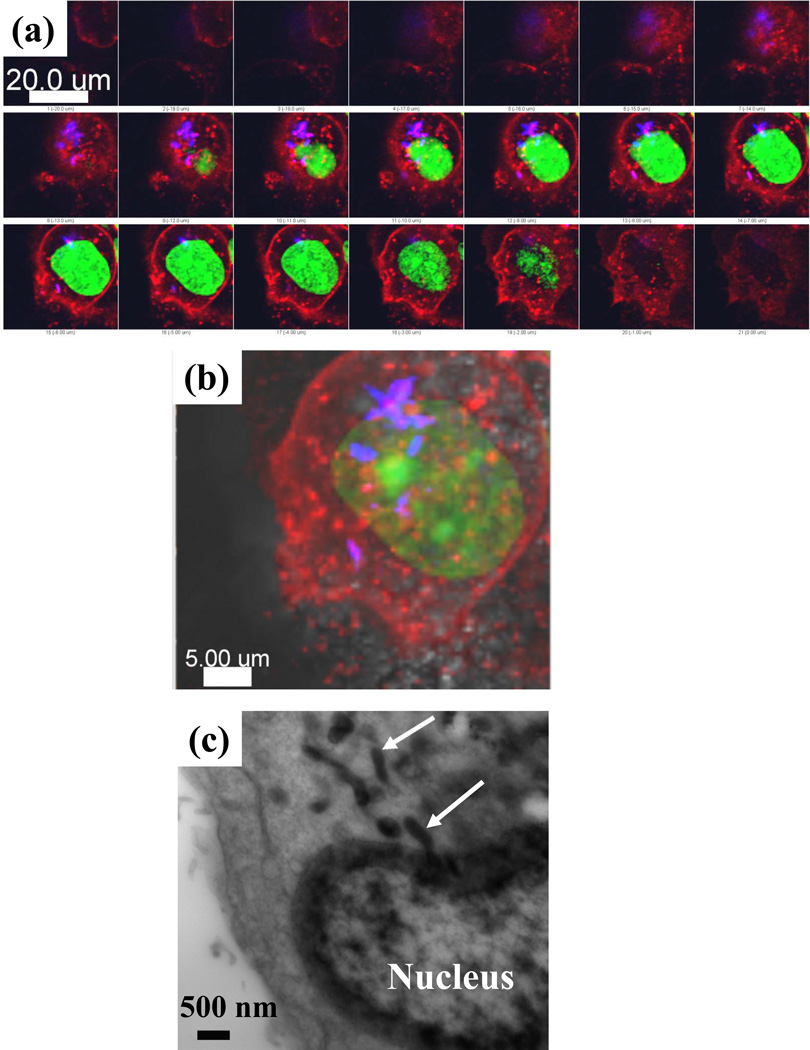
As an inhibitor of one of the nuclear enzymes, topoI, CPT is thought to localize inside the nucleus.28,46 While that may be the case for soluble forms of CPT, nanorods of CPT were found to accumulate in the perinuclear region (Fig. 4a and 4b). These observations were further validated with transmission electron microscopy. CPT was found in endosomes distributed from the plasma membrane close to the nucleus (Fig. 4c and SI Fig.s 7a and 7b). No such particles were found in the cells treated with PBS (SI Fig. 7c). Nuclear entry of CPT could not be seen in the TEM images. It is possible that small amounts of CPT dissolve with the cells and diffuse across the nuclear membrane into the nucleus.
Figure 4.
(a) and (b) Subcellular localization of CPT (blue) in live BT-474 breast cancer cells after 2 h CPT- Alexa 594 TTZ nanoparticle exposure followed by 24 h incubation in cell culture medium at 37°C. The cell nuclei were stained with green SYTO13 (Molecular Probes). (a) Projections of 1µm z-stacks showing the appearance of CPT nanoparticles close to the nucleus in the deeper sections of the cells. No CPT was found at the top or bottom cell sections. (b) The convergence of the z stacks and DIC image. (c) TEM image of BT-474 cell section incubated for 24 h with an initial exposure to CPT-TTZ-DOX nanoparticles for 2 h. Arrows are showing localization of rod-shaped nanoparticles close to the nucleus.
DOX that was once associated with CPT-TTZ nanorods, on the other hand, readily entered the nuclei of the cells, while leaving CPT nanorods outside the nucleus (Fig. 5). The precise time at which DOX dissociated from the nanorods is not clear. It is possible that DOX dissociated from the particles prior to their cellular entry and then diffused in the molecular form into the nucleus. Another possibility is that dissociation of DOX from nanorods occurred within the endosomes, followed by its diffusion into the nucleus. Free DOX, when incubated with a drug cocktail of CPT-DMSO and TTZ solution simultaneously, was also detected in the nuclei of the cells (SI Fig. 4c). However, quantitative analysis showed that the average fluorescence intensity of DOX per cell (42.9 ± 5.1) in CPT-TTZ-DOX is almost 1.7 times higher than that of free DOX (25.1 ± 10) per cell indicating higher accumulation of DOX inside the nucleus using CPT-TTZ-DOX. These results verify that the combination of CPT-TTZ-DOX nanoparticles complement sub-cellular distribution to attribute to better efficacy than free drugs.
Figure 5.
(a) Nuclear localization of red fluorescent DOX in BT-474 cells after 2 h incubation with CPT-TTZ-DOX particles. Cells were re-incubated in cell culture medium at 37°C for 24 h before confocal imaging. The blue signals show accumulation of CPT in the cytoplasm. (b) Overlay of the fluorescence images with DIC.
In vitro cell growth inhibition by CPT-TTZ-DOX
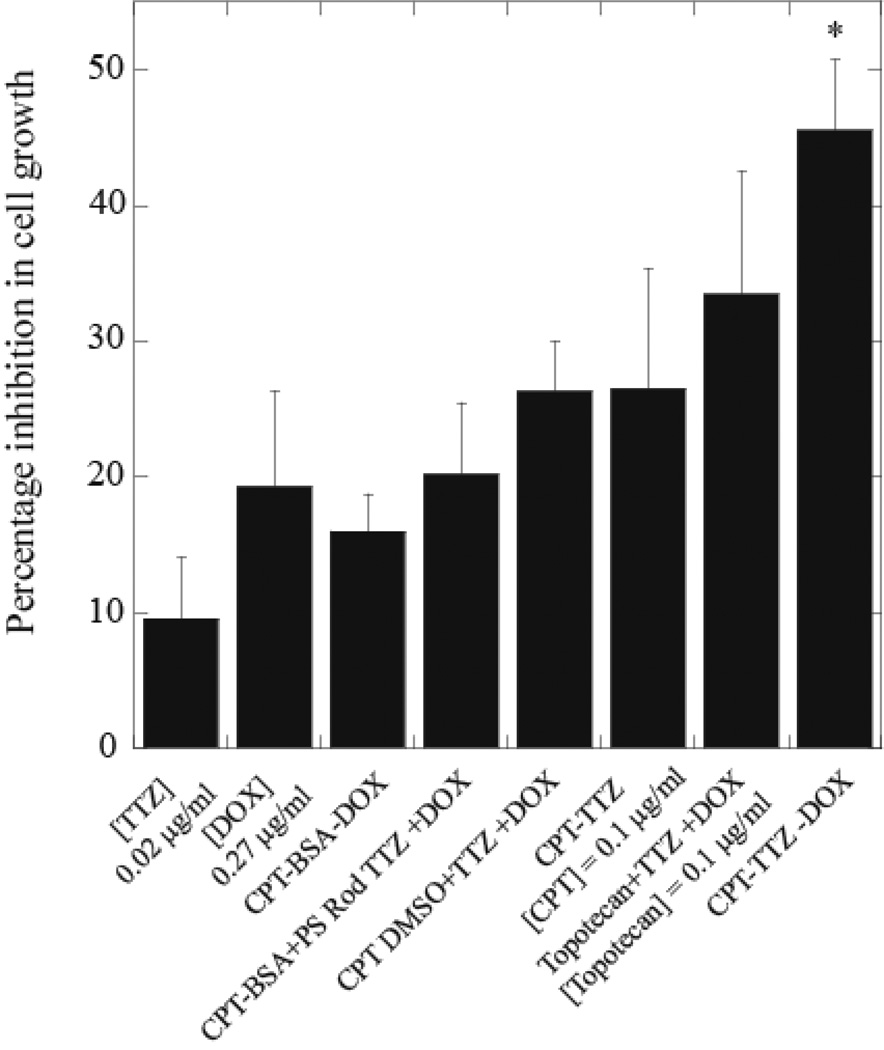
The effect of CPT-TTZ-DOX on in vitro grown inhibition of BT-474 cells was assessed. TTZ alone has an IC50 of about 1000µg/ml, above which the cell growth inhibition does not exhibit much dependence on concentration (SI Fig. 8a). The IC50 values for CPT and DOX are 1 and 4µg/ml, respectively (SI Fig 8b and c). Theoretical potency ( Dm ) of CPT, TTZ and DOX and the shape (m) of the dose effect curve of each drug are described in SI Text 4 and shown in SI Fig. 8d–8f. The combination CPT-TTZ-DOX yielded 45.5 ± 5.2% cell growth inhibition at concentrations of TTZ, CPT and DOX of 0.02, 0.1 and 0.27µg/ml, respectively (Fig. 6), which are 10–50,000 times lower than those required to accomplish similar inhibition with individual drug treatments (SI Fig. 8). Individual drug concentration at the same value as that used in CPT-TTZ-DOX nanorods induced less than 20% growth inhibition (Fig. 6); CPT-BSA-DOX particles induced only 16 ± 2.7% % inhibition confirming the role of TTZ in the activity of CPT-TTZ-DOX. Simultaneous delivery of CPT-BSA nanoparticles, TTZ-coated polystyrene nanoparticles (SI Fig. 9a and SI Text 5 for the preparation) and free DOX also showed lower growth suppression (20.2 ± 5.3%) than CPT-TTZ-DOX formulation suggesting the CPT-TTZ nanorod assisted combination therapies. However, CPT-TTZ without DOX induced only 26.5 ± 8.9% inhibition demonstrating that the addition of DOX to CPT-TTZ enhanced efficacy. The combination using the same concentrations of soluble CPT in DMSO or a water soluble form of CPT (topotecan), TTZ solution and free DOX inhibited cell growth by 26.3–30% (Fig. 6), which is significantly less than that induced by CPT-TTZ-DOX single formulation. The synergy for CPT-TTZ-DOX is also specific for Her2 overexpressing cancer cells and is not seen in MDA-MB-231 cell line expressing lower or no Her2 (SI Fig. 9b). The combination index (CI) based on the Chou-Talalay equation was calculated to be 0.17 ± 0.03 using, indicating highly synergistic actions of three drugs.
Figure 6.
Effect of CPT-TTZ-DOX particles on cell growth inhibition of BT-474 cells. Data are shown as a percent inhibition of cell growth compared to PBS-treated control cells. The number of live cells was determined using calcein-AM (Molecular Probes). BT-474 cells were treated with 0.02µg/ml TTZ solution, 0.27µg/ml DOX solution, CPT-BSA-DOX, CPT-TTZ and CPT-TTZ-DOX nanoparticles for 2 h followed by 72 h incubation in medium. Cells were also treated with the same concentrations of triple drug cocktails of CPT-BSA, TTZ-coated polystyrene and free DOX; soluble CPT in DMSO, TTZ solution and free DOX; and a soluble form of CPT (topotecan), TTZ solution and free DOX. Columns represent mean values of three replicates. CPT-TTZ-DOX treatment is statistically significant (p≤0.05) compared with other treatments (SI Table II) as indicated by the asterisk.
Effect on cell cycle
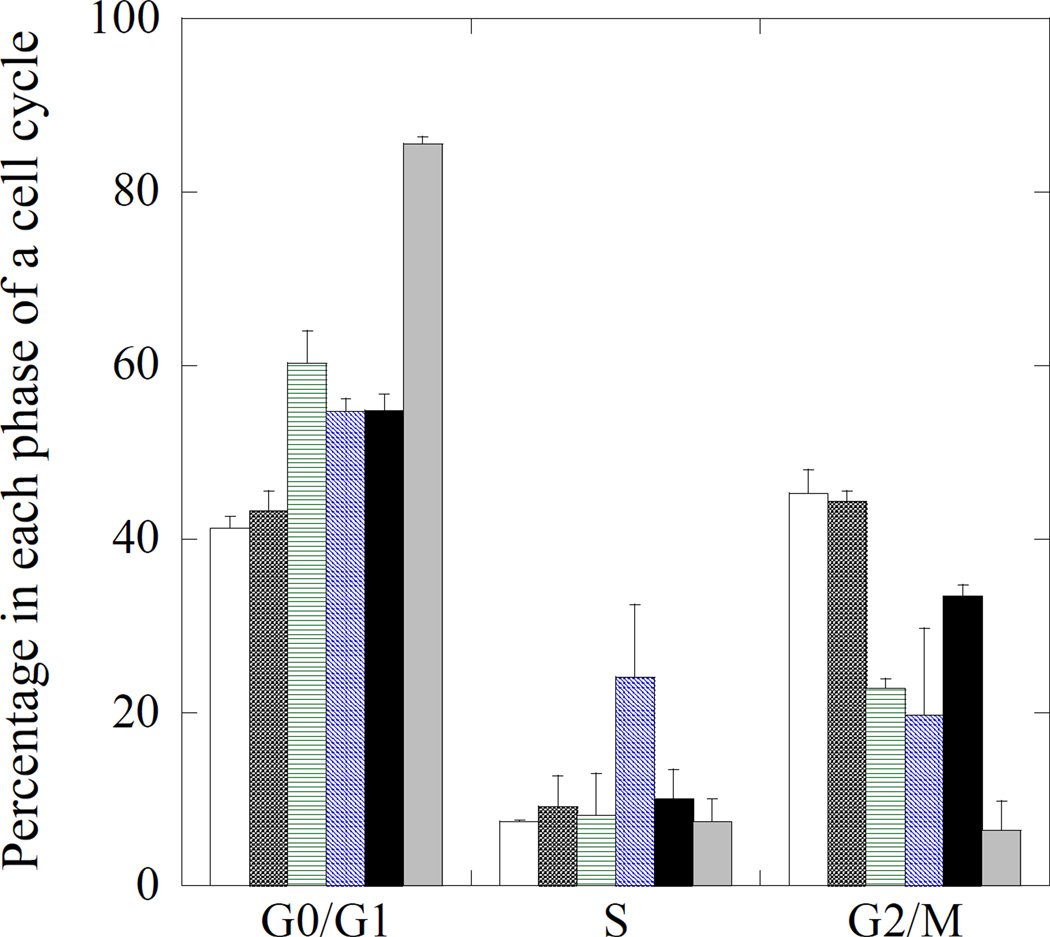
Mechanistically, TTZ, CPT and DOX have been reported to arrest cells in the G0/G1 (resting phase), S (DNA synthesis) and G2/M (prior to cell division) phases, respectively. 46,48–50 Cell populations in each phase after exposure to CPT-TTZ-DOX were determined (Fig. 7 and SI Fig. 10). A high concentration (10mg/ml) of TTZ solution was used as a positive control that arrested 85.6% cells in the G0/G1 phase (grey bars) compared to 41% in the absence of any drug (open bars). TTZ, adsorbed on polystyrene nanorods, was used as particulate form of the antibody20. The physical properties of polystyrene nanorods were comparable to CPT nanorods (comparable to CPT nanorods SI Fig. 9a). TTZ nanorods, either polystyrene (hatched), CPT (two-way crossed) or CPT-DOX (black bars), arrested 55–60% cells in the G0/G1 phase indicating that TTZ retains its function after adsorption to nanoparticle surfaces (Fig. 7 and SI Fig. 10). BSA coated polystyrene rods (star-crossed) did not exhibit elevated population in the G0/G1 phase compared to untreated control confirming that neither polystyrene or BSA affect cell cycle. CPT-TTZ nanorods arrested 24% cells in the S phase compared to < 10% for controls, thus confirming preservation of CPT activity in the antibody-coated nanorod form. CPT-TTZ-DOX reduced the population in the S phase, but increased the population in the G2/M phase compared to TTZ-polystyrene rods and CPT-TTZ indicating the effect elicited by DOX. However, the percentage of cells in the G2/M phase due to CPT-TTZ-DOX is lower than that of the untreated cells. Combined together, the effect of TTZ appears to be dominant in CPT-TTZ-DOX combination followed by DOX and CPT in that order.
Figure 7.
Effect on cell cycle in BT-474 cells. Cells were grown overnight in T25 flasks, and incubated with PBS (open bars), BSA coated polystyrene rod shape nanoparticles (crossed), TTZ-coated polystyrene nanorods (horizontal), TTZ-coated CPT nanorods (hatched), CPT-TTZ-DOX (black bars) and TTZ solution (10mg/ml) (grey). After 2h incubation, cells were incubated in fresh medium for 72 h, trypsinized, resuspended in HBSS, and stained with Vybrant DyeCycle Violet stain. At least 50,000 cells were analyzed by flow cytometry. The quantitative data of cell cycle distribution represents mean ± standard deviation.
DISCUSSION
Understanding the mechanisms of drug penetration into the cell and subcellular compartments has both pharmacodynamic and clinical applications. To elicit a therapeutic response, drug molecules must penetrate the cell surface and initiate their actions in cellular organelles. In this study, we show efficient intracellular delivery of a hydrophobic drug, CPT, and nuclear delivery of DOX using a pure drug nanoparticle construct, CPT-TTZ-DOX. TTZ induces the endocytosis of CPT. The results are consistent with the constitutive internalization and rapid recycling rate of TTZ-Her2 receptor protein complex to the plasma membrane.22 The endocytic sorting of TTZ-Her2 complexes in early and recycling endosomes (tubulovesicular compartment) have been reported at 16.6% and 70.5%, respectively after 3 h incubation in SK-Br-3 cells.22 TTZ-coated gold nanorods (60 × 13 nm) have been found to reside mostly in the early endosomes and then in lysosomes of SK-Br-3 cells after 6 h.51 However, our results show CPT localization close to the nucleus after 24 h, but majority of TTZ recycles back to the plasma membrane in the following mechanisms. The early endosomes carrying Tf and CPT-TTZ individually fuse and form a dynamic tubulovesicular endosomal compartment in which Tf, CPT and TTZ are sorted out with the help of sorting nexin-4 (SNX4) protein.22,52 The apo-Tf (Tf after releasing its iron content), TTZ and their respective receptors are sorted out in the tubular compartments of the tubulovesicular endosome, pinched off from the vesicular compartment, and recycled back to the plasma membrane. CPT nanorods remain in the vesicular compartments of the endosome, are transported deeper in the cytoplasm, and matured to late endosomes.
Free TTZ was not recycled but instead retained in endosomes demonstrating the significance of endosomal sorting in presence of CPT nanorods. The precise reason of TTZ recycling to the plasma membrane in case of CPT-TTZ, but not for free TTZ, is unknown; however, two reasons are possible. TTZ, when delivered in the soluble form, is likely released from Her2 receptors in the endosomes, thus preventing its recycling. On the other hand, it is possible that TTZ attached to CPT cannot be released from Her2 due to conformational changes in TTZ by hydrophobic interactions between CPT and TTZ, thus allowing its recycling. Another possibility is that free TTZ and CPT-TTZ arrest cell cycles in different stages, thus impacting the extent of TTZ recycling. Free TTZ arrests cells in the G0/G1 which is a rather quiescent phase where endosome recycling to the cell surface may be inhibited or slowed. In contrast, CPT-TTZ arrests cells in S phase where cells are more active. In addition to the TTZ-mediated endocytosis and cellular localization, the shape of CPT nanorods may also contribute to the enhanced uptake and intracellular sorting. Multivalent interactions between the rod-shaped CPT-TTZ nanoparticles and Her2 receptor proteins likely increase the binding affinity of the particles to the cell surface and eventually enhance intracellular delivery.8,20,53 Theoretical models as well as in vivo biodistriubtion studies have suggested the benefits of using elongated particles for enhanced targeting ability and receptor-mediated cellular uptake.18,54–56
Previous studies in the literature have reported that size and shape impact intracellular uptake18,57–59 and distribution of particles.56,60 Intracellular trafficking of non-spherical particles conjugated with targeting ligands have different trafficking pathway compared to that of spherical particles.56,58 Rod-shaped nanoparticles are reported to target the nucleus, while hexagonal sheet-like nanoparticles retain in the cytoplasm.61 This shape-dependent intracellular transport behavior is important to deliver drugs to the cytoplasm for protecting them from lysosomal degradation. In a separate study, Kolhar et al showed that cytoplasmic transport and accumulation of particles at the perinuclear region depends on their size and shape.60 While the present study did not explore the effects of size and shape, it is possible that similar intracellular distribution of CPT, TTZ and DOX may be observed using other nanoparticle size and shapes.
The solution form of CPT is expected to enter the nucleus and exhibit its activity as a nuclear topoI enzyme inhibitor.28 In the nanorod form, however, a majority of CPT was found to reside outside the nucleus, most likely within the late endosomes. Two water soluble CPT analogs, topotecan and gimatecan are reported to localize in the mitochondria and endoplasmic reticulum, and lysosomes, respectively instead of the nucleus in HT-29 colon cancer cells.62 Localization of CPT in the acidic lysosomes can be beneficial for enhancing the stability of the lactone ring of CPT in acidic pH46 and the intracellular release of the drug within the cell. Some CPT could have entered the nucleus but not detected within the detection limit of the confocal microscopy and TEM.
DOX entered the nucleus most possibly via the passive diffusion through the nuclear pore complexes of the nuclear membrane.63 Several different approaches have been taken to promote nuclear delivery of DOX using nuclear localization signal (NLS)-conjugated poly (d,l-lactide-co-glycolide)(PLGA) nanoparticles,64 NLS-conjugated glycol chitosan,65 PEGylated liposomes,7 micelles,66 and polymers.67 However, many of these approaches suffer from DOX protonation in the acidic pH.68 CPT-TTZ-DOX nanoparticles led to a substantial accumulation of DOX inside the nucleus without significant detectable amounts of DOX in the cytoplasm, suggesting negligible DOX protonation. In addition, DOX binding to DNA has been reported to quench its fluorescence intensity, while DOX fluorescence intensity is enhanced when resides in the cytoplasm.69 In our study, we found most of the DOX entered the nucleus of the cells which might cause lower DOX fluorescence intensity upon binding to DNA. Although the half-life of DOX release from the particles is ~ 2 h under physiological conditions, this time is significantly longer than the blood circulation half-life (5–10 min) of free DOX.70 Upon in vivo administration, CPT-TTZ-DOX are expected to reach the tumor site within 30 min after administration still carrying with sufficient levels of DOX and release the drug at the tumor site. Detailed pharmacokinetics and biodistribution studies are necessary to further assess this possibility.
The cell growth inhibition using CPT-TTZ-DOX was attributed to the cell cycle arrest showing a higher proportion of cells in the G0/G1, S and G2/M phases than the respective controls. TTZ is known to induce cell cycle arrest in the G1 phase with a reduction of cell proliferation and induction of apoptosis.48 CPT is an S phase specific DNA-topoI complex intercalator49 and DOX tends to arrest cells in the G2/M cell cycle.50,71 The cell cycle data reported here show that individual components of CPT-TTZ-DOX nanoparticles retain their activity in the nanoparticulate form. In vivo investigations are necessary to confirm therapeutic benefits of this combination.
CPT itself has not been used for cancer therapy due to its insolubility in aqueous phase. Hydrophobic CPT also shows limited interactions with cell membrane and diffusion inside the cytoplasm (SI Fig. 4c). However, rod-shaped CPT nanorods, when coated by TTZ, exhibit significant intracellular uptake (Fig. 2vs. SI Fig. 4c). In vitro tests in BT-474 cells showed that CPT-TTZ-DOX can act synergistically at respective doses of 0.1, 0.02 and 0.27 µg/ml (or same numbers in mg/kg assuming the same concentration is to be achieved in vivo). CPT concentrations between 0.5 to 4.0 mg/kg have been reported to increase the life time of mice beating L1210 leukemia tumors.72 TTZ doses up to 20 mg/kg have been used to disrupt Her2-Her3 association in BT-474-M1 tumor xenografts in mice.73 Free DOX doses of 9 and 16 mg/kg have been used to study pharmacokinetics of the drug in mammary carcinoma (4T1) implanted mice.74 The doses used in this in vitro study are significantly lower than those reported in the literature for in vivo studies.
The size of CPT nanorods can be further reduced to facilitate its in vivo use. Specifically, the length of CPT nanorods was reduced from 509.5±202.6 × 52.7±18 nm to ~290±88 × 47.4±19.1 nm by modifying the synthesis procedure (SI Fig. 11 and SI Text 6). These newer generation CPT nanorods can be functionalized with TTZ using the same method as those used for longer nanorods. Nanoparticle with dimensions of 300 nm have been used for in vivo studies without causing any toxicity and aggregation problems.75–77 This size is within the cut-off size of permeation across the vascular wall. Literature studies have shown that doxorubicin-loaded polymeric spheres (diameter: 300 nm) exhibit high accumulation at tumor site after 1–3 days of intravenous injection.77 Liposomes between 100–400 nm have also been reported to penetrate tumors in mice xenografts.78 We have recently showed that rod-shaped nanoparticles of ~500nm bind to lung and brain endothelial cells of mice better than spheres when coated with antibodies.79 Therefore, it can be anticipated that the CPT-TTZ-DOX holds significant promise for in vivo efficacy although we agree that direct in vivo tests are necessary to confirm the efficacy.
CONCLUSION
In summary, we have formulated rod-shaped drug nanoparticles using three anti-cancer drugs to combine active targeting of cancer cells with improved therapeutic activity. The distinctive intracellular distribution of the drugs makes them suitable for multiple cytoplasmic targeting as well as synergistic efficacy even at very low drug concentrations. The in vitro tests offer detailed insights into the mechanisms of synergy. We expect that such in vitro studies will contribute to bridging the gap between in vitro design/characterization, and in vivo therapeutic evaluations.
METHODS
Preparation of camptothecin (CPT)-trastuzumab (TTZ)-doxorubicin (DOX) nanoparticles
First, we prepared CPT nanorods using solvent diffusion method, and adsorbed TTZ and DOX on the surfaces of CPT nanorods. Briefly, 1ml of 1mg/ml CPT (Sigma-Aldrich) DMSO solution was injected into 50ml of 1% polyvinyl alcohol (PVA; 13–23k; Sigma) water mixture using a syringe pump (kd Scientific Inc.). The mixture was stirred under 300rpm at room temperature (22°C). CPT nanorods formed at the boundary where DMSO diffused slowly into water. The nanorods were washed using miliQ water and centrifuged three times at 11,000 rcf for 1 h. The concentrations of CPT nanorods were measured by reading absorbance at 366nm using a spectrophotometer (Tecan Saffire) and a CPT calibration curve.
TTZ (Gennentech), was conjugated with Alexa Fluor 594 dye using a protein labeling kit (Invitrogen). The moles of Alexa 594 dye per mole of TTZ protein was calculated 4.7 ± 0.09, which is in the acceptable range (2–6 moles) according to the vendor’s protocol (Invitrogen). A 100µl labeled or unlabeled TTZ solution (1mg/ml) was added to 1ml of 1mg/ml CPT nanorods and incubated overnight at 4°C. TTZ adsorbed CPT nanorods were purified by a threefold centrifugation, and re-dispersion in phosphate buffer saline (PBS) buffer containing 0.1% BSA and 0.01% sodium azide. The supernatants collected during the centrifugation were analyzed using micro-BCA assay kit (Pierce) to measure the unbound TTZ. TTZ adsorption efficiency was calculated as the difference between the initial amount and the unbound antibody per milligram of CPT nanorods. Bovine serum albumin (Sigma) and Alexa 594 conjugated anti-human IgG (Molecular Probes) were adsorbed on CPT nanorods as controls.
Finally, DOX (1000µg; Sigma) solution was added to 500µg CPT containing CPT-TTZ nanorods and incubated overnight at 4°C in a rotator. The mixture was centrifuged three times at 1000rcf for 10 min where the supernatants were collected to measure the unbound DOX and CPT. A DOX standard calibration curve was plotted using standard concentrations of DOX dissolved in water. Fluorescence intensity of the DOX standards and supernatants were measured using an excitation/emission of 471±9nm /556±25nm. The amount of encapsulated DOX was calculated by subtracting the amount of DOX in the supernatants from the initial amount of DOX used.
Morphology of CPT nanorods, CPT-TTZ and CPT-TTZ-DOX was visualized using a scanning electron microscope (SEM). The surface charges of the nanoparticles suspended in PBS were measured as zeta potential using a Nanoseries Zetasizer (Malvern).
DOX release from CPT-TTZ-DOX nanoparticles
Freshly prepared nanoparticles were resuspended in 1ml of 10% fetal bovine serum (FBS) containing phosphate buffer saline (PBS) at pH 7.4 and incubated at 37°C. Nanoparticles were centrifuged to collect the supernatants, re-suspended in 10%FBS-PBS and further incubated. The release of DOX was measured at 0, 0.5, 1, 2 and 24 h by fluorescence spectrophotometry (ex/em = 471±9nm/556±25nm). The percentage of DOX release was calculated as , where Ft, Fi and Ftotal are the fluorescence intensities at any time t, initial DOX and total DOX solution.
Intracellular localization of CPT-TTZ and CPT-TTZ-DOX
Intracellular uptake of CPT-TTZ was studied in BT-474 breast cancer cells (ATCC) by confocal microscope analysis. Her2 overexpressing BT-474 cells were cultured in ATCC Hybricare medium supplemented with 10% FBS at 37°C and 5% CO2 in a humidified incubator. Cells were seeded in an 8-well chambered coverglass (Lab-Tek II) at a density of 10,000 cells in 200µl medium. After overnight growth, cells were incubated with CPT-Alexa 594 conjugated TTZ, CPT-BSA, CPT-Alexa 594 IgG and CPT nanorods alone where CPT, TTZ, BSA and IgG concentrations were 300, 50, 36 and 50 µg/ml, respectively. In case of CPT-TTZ-DOX nanoparticles incubation, the concentrations were 100, 16.7 and 260 µg/ml, respectively. To exclude the possibility of non-specific cellular interactions by TTZ-coated nanoparticles, cells were pre-incubated with 100 µg/ml TTZ for 30 min and then treated with Alexa 594 CPT-TTZ particles. To verify the dependence of intracellular distribution of three drugs on nanoparticle geometry, cells were incubated with a drug cocktail of soluble CPT (solubilized in DMSO), TTZ and DOX solution. Soluble CPT and Alexa 594 TTZ were used to interpret the localization of free TTZ. The drugs were added at the same concentrations as they were in the nanoparticles with every 15 min intervals by incubating the cells at 37°C. After 2 h of total incubation at 37°C, cells were washed three times using PBS. For recycling endosome staining, cells were incubated further for 1 h with 10µg/ml Alexa 488 conjugated transferrin (Molecular Probes) and washed prior to imaging. Transferrin was added later to avoid its potential association with CPT-TTZ nanorods outside the cells (in the medium) which could confound the interpretation. To image the intracellular localization of CPT-TTZ and CPT-TTZ-DOX after 24 h, cells were washed after 2 h of particle exposure and re-incubated in fresh medium. To stain the cell nuclei, cells were incubated with 1µM green fluorescent nuclear stain, SYTO13 (Molecular Probes) for 1 h before washing. Imaging was performed within 1µm inner sections of cells by sequential scanning using an Olympus confocal microscope (Fluoview 1000) equipped with a 60x silicone oil immersion objective. Fast sequential line scanning was carried out using acousto-optic tunable filters (AOTF) to avoid any crosstalk between image channels. AOTF rapidly turns on and off different laser lines in a few milliseconds that excites only one fluorophore at a time eliminating any crosstalk between image channels. In addition, control images were taken individually at the very beginning of the experiments to set up the excitation/emission settings. CPT, Alexa 488, SYTO 13, DOX, and Alexa 594 were excited with 405 diode, 488 Argon, 488 Argon, 488 Argon and 559 diode line lasers, respectively and the emissions were collected at 461 ± 20, 520 ± 25, 510 ± 10, 575 ± 25 and 618 ± 50 nm wavelengths, respectively. The z-stacks of fluorescence images were merged and analyzed using Imaris (Bitplane) software. Fluorescence intensities of Alexa 594 and DOX per cell were analyzed for at least 10 cells.
Quantitative colocalization analysis
Colocalization analysis was done using Imaris, ImageJ 1.37a software (National Institute of Health; http://rsb.info.nih.gov/ij/) and its plugin, intensity correlation analysis. The RGB color images were converted into 8-bit colors using ImageJ. The 8-bit red and green channels for three independent samples were used to calculate the Pearson’s correlation coefficient (Rr), overlap coefficient (R), and colocalization coefficients, m for each channel. Rr measures if there is any correlation between two signal intensity in the range between −1 to 1, where −1, 0 and 1 indicate inverse, none and positive correlations between channels. R indicates the percent overlap of selected channels. Coefficient m describes the contribution of each pixel during colocalization. The percent of colocalizaton were calculated from these three coefficients.
Transmission Electron Microscopy (TEM)
BT474 cells were incubated with 300µg/ml CPT-TTZ-DOX nanoparticles for 2 h followed by fresh cell culture medium for 24 h. Cells were post-fixed using 2% glutaraldehyde solution in 0.1M cacodylate buffer, stained using 1% osmium tetraoxide, dehydrated in graded ethanol series, infiltrated with propylene oxide/Spurr resin mixture and embedded in Spurr resin. The embedded cells were cut into 90 nm ultra-thin sections using an ultramicrotome and a glass knife. Three to four sections were mounted on 200 mesh copper grids, stained with uranyl acetate and lead citrate, and imaged in a JEOL 123 transmission electron moicroscope operated at 80kV.
In vitro cytotoxicity of CPT-TTZ-DOX
In vitro activity of CPT-TTZ-DOX in BT-474 cells and Her2-negative MDA-MB-231 cells was analyzed using calcein AM of the live-dead assay kit (Invitrogen). BT-474 and MDA-MB-231 cells were seeded in 96-well plates at a density of 10,000 cells in 200µl Hybricare medium (ATCC) and DMEM (ATCC), respectively in 96-well plates, allowed to attach overnight and treated with CPT-TTZ-DOX particles for 2 h with final doses of CPT, TTZ and DOX as 0.1, 0.02 and 0.27µg/ml, respectively. The following controls were used in BT-474 cells: PBS, TTZ (0.02 µg/ml) alone, DOX (0.27 µg/ml) alone, CPT-TTZ particles (0.1 and 0.02 µg/ml of CPT and TTZ, respectively) and CPT-BSA-DOX particles (0.1, 0.012 and 0.27µg/ml of CPT, BSA and DOX, respectively). To test if nanoparticle geometry influence the drug activity, BT-474 cells were incubated with the following controls by adding the drugs simultaneously at 15 min intervals: (i) soluble CPT in DMSO, TTZ solution and free DOX; (ii) a soluble form of CPT (topotecan), TTZ solution and free DOX; and (iii) CPT-BSA, TTZ coated PS nanorods and free DOX. Cells were incubated for the indicated time interval before adding each drug. After 2 h total incubation, medium was replaced with fresh medium, and the cells were further incubated for 72 h. Live cells were then measured using the live-dead assay kit (Invitrogen) and analyzed using a plate reader (Tecan Saffire). For quantification of the number of live cells, the medium was removed, and calcein AM (1µM) in PBS was added to the cells and incubated at room temperature for 30 min. Fluorescence intensities of calcein AM (ex/em 495/530±25nm) was measured using the plate reader. Fluorescence backgrounds of PBS were subtracted from each well. Assays were performed at least in triplicates in three independent experiments. The results are expressed as percentage inhibition in cell growth relative to growth of PBS treated control cells. Similarly, individual dose response curves of TTZ, CPT and DOX were also performed to determine inhibitory drug concentrations (IC50) to stop 50% cell growth. From the resulting curves of individual drug treatment and CPT-TTZ-DOX effects, the combination index (CI) for CPT-TTZ-DOX was calculated using the Chou-Talalay method47
| (1) |
In this analysis, synergy is defined when CI <1. Two-tailed, type 3 (samples with different variances) statistical testes were used to determine if the effect due to CPT-TTZ-DOX were significantly different from other data sets (e.g. CPT-TTZ, CPT-BSA-DOX etc.).
Cell cycle analysis
To determine the growth arrest induced by CPT-TTZ-DOX particles, we performed the DNA counts in BT-474 cells after exposure to polystyrene nanorods-TTZ, CPT-TTZ and CPT-TTZ-DOX. Subconfluent monolayers of BT-474 cells (100,000) were grown in T-25 flasks in 5ml medium. Cells were incubated with TTZ-coated polystyrene rods (100 µg/ml), CPT nanorods (10µg/ml), CPT(10µg/ml)-DOX (2.7µg/ml) particles, PBS and a high concentration (10mg/ml) of TTZ solution (a positive control) for 2 h. TTZ concentrations on polystyrene particles and CPT were 12.5 and 0.2µg/ml, respectively. BSA-coated polystyrene rod was used as the negative control. The cells were then incubated in fresh medium for 72 h, trypsinized and resuspended in 500 µl Hanks’ Balanced Salt Solution (HBSS). A DNA binding fluorescent dye and cell-permeant stain, Vybrant DyeCycle Violet was added to the cell suspension at a concentration of 1µM and incubated at 37°C for 30 min by wrapping aluminum foils around the sample tubes. The cells were then analyzed on a BD FACSAria flow cytometer (BD Biosciences) using a UV light source. A minimum of 50,000 cells was analyzed for each sample in duplicates in two independent experiments.
Supplementary Material
Acknowledgements
Authors thank M. H. Bakker for his assistance in modifying the CPT preparation method to obtain shorter length CPT nanorods. We acknowledge the 2012 Daryl and Marguerite Errett Discovery Award and funding from Genentech Inc. This work made use of MRL Central Facilities supported by the MRSEC program of the National Science Foundation under award no. MR05-20415, and MCDB microscope facility funded by NIH Grant Number: 1 S10 OD010610-01A1. The authors thank Dr. G. P. Lewis and K. A. Linberg for their expert technical assistance with electron microscopy.
Footnotes
Supporting Information Available
Supporting data, experimental methods, detailed descriptions, figures and tables. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Sun B, Ranganathan B, Feng S-S. Multifunctional Poly(D,L-Lactide-Co-Glycolide)/Montmorillonite (PLGA/MMT) Nanoparticles Decorated by Trastuzumab for Targeted Chemotherapy of Breast Cancer. Biomaterials. 2008;29:475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of Active Targeting in Solid Tumors with Transferrin-Containing Gold Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mi Y, Liu X, Zhao J, Ding J, Feng SS. Multimodality Treatment of Cancer with Herceptin Conjugated, Thermomagnetic Iron Oxides and Docetaxel Loaded Nanoparticles of Biodegradable Polymers. Biomaterials. 2012;33:7519–29. doi: 10.1016/j.biomaterials.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 4.Fabbro C, Ali-Boucetta H, Ros TD, Kostarelos K, Bianco A, Prato M. Targeting Carbon Nanotubes against Cancer. Chem. Commun. 2012;48:3911–3926. doi: 10.1039/c2cc17995d. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 6.Koo OM, Rubinstein I, Onyuksel H. Camptothecin in Sterically Stabilized Phospholipid Micelles: A Novel Nanomedicine. Nanomedicine. 2005;1:77–84. doi: 10.1016/j.nano.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Yu Y, Dai W, Lu J, Cui J, Wu H, Yuan L, Zhang H, Wang X, Wang J, et al. The Use of a Tumor Metastasis Targeting Peptide to Deliver Doxorubicin-Containing Liposomes to Highly Metastatic Cancer. Biomaterials. 2012;33:8451–8460. doi: 10.1016/j.biomaterials.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Tsai C-P, Chen C-Y, Hung Y, Chang F-H, Mou C-Y. Monoclonal Antibody-Functionalized Mesoporous Silica Nanoparticles (MSN) for Selective Targeting Breast Cancer Cells. J. Mater. Chem. 2009;19:5737–5743. [Google Scholar]
- 9.Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting Cancer Cells Using Plga Nanoparticles Surface Modified with Monoclonal Antibody. J. Controlled Release. 2007;120:18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted Nanoparticle-Aptamer Bioconjugates for Cancer Chemotherapy in Vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum Dot-Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z, Levy-Nissenbaum E, Alexis F, Lupták A, Teply BA, Chan JM, Shi J, Digga E, Cheng J, Langer R, et al. Engineering of Targeted Nanoparticles for Cancer Therapy Using Internalizing Aptamers Isolated by Cell-Uptake Selection. ACS Nano. 2012;6:696–704. doi: 10.1021/nn204165v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dam DHM, Lee JH, Sisco PN, Co DT, Zhang M, Wasielewski MR, Odom TW. Direct Observation of Nanoparticle-Cancer Cell Nucleus Interactions. ACS Nano. 2012;6:3318–3326. doi: 10.1021/nn300296p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting Quantum Dots to Surface Proteins in Living Cells with Biotin Ligase. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-Dependent Endocytosis of Nanoparticles. Adv. Biomater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo J-W, Doshi N, Mitragotri S. Endocytosis and Intracellular Distribution of Plga Particles in Endothelial Cells: Effect of Particle Geometry. Macromol. Rapid Commun. 2010;31:142–148. doi: 10.1002/marc.200900592. [DOI] [PubMed] [Google Scholar]
- 18.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The Effect of Particle Design on Cellular Internalization Pathways. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11613–8. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vácha R, Martinez-Veracoechea FJ, Frenkel D. Receptor-Mediated Endocytosis of Nanoparticles of Various Shapes. Nano Lett. 2011;11:5391–5395. doi: 10.1021/nl2030213. [DOI] [PubMed] [Google Scholar]
- 20.Barua S, Yoo JW, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S. Particle Shape Enhances Specificity of Antibody-Displaying Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3270–3275. doi: 10.1073/pnas.1216893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of Nanoparticles to the Clathrin-Mediated Endocytic Pathway. Biochem. Biophys. Res. Commun. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 22.Austin CD, De Mazière AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and Sorting of Erbb2 and the Site of Action of Cancer Therapeutics Trastuzumab and Geldanamycin. Mol. Biol. Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand Binding and Kinetics of Folate Receptor Recycling in Vivo: Impact on Receptor-Mediated Drug Delivery. Mol. Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small. 2010;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 25.Sahoo SK, Ma W, Labhasetwar V. Efficacy of Transferrin-Conjugated Paclitaxel-Loaded Nanoparticles in a Murine Model of Prostate Cancer. Int. J. Cancer. 2004;112:335–340. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- 26.Sahoo SK, Labhasetwar V. Enhanced Antiproliferative Activity of Transferrin-Conjugated Paclitaxel-Loaded Nanoparticles Is Mediated Via Sustained Intracellular Drug Retention. Mol. Pharmaceutics. 2005;2:373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 27.Jaxel C, Capranico G, Kerrigan D, Kohn KW, Pommier Y. Effect of Local DNA Sequence on Topoisomerase I Cleavage in the Presence or Absence of Camptothecin. J. Biol. Chem. 1991;266:20418–23. [PubMed] [Google Scholar]
- 28.Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Structure-Activity Study of the Actions of Camptothecin Derivatives on Mammalian Topoisomerase I: Evidence for a Specific Receptor Site and a Relation to Antitumor Activity. Cancer Res. 1989;49:1465–1469. [PubMed] [Google Scholar]
- 29.Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, Burgin AB. Structures of Three Classes of Anticancer Agents Bound to the Human Topoisomerase I-DNA Covalent Complex. J. Med. Chem. 2005;48:2336–2345. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA, Girish S, et al. Phase Ii Study of the Antibody Drug Conjugate Trastuzumab-Dm1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (Her2) -Positive Breast Cancer after Prior Her2-Directed Therapy. J. Clin. Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 31.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of Her2-Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 32.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, et al. Multinational Study of the Efficacy and Safety of Humanized Anti-Her2 Monoclonal Antibody in Women Who Have Her2-Overexpressing Metastatic Breast Cancer That Has Progressed after Chemotherapy for Metastatic Disease. J. Clin. Oncol. 1999;17:2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 33.Croxtall JD, McKeage K. Trastuzumab: In Her2-Positive Metastatic Gastric Cancer. Drugs. 2010;70:2259–2267. doi: 10.2165/11205900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC; Shen, L; Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in Combination with Chemotherapy Versus Chemotherapy Alone for Treatment of Her2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (Toga): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of Chemotherapy Plus a Monoclonal Antibody against Her2 for Metastatic Breast Cancer That Overexpresses Her2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 36.Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, et al. Preoperative Therapy with Trastuzumab and Paclitaxel Followed by Sequential Adjuvant Doxorubicin/Cyclophosphamide for Her2 Overexpressing Stage Ii or Iii Breast Cancer: A Pilot Study. J. Clin. Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 37.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A, et al. Randomized Phase Ii Trial of the Efficacy and Safety of Trastuzumab Combined with Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2 D Positive Metastatic Breast Cancer Administered as First-Line Treatment: The M77001 Study Group. J. Clin. Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 38.Jackisch C. Her-2-Positive Metastatic Breast Cancer: Optimizing Trastuzumab-Based Therapy. Oncologist. 2006;11:34–41. doi: 10.1634/theoncologist.11-90001-34. [DOI] [PubMed] [Google Scholar]
- 39.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab Plus Adjuvant Chemotherapy for Operable Her2-Positive Breast Cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 40.Bodley A, Liu LF, Israel M, Seshadri R, Koseki Y, Giuliani FC, Kirschenbaum S, Silber R, Potmesil M. DNA Topoisomerase Ii-Mediated Interaction of Doxorubicin and Daunorubicin Congeners with DNA. Cancer Res. 1989;49:5969–5978. [PubMed] [Google Scholar]
- 41.Blum RH, Carter SK. Adriamycin. Ann. Intern. Med. 1974;80:249–259. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- 42.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 43.Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK, et al. Synthesis of Water-Soluble (Aminoalkyl)Camptothecin Analogs: Inhibition of Topoisomerase I and Antitumor Activity. J. Med. Chem. 1991;34:98–107. doi: 10.1021/jm00105a017. [DOI] [PubMed] [Google Scholar]
- 44.Luzzio MJ, Besterman JM, Emerson DL, Evans MG, Lackey K, Leitner PL, McIntyre G, Morton B, Myers PL, Peel M, et al. Synthesis and Antitumor Activity of Novel Water Soluble Derivatives of Camptothecin as Specific Inhibitors of Topoisomerase I. J. Med. Chem. 1995;38:395–401. doi: 10.1021/jm00003a001. [DOI] [PubMed] [Google Scholar]
- 45.Nawara K, Romiszewski J, Kijewska K, Szczytko J, Twardowski A, Mazur M, Krysinski P. Adsorption of Doxorubicin onto Citrate-Stabilized Magnetic Nanoparticles. J. Phys. Chem. C. 2012;116:5598–5609. [Google Scholar]
- 46.Pommier Y, Redon C, Rao VA, Seiler JA, Sordet O, Takemura H, Antony S, Meng L, Liao Z, Kohlhagen G, et al. Repair of Checkpoint Response to Topoisomerase I-Mediated DNA Damage. Mutat. Res., FundamMol. Mech. Mutagen. 2003;532:173–203. doi: 10.1016/j.mrfmmm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Chou T-C, Talalay P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.Dean-Colomb W, Esteva FJ. Her2-Positive Breast Cancer: Herceptin and Beyond. Eur. J. Cancer. 2008;44:2806–2812. doi: 10.1016/j.ejca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Hsiang Y-H, Lihou MG, Liu LF. Arrest of Replication Forks by Drug-Stabilized Topoisomerase I-DNA Cleavable Complexes as a Mechanism of Cell Killing by Camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 50.Ling YH, el-Naggar AK, Priebe W, Perez-Soler R. Cell Cycle-Dependent Cytotoxicity, G2/M Phase Arrest, and Disruption of P34cdc2/Cyclin B1 Activity Induced by Doxorubicin in Synchronized P388 Cells. Mol. Pharmacol. 1996;49:832–841. [PubMed] [Google Scholar]
- 51.Chen J, Irudayaraj J. Quantitative Investigation of Compartmentalized Dynamics of Erbb2 Targeting Gold Nanorods in Live Cells by Single Molecule Spectroscopy. ACS Nano. 2009;3:4071–4079. doi: 10.1021/nn900743v. [DOI] [PubMed] [Google Scholar]
- 52.Colin JT, Anna CR, Krysten JP, Thomas W, Jacqueline O, Naomi A, Jez GC, Joachim K, David JS, Peter JC. Snx4 Coordinates Endosomal Sorting of Tfnr with Dynein-Mediated Transport into the Endocytic Recycling Compartment. Nat. Cell Biol. 2007;9:1370–1380. doi: 10.1038/ncb1656. [DOI] [PubMed] [Google Scholar]
- 53.Humblet V, Misra P, Bhushan KR, Nasr K, Ko Y-S, Tsukamoto T, Pannier N, Frangioni JV, Maison W. Multivalent Scaffolds for Affinity Maturation of Small Molecule Cell Surface Binders and Their Application to Prostate Tumor Targeting. J. Med. Chem. 2008;52:544–550. doi: 10.1021/jm801033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decuzzi P, Ferrari M. The Adhesive Strength of Non-Spherical Particles Mediated by Specific Interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Decuzzi P, Ferrari M. The Receptor-Mediated Endocytosis of Nonspherical Particles. Biophys. J. 2008;94:3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of Icam-1-Targeted Carriers. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin H, Heller DA, Sharma R, Strano MS. Size-Dependent Cellular Uptake and Expulsion of Single-Walled Carbon Nanotubes: Single Particle Tracking and a Generic Uptake Model for Nanoparticles. ACS Nano. 2009;3:149–158. doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 58.Park J-H, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Systematic Surface Engineering of Magnetic Nanoworms for in Vivo Tumor Targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X, Teng X, Chen D, Tang F, He J. The Effect of the Shape of Mesoporous Silica Nanoparticles on Cellular Uptake and Cell Function. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 60.Kolhar P, Mitragotri S. Polymer Microparticles Exhibit Size and Shape Dependent Accumulation around the Nucleus after Endocytosis. Adv. Funct. Mater. 2012;22:3759–3764. [Google Scholar]
- 61.Xu ZP, Niebert M, Porazik K, Walker TL, Cooper HM, Middelberg APJ, Gray PP, Bartlett PF, Lu GQ. Subcellular Compartment Targeting of Layered Double Hydroxide Nanoparticles. J. Controlled Release. 2008;130:86–94. doi: 10.1016/j.jconrel.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 62.Croce AC, Bottiroli G, Supino R, Favini E, Zuco V, Zunino F. Subcellular Localization of the Camptothecin Analogues, Topotecan and Gimatecan. Biochem. Pharmacol. 2004;67:1035–1045. doi: 10.1016/j.bcp.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 63.Poon IKH, Jans DA. Regulation of Nuclear Transport: Central Role in Development and Transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 64.Misra R, Sahoo SK. Intracellular Trafficking of Nuclear Localization Signal Conjugated Nanoparticles for Cancer Therapy. Eur. J. Pharm. Sci. 2010;39:152–163. doi: 10.1016/j.ejps.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Xie X, Zheng M, Yu L, Zhang L, Zhao J, Jiang D, Che X. Fabrication and Characterization of Nuclear Localization Signal-Conjugated Glycol Chitosan Micelles for Improving the Nuclear Delivery of Doxorubicin. Int. J. Nanomed. 2012;7:5079–5090. doi: 10.2147/IJN.S36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gillies ER, Fréchet JMJ. Ph-Responsive Copolymer Assemblies for Controlled Release of Doxorubicin. Bioconjugate Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 67.Dong D-W, Tong S-W, Qi X-R. Comparative Studies of Polyethylenimine– Doxorubicin Conjugates with Ph-Sensitive and Ph-Insensitive Linkers. J. Biomed. Mater. Res., Part A. 2013;101A:1336–1344. doi: 10.1002/jbm.a.34450. [DOI] [PubMed] [Google Scholar]
- 68.Dalmark M, Johansen P. Molecular Association between Doxorubicin (Adriamycin) and DNA-Derived Bases, Nucleosides, Nucleotides, Other Aromatic Compounds, and Proteins in Aqueous Solution. Mol. Pharmacol. 1982;22:158–165. [PubMed] [Google Scholar]
- 69.Mohan P, Rapoport N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharmaceutics. 2010;7:1959–1973. doi: 10.1021/mp100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert J, Illiadis A, Hoerni B, Cano J-P, Durand M, Lagarde C. Pharmacokinetics of Adriamycin in Patients with Breast Cancer: Correlation between Pharmacokinetic Parameters and Clinical Short-Term Response. Eur. J. Cancer Clin. Oncol. 1982;18:739–745. doi: 10.1016/0277-5379(82)90072-4. [DOI] [PubMed] [Google Scholar]
- 71.Potter AJ, Gollahon KA, Palanca BJA, Harbert MJ, Choi YM, Moskovitz AH, Potter JD, Rabinovitch PS. Flow Cytometric Analysis of the Cell Cycle Phase Specificity of DNA Damage Induced by Radiation, Hydrogen Peroxide and Doxorubicin. Carcinogenesis. 2002;23:389–401. doi: 10.1093/carcin/23.3.389. [DOI] [PubMed] [Google Scholar]
- 72.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA, Plant Antitumor Agents I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca Acuminata1,2. J. Am. Chem. Soc. 1966;88:3888–3890. [Google Scholar]
- 73.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-Independent Her2/Her3/Pi3k Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the Pi3k Inhibitor Gdc-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 74.Laginha KM, Verwoert S, Charrois GJR, Allen TM. Determination of Doxorubicin Levels in Whole Tumor and Tumor Nuclei in Murine Breast Cancer Tumors. Clin. Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 75.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 76.Susanne Wiegand TH, Annette Ramaswamy, Andreas M, Sesterhenn, Christian Bergemann, Jochen A, Werner, Andreas S. Lübbe Evaluation of the Tolerance and Distribution of Intravenously Applied Ferrofluid Particles of 250 and 500 nm Size in an Animal Model. J. Drug Targeting. 2009;17:194–199. doi: 10.1080/10611860802582467. [DOI] [PubMed] [Google Scholar]
- 77.Hyung Park J, Kwon S, Lee M, Chung H, Kim J-H, Kim Y-S, Park R-W, Kim I-S, Bong Seo S, Kwon IC, et al. Self-Assembled Nanoparticles Based on Glycol Chitosan Bearing Hydrophobic Moieties as Carriers for Doxorubicin: In Vivo Biodistribution and Anti-Tumor Activity. Biomaterials. 2006;27:119–126. doi: 10.1016/j.biomaterials.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 78.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 79.Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using Shape Effects to Target Antibody-Coated Nanoparticles to Lung and Brain Endothelium. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.