Abstract
Background: CD44 is a potentially interesting prognostic marker and therapeutic target in non-small cell lung cancer (NSCLC). Although the expression of CD44 has been reported to correlate with poor prognosis of NSCLC in most literatures, some controversies still exist. Since the limited patient numbers within independent studies, here we performed a meta-analysis to clarify the correlations between CD44 expression and prognosis and clinicopathological features in NSCLC. Methods: Relevant literatures were identified using PubMed, EMBASE and CNKI (China National Knowledge Infrastructure) databases (up to February 2014). Data from eligible studies were extracted and included into meta-analysis using a random effects model. Studies were pooled. Summary hazard ratios (HR) and clinical parameters were calculated. Results: We performed a final analysis of 1772 patients from 23 evaluable studies for Prognostic Value and 2167 patients from 28 evaluable studies for clinicopathological features. Our study shows that the pooled hazard ratio (HR) of overexpression CD44-V6 for overall survival in NSCLC was 1.63 [95% confidence interval (CI): 1.20-2.21] by univariate analysis and 1.29 (95% CI: 0.71-2.37) by multivariate analysis.The pooled HR of overexprssion panCD44 for overall survival in NSCLC was 1.53 (95% CI: 0.58-4.04) by univariate analysis and 3.00 (95% CI: 1.53-5.87) by multivariate analysis. Overexpression of CD44-V6 is associated with tumor differentiation (poor differentiation, OR = 1.66, 95% CI: 1.12-2.45), tumor histological type [squamous cell carcinomas (SCC), OR = 2.6, 95% CI: 1.63-5.02], clinical TMN stage (TMN stage III, OR = 2.22, 95% CI: 1.44-3.43) and lymph node metastasis (N1-3, 3.52, 95% CI: 2.08-5.93) in patients with NSCLC. However, there was no significant association between CD44-V6 and tumor size [T category, OR = 1.42, 95% CI: 0.73-2.78]. Conclusion: Our meta-analysis showed that CD44-V6 is an efficient prognostic factor for NSCLC. Overexpression of CD44-V6 was significantly associated with tumor differentiation, tumor histological type, clinical TMN stage and lymph node metastasis. However, there was no significant association between CD44-V6 and tumor size. Large prospective studies are now needed to confirm the clinical utility of CD44 as an independent prognostic marker.
Keywords: Non-small cell lung cancer (NSCLC), CD44, panCD44, CD44-V6, clinicopathological features, overall survival, meta-analysis
Introduction
Lung cancer is one of the most common human cancers and the leading cause of cancer-related deaths with non-small cell lung cancer (NSCLC) accounting for 80% of all primary lung cancers. Despite early detection screening protocols, improved surgical techniques, and advanced radio and chemotherapeutic regimens, little progress has been made in altering the natural progression of the disease, the 5-year survival rate of patient with NSCLC is only 15% [1]. Thus, the lack of major improvements in the 5-year survival rate of NSCLC has driven the search for new strategies aimed at improving lung cancer management. Current knowledge regarding NSCLC is not a single disease but a collection of diseases with distinct pathogeneses by molecular mechanisms. Genetic and epigenetic alternations play an integral role in the transformation, promotion and progression of cancer [2].
A number of cell surface markers, including CD133, CD24 and CD44, are responsible for tumor initiation, progression, metastasis and drug resistance [3]. It was reported that overexpression of these markers indicated bad clinical features and poor prognosis [4-6]. CD44 includes standard form of CD44 (CD44s) and its variant isoforms. CD44 has been accepted as a promising prognostic indicator in solid tumors, and it was revealed to be a target of the Wnt pathway [7], which is accepted as a key pathway for the stemness maintenance of cancer stem cell (CSC) markers. The CSC hypothesis also has been connected to a number of solid tumors including lung cancer and other types of cancers, particularly NSCLC, harbor CSC populations [8-10]. The CD44 is a polymorphic family of cell surface glycoproteins with a cytoplasmic domain and seven extracellular domains, a transmembrane domain [11]. CD44 is with a variety of functions including participation in cell adhesion and migration as well as modulation of cell-matrix interactions. Expression of CD44 has been shown in both normal and neoplastic tissue and holds promise as a prognostic indicator. The prognostic value of CD44 for patients with cancer has been reported in various solid tumors, including breast cancer, colon cancer, and lung cancer [12-14].
The effect of abnormalities expression of CD44 has been investigated for NSCLC by using univariate or multivariate analysis, however, the relationships between CD44 and prognosis value were still controversial mainly because of the limited patient numbers of independent reports. Based on the discordant results obtained by numbers of studies, we conducted this meta-analysis to quantify the prognostic impact of CD44 expression on overall survival and clinicopathological features among patients with NSCLC.
Materials and methods
Literature search
A literature search via PubMed, EMBASE and CNKI (China National Knowledge Infrastructure) databases was conducted to find articles that evaluated the role of CD44 in NSCLC. The keywords and text words were used as follows: (1) CD44, and (2) non-small cell lung cancer or NSCLC or lung cancer or lung carcinoma or carcinoma of lung and (3) outcome or survival or prognosis.
Study selection
All languages were included, and all eligible articles that examined the association between the expression of CD44 and overall survival or CD44 and clinicopathological features were gathered. However, the papers which only have abstracts were excluded because of insufficient data for meta-analysis. Therefore, we first read the titles of the publications and the abstracts to find exactly those articles that examined the relationship between CD44 and overall survival (OS) or CD44 and clinicopathological features in patients with NSCLC. After the abstracts met these conditions, the full texts were analyzed and included into our meta-analysis according to the following criteria: (1) an original paper (2) expression levels of CD44 were compared to patient’s overall survival; (3) expression of the proteins were evaluated in tumor tissues by immunohistochemistry (IHC) or reverse transcription and polymerase chain reaction (RT-PCR) analysis; (4) studies that reported a hazard ratio (HR)and confidence interval (CI) or could be calculated from the sufficient data; (5) expression levels of CD44 were compared to patient’s clinicopathological features; (6) including three or more clinical characteristics of lymph node metastasis, TMN stage, tumor size, tumor differentiation and histological type (5) if the same group of patients were used to analyze more than once, the most complete research was selected for our study.
The major exclusion criteria were (1) reviews, non-original articles, abstracts and letters; (2) non-CD44 or NSCLC; (3) duplication of a previous publication; and (4) examination of specimens collected after chemotherapy or radiotherapy.
Data extraction
Two reviewers (Zhuang Luo and Rong-rong Wu) independently checked all articles and extracted data in separate databases. The following information were collected from each study: first author’s name, type of CD44, year of publication, ethnicity, sample size, laboratory methodology, cut-off value, lymph node metastasis, tumor size, tumor differentiation, histological type, clinical TMN stage and HR with 95% CI. Disagreements were resolved through discussion among the authors.
Statistical analysis
The intensity of relationship between the expression levels of CD44 and overall survival were described as HRs. Overexpression of CD44 indicated poor prognosis in patients with NSCLC if HR > 1 with the 95% CI did not overlap 1. From some published researches, HR and 95% CI could be directly obtained by using univariate or multivariate survival analysis. Otherwise, HR and 95% CI were calculated by Kaplan-Meier survival curves using the software Engauge Digitizer Version 4.1 (http://digitizer.sourceforge.net/) and the method presented by Parmar et al. before [15,16]. Then, extracted data were utilized to reconstruct the HR and its variance (GraphPad Software, Inc, La Jolla, CA, USA).
The pooled HR corresponding to the 95% CI was used to assess the prognostic value of CD44 in patients. Statistical heterogeneity was tested by Cochrane’s Q test (Chi-squared test; Chi2) and inconsistency (I2) [17,18]. If there was no obvious heterogeneity, the fixed-effects model (Mantel-Haenszel method) was used to estimate the pooled HR; otherwise, the random-effects model (DerSimonian and Laid method) was used [15]. We assessed the possibility of publication bias using a funnel plot and tested it with Egger’s test [19]. A P value less than 0.05 was considered statistically significant. STATA 12.0 (STATA Corp., College, TX) was used to perform statistical analysis.
Results
Characteristics of eligible studies
One hundred fifty, 10, 138 articles were retrieved from PubMed, EMBASE and CNKI electronic database according to our defined keywords and text words, respectively (Figure 1). Then, via careful reading the abstracts, 81 researches that focused on the association between the expression of CD44 and survival were included in our full-text review process. After reading the full-text researches, 43 papers had to be excluded because data were not extractable or could not provide enough information about overall survival or complete clinicopathological features. As a result, 38 eligible studies were included in this meta-analysis [20-57]. Twenty Three studies including 1772 cases were available for our meta-analysis for the expression of CD44 and prognosis. Among all the included studies, twenty four studies including 1871 cases were available for our meta-analysis for the expression of CD44 and clinicopathological features. The individual characteristics and results of eligible prognostic studies evaluating surviving are summarized in Table 1. Main clinicopathological features and results of eligible studies are summarized in Table 2.
Figure 1.
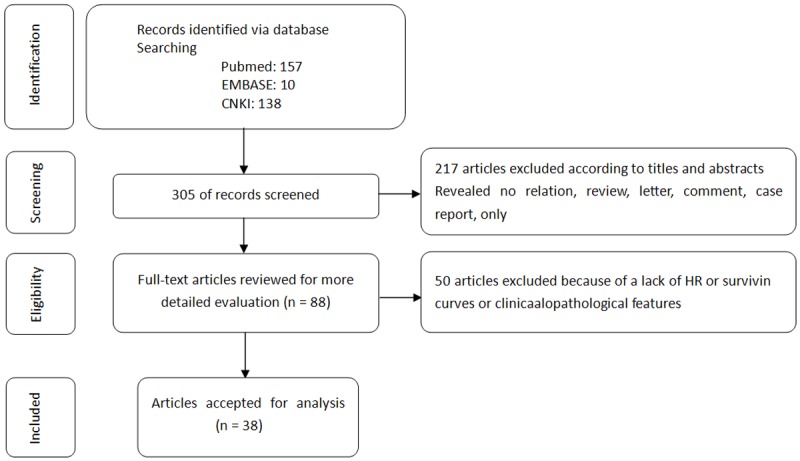
Flow chart summarizing the literature search and study selection.
Table 1.
Individual characteristics and results of eligible prognostic studies evaluating survival
| First Author | CD44 type | Year | Ethnicity | case | Method | Cutt off value | Univariate HR | Univariate 95% CI | Muti-variate HR | Muti-variate 95% CI | SCC/ADC/Others | Stage (I/II/III/IV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clarke MR | pan-CD44 | 1995 | America | 31 | IHC | > 0% | 0.45 | 0.28-0.73 | NA | NA | NA | 31/0/0/0 |
| Hirata T | CD44-V6 | 1998 | Japan | 69 | IHC | ≥ 20% | 2.42 | 0.91-6.43 | 2.55 | 1.31-4.98 | 25/43/1 | 69/0/0/0 |
| Fukuse T | CD44-V6 | 1999 | Japan | 34 | IHC | ≥ 20% | 0.95 | 0.50-1.81 | 0.29 | 0.05-1.63 | 12/21/1 | NA |
| Pirinen R | CD44-V6 | 2000 | Finlang | 231 | IHC | > 66% | NA | NA | 2 | 1.20-3.0 | 146/58/27 | 149/78/0/0 |
| Ramasami S | CD44-V6 | 2000 | UK | 120 | IHC | ≥ 10% | 0.59 | 0.40-0.87 | NA | NA | 0/120/0 | 69/35/16/0 |
| Zhang HZ | CD44-V6 | 2001 | China | 42 | IHC | > 0% | 1.59 | 0.92-2.75 | NA | NA | 15/27/0 | 25/12/4/1 |
| Zhang GR | CD44-V6 | 2003 | China | 62 | IHC | ≥ 10% | 2.81 | 1.75-4.51 | NA | NA | 33/29/0 | 9/26/27/0 |
| Zhang YC | CD44-V6 | 2003 | China | 79 | IHC | > 0% | 0.82 | 0.48-1.42 | NA | NA | 42/37/0 | 32/32/15/0 |
| Cheng C | pan-CD44 | 2004 | China | 32 | IHC | > 0% | NA | NA | 1.217 | 0.054-2.87 | 19/12/1 | 0/0/32/0 |
| Zhang L | CD44-V6 | 2004 | China | 43 | IHC | ≥ 10% | 3.2 | 1.59-6.45 | NA | NA | 24/19/0 | 17/12/14/0 |
| Liu B | CD44-V6 | 2005 | China | 108 | IHC | > 4 score | 1.5 | 1.13-1.98 | NA | NA | 56/44/8 | NA |
| Wu QP | CD44-V6 | 2005 | China | 52 | IHC | > 0% | 1.53 | 0.92-2.53 | 1.88 | 1.15-2.76 | 31/21/0 | 19/20/12/1 |
| Le QT | CD44-V6 | 2006 | Ireland | 20 | IHC | > 0% | 1.98 | 0.56-6.95 | NA | NA | 2/10/8 | 12/8/0/0 |
| Li GH | pan-CD44 | 2007 | China | 36 | RT-PCR | NA | 2.15 | 0.95-4.85 | NA | NA | 19/13/4 | 25/0/11/0 |
| Weng MX | CD44-V6 | 2008 | China | 86 | IHC | > 5 score | 1.68 | 1.32-2.14 | NA | NA | 50/36/0 | 47/18/21/0 |
| Xie ZM | CD44-V6 | 2008 | China | 80 | IHC | > 0% | NA | NA | 1.87 | 0.298-4.72 | 28/52/0 | 80/0/0/0 |
| Wu Y | pan-CD44 | 2009 | China | 36 | RT-PCR | > 0.5 | 1.11 | 0.24-5.13 | NA | NA | 7/19/2 | 12/0/9/0 |
| Zhang XH | CD44-V6 | 2009 | China | 46 | IHC | > 0% | 2.43 | 1.11-5.32 | NA | NA | 15/27/4 | NA |
| Meng L | CD44-V6 | 2010 | China | 56 | IHC | ≥10% | 3.41 | 2.08-5.57 | NA | NA | 32/24/0 | 22/34/0/0 |
| Situ DR | CD44-V6 | 2010 | China | 190 | IHC | > 0% | 0.318 | 0.14-0.15 | 0.325 | 0.14-0.77 | 71/60/59 | 190/0/0/0 |
| Ko YH | pan-CD44 | 2011 | South Korea | 82 | IHC | > 3 score | 3.32 | 2.12-5.2 | 3.152 | 1.26-7.91 | 0/82/0 | 43/18/21/0 |
| Wei MC | CD44-V6 | 2011 | China | 60 | IHC | ≥ 4 score | 3.03 | 1.70-5.41 | NA | NA | 33/27/0 | 60/0/0/0 |
| Okudela K | pan-CD44 | 2012 | Japan | 177 | IHC | ≥ 10% | 2.29 | 0.89-5.90 | 3.73 | 1.2-11.58 | NA | 177/0/0/0 |
Abbreviation: P/N, positive expression/negative expression; IHC, immunohistochemistry; SCC, Squamous cell carcinoma; ADC, Adenocarcinoma; HR, hazard ratio; NA, no available or no applicable.
Table 2.
Main clinicopathological features and results of eligible studies
| First Author | CD44 type | Year | Ethnicity | case | lymph node metastasis | TMN stage | Tumor size | Tumor differentiation | Histological type | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CD44+ | CD44- | CD44+ | CD44- | CD44+ | CD44- | CD44+ | CD44- | CD44+ | CD44- | |||||
|
| ||||||||||||||
| N/P | N/P | I II/III IV | I II/III IV | < 3 cm/> 3 cm | < 3 cm/> 3 cm | I II/III | I II/III | SCC/ADC | SCC/ADC | |||||
| Miyoshi T | CD44-V6 | 1997 | Japan | 61 | 13/23 | 19/6 | 15/21 | 13/12 | 7/29 | 13/12 | NA | NA | NA | NA |
| Dai BJ | CD44-v6 | 1998 | China | 62 | 19/24 | 4/4 | 20/23 | 14/5 | 17/26 | 10/9 | NA | NA | 19/17 | 6/10 |
| Hirata T | CD44-v6 | 1998 | Japan | 69 | 14/6 | 37/12 | NA | NA | NA | NA | NA | NA | 13/1 | 12/35 |
| Zhao HY | CD44-V6 | 1998 | China | 96 | 26/31 | 25/3 | 25/14 | 35/16 | NA | NA | 31/26 | 17/11 | NA | NA |
| Zhao ZS | CD44-v6 | 1999 | China | 62 | 24/18 | 21/1 | 20/22 | 17/3 | 9/33 | 14/6 | 24/18 | 15/5 | 11/20 | 8/7 |
| Pirinen R | CD44-V6 | 2000 | Finlang | 231 | 67/17 | 88/54 | NA | NA | 19/65 | 44/98 | 46/30 | 65/56 | 72/6 | 74/52 |
| Zhang HZ | CD44-V6 | 2001 | China | 42 | 8/21 | 10/3 | 41754 | 12/1 | NA | NA | NA | NA | NA | NA |
| Wang ZT | CD44-v6 | 2002 | China | 147 | 54/40 | 31/22 | 63/31 | 32/21 | NA | NA | 83/11 | 38/15 | 49/35 | 14/31 |
| Sun L | CD44-v6 | 2002 | China | 90 | 23/36 | 21/10 | 10/36 | 21/23 | NA | NA | 28/15 | 25/19 | NA | NA |
| Zuo WL | CD44-v6 | 2003 | China | 79 | 15/33 | 23/8 | NA | NA | NA | NA | NA | NA | 20/28 | 11/10 |
| Gao K | CD44-v6 | 2003 | China | 74 | 14/19 | 30/11 | 9/24 | 21/20 | NA | NA | 21/12 | 25/16 | NA | NA |
| Zhang GR | CD44-V6 | 2003 | China | 62 | 5/38 | 7/12 | 18/25 | 17/2 | 21/22 | 8/11 | NA | NA | 21/22 | 11/7 |
| Zhang YC | CD44-V6 | 2003 | China | 79 | 21/20 | 22/14 | 35/6 | 29/9 | 11/30 | 10/28 | NA | NA | 28/6 | 4/20 |
| Zhang Q | CD44-v6 | 2004 | China | 116 | 36/23 | 43/3 | 45/14 | 44/2 | 19/40 | 27/19 | 38/20 | 40/6 | 46/13 | 23/23 |
| Zhang L | CD44-V6 | 2004 | China | 43 | 6/21 | 12/4 | 6/21 | 11/5 | NA | NA | NA | NA | 14/13 | 10/6 |
| Lin H | CD44-v6 | 2005 | China | 101 | 15/65 | 16/5 | 45/35 | 11/6 | 19/61 | 5/16 | 42/38 | 11/10 | 45/35 | 14/7 |
| Wu GZ | CD44-v6 | 2005 | China | 50 | NA | NA | 14/13 | 16/7 | NA | NA | 20/6 | 20/4 | 29/6 | 3/6 |
| Wu QP | CD44-V6 | 2005 | China | 52 | 1/18 | 13/20 | 22/8 | 17/4 | NA | NA | 20/16 | 15/1 | 28/8 | 3/13 |
| YU J | CD44-V6 | 2006 | China | 69 | 12/26 | 25/6 | 27/11 | 15/16 | NA | NA | 9/29 | 4/27 | 25/13 | 10/21 |
| Li GH | pan-CD44 | 2007 | China | 36 | 2/19 | 8/7 | 12/9 | 13/2 | 5 | 4/11 | 20/1 | 12/3 | 12/7 | 7/6 |
| Yang Yj | CD44-V6 | 2007 | China | 65 | NA | NA | 18/25 | 20/2 | NA | NA | 16/27 | 12/10 | 30/13 | 5/17 |
| Weng MX | CD44-V6 | 2008 | China | 86 | 22/16 | 38/10 | 23/15 | 42/6 | NA | NA | 23/26 | 27/19 | 27/11 | 23/25 |
| Eren B | CD44-V6 | 2008 | Turkey | 33 | 15/3 | 6/9 | 3/15 | 3/12 | NA | NA | NA | NA | 16/1 | 7/8 |
| Wu Y | pan-CD44 | 2009 | China | 36 | 2/19 | 8/7 | 12/9 | 13/2 | 5/16 | 4/11 | NA | NA | 12/7 | 7/6 |
| Zhang XH | CD44-V6 | 2009 | China | 46 | 14/12 | 18/2 | NA | NA | 11/16 | 10/9 | NA | NA | NA | NA |
| Yuan H | pan-CD44 | 2010 | China | 75 | 14/35 | 15/11 | 17/32 | 20/6 | NA | NA | 22/19 | 21/15 | NA | NA |
| Meng L | CD44-V6 | 2010 | China | 56 | 7/26 | 15/8 | 8/26 | 15/8 | NA | NA | NA | NA | 18/15 | 14/9 |
| Ko YH | pan-CD44 | 2011 | South Korea | 149 | 59/43 | 24/17 | 78/29 | 32/10 | 33/92 | 13/28 | 18/89 | 7/35 | 66/41 | 6/36 |
Abbreviation: N/P, negative expression/positive expression; SCC, Squamous cell carcinoma; ADC, Adenocarcinoma; I II/III IV, TMN stageIand TMN stage II/TMN stage III and TMN stage IV; Tumor differentiation I II/III, well and moderate differentiation/poor differentiation; NA, no available or no applicable.
Impact of CD44 expression on OS in NSCLC
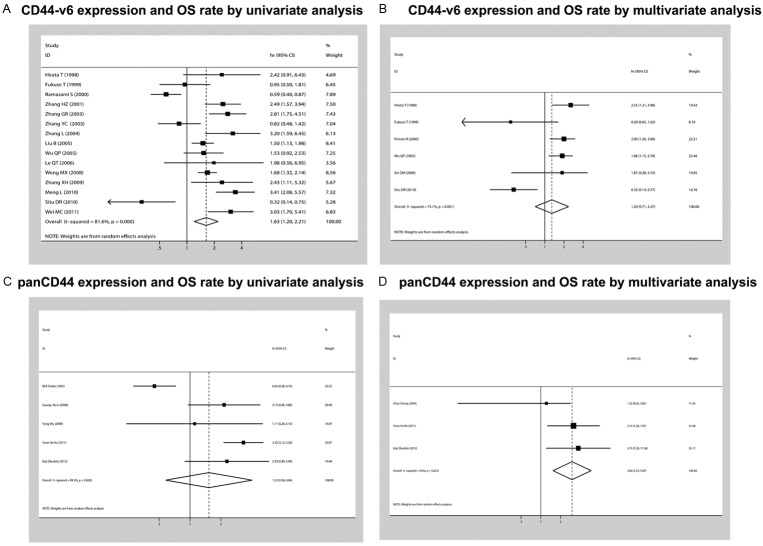
We evaluated whether CD44v6 and panCD44 expression levels were associated with the overall survival in patients with NSCLC. Of the 23 trials evaluable for systematic review, 15 and 5 could be included in meta-analysis by univariate and multivariate analysis effect of CD44v6 on overall survival due to sufficient data to estimate the HR and 95% CI. Only 5 and 3 could be included in meta-analysis by univariate and multivariate analysis effect of panCD44 on overall survival.
The relationship between CD44 expression and NSCLC prognosis is illustrated in Figure 2. Seventeen studies(including a total of 1378 patients) that demonstrated the association of CD44-V6 expression and OS rate were obtained from the published information. According to univariate analysis of CD44-V6 expression and OS rate in fifteen studies(including a total of 1067 patients), with a combined HR of 1.63 (95% CI: 1.20-2.21, P = 0.000, I2 = 81.6%, random-effect). According to multivariate analysis of CD44-V6 expression and OS rate in six studies(including a total of 656 patients), with a combined HR of 1.29 (95% CI: 0.71-2.37, P = 0.00, I2 = 75.1%). Six studies (including a total of 394 patients) that demonstrated the association of panCD44 expression and OS rate were obtained from the published information. Furthermore, according to univariate analysis of panCD44 expression and OS rate in five studies(including a total of 362 patients), there was no significant disparities for the effective value of OS was determined (HR of 1.53, 95% CI: 0.58-4.04, P = 0.0000, I2 = 89.5%). According to multivariate analysis of panCD44 expression and OS rate in three studies (including a total of 291 patients), with a combined HR of 3.00 (95% CI: 1.53-5.87, P = 0.623, I2 = 0.0%).
Figure 2.
Forest plot showing the combined relative HR from the random-effects model for overall survival by univariate and multivariate analysis such as (A), CD44-v6 expression and OS rate by univariate analysis (B), CD44-v6 expression and OS rate by multivariate analysis (C), panCD44 expression and OS rate by univariate analysis (D), panCD44 expression and OS rate by multivariate analysis.
Univariate HR of subgroup analyses for CD44-v6 on NSCLC survival are ummarized in the Table 3. When grouped according to the ethnicity, combined HRs of Asian and Non-Asian written were 1.78 (CI: 1.34-2.35) and 0.53 (CI: 0.29-2.92), respectively. In the subgroup analysis according to Written Language the combined HR was 0.88 (CI: 0.47-1.63) for English written and 2.05 (CI: 1.59-2.64) for non English written. When the survival data calculated indirectly from Kaplan-Meier based survival curve were pooled, the combined HR was 1.83 (95% CI: 1.26-2.65), also suggesting CD44-v6 status was of prognostic value. Although we also observed statistically significant effects of CD44-v6 expression on survival from studies reported I-III with an HR of 1.61 (95% CI: 1.01-2.58) from 7 studies reported, when we aggregated the studies that reported results for I-II early-stage NSCLC, the combined HR were 1.78 (95% CI: 0.78-4.09). Moreover, when the survival data calculated by follow-up time, combined HRs of 36 months and 60 months showed an inverse effects on survival (HR 1.79 CI: 0.9-3.58 and 1.82 CI: 1.29-2.56, respectively). Finally, grouped according to the positive threshold for CD44-v6 expression, as defined by the studies’ authors, the combined HR of 0% and 10% cut off value were 1.31 (95% CI: 0.73-2.35) and 2.03 (95% CI: 0.79-5.22), respectively.
Table 3.
Summarized Univariate HR of overall and subgroup analyses for CD44v6 on NSCLC survival
| N. of studies | Number of patients | Random effects HR (95% CIs) | Heterogeneity test | ||
|---|---|---|---|---|---|
|
| |||||
| chi-squared | I2 | P-value | |||
| Written Language | |||||
| English written | 5 | 0.88 (0.47-1.63) | 13.6 | 70.60% | 0.678 |
| Non English written | 10 | 2.05 (1.59-2.64) | 28.1 | 67.90% | 0.0001 |
| HR Estimate | |||||
| HR | 2 | 0.73 (0.16-2.3) | 9.58 | 89.6 | 0.683 |
| Sur. Curve | 13 | 1.83 (1.26-2.65) | 62.14 | 82.3 | 0.001 |
| Ethnicity | |||||
| Asian | 13 | 1.78 (1.34-2.35) | 48.26 | 75.10% | 0.0001 |
| Non-Asian | 2 | 0.53 (0.29-2.92) | 3.24 | 69.20% | 0.892 |
| Cutoff value | |||||
| 0% | 6 | 1.31 (0.73-2.35) | 23.54 | 78.80% | 0.369 |
| 10% | 4 | 2.03 (0.79-5.22) | 43.56 | 93.10% | 0.141 |
| Tumor stage | |||||
| I-II | 5 | 1.78 (0.78-4.09) | 23.85 | 83.20% | 0.7114 |
| I-III | 7 | 1.61 (1.01-2.58) | 43.52 | 86.20% | 0.0400 |
| Follow up time | |||||
| 36 months | 4 | 1.79 (0.9-3.58) | 12.05 | 75.10% | 0.0035 |
| 60 months | 9 | 1.82 (1.29-2.56) | 35.88 | 77.70% | 0.1915 |
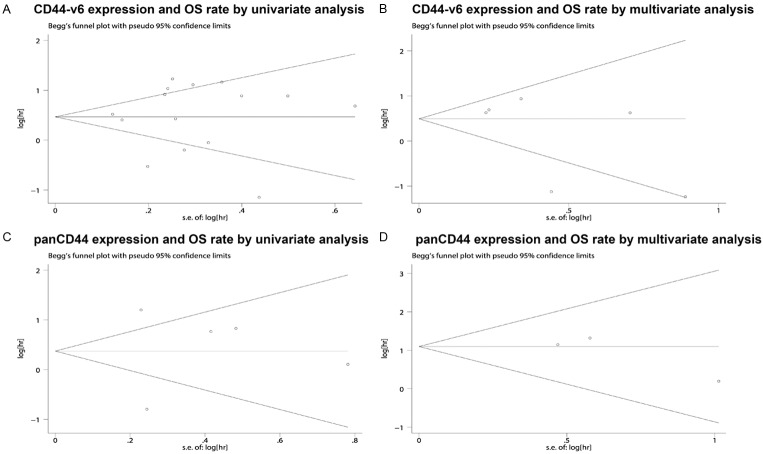
Publication bias statistics were determined using the method of Begg’s test (Figure 3) between CD44 (including CD44-v6 and pan-CD44) expression and NSCLC prognosis. In all included studies, no funnel plot asymmetry was found Sensitively analysis was performed to investigate the effect of every study on the overall meta-analysis by omitting one study each time, and the omission of any study made no significant difference, demonstrating that our results were statistically reliable.
Figure 3.
Begg’s test results of overall survival rate such as (A), CD44-v6 expression and OS rate by univariate analysis (B), CD44-v6 expression and OS rate by multivariate analysis (C), panCD44 expression and OS rate by univariate analysis (D), panCD44 expression and OS rate by multivariate analysis.
Correlation of CD44-v6 expression with clinicopathological parameters
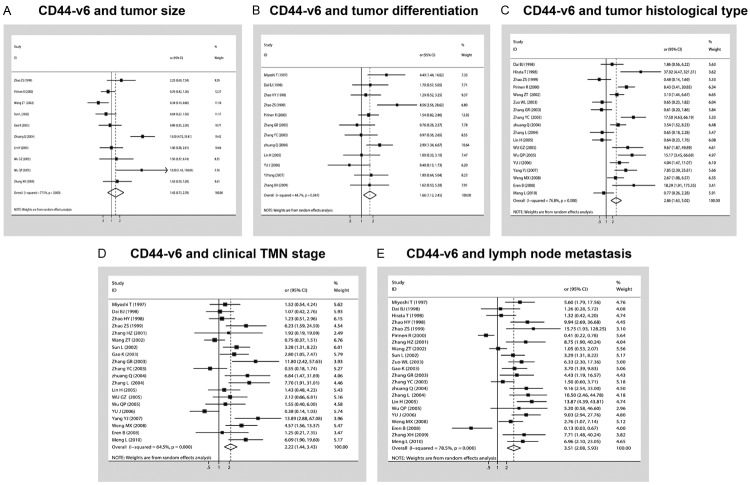
The forest plot of OR was assessed for association between CD44-v6 and clinicopathological features such as tumor category (A), tumor size (B), tumor differentiation (C), tumor histological type (D), clinical TMN stage (E), lymph node metastasis in Figure 4. In pooled analysis, CD44-v6 expression was significantly associated with tumor differentiation [poor differentiation, OR (odds ratio) = 1.66, 95% CI: 1.12-2.45 and, P = 0.00001 and I2 = 76.8, random-effect], tumor histological type (SCC, OR = 2.6, 95% CI: 1.63-5.02 and P = 0.014 and I2 = 51.3, random-effect), clinical TMN stage (TMN stage III, OR = 2.22, 95% CI: 1.44-3.43, P = 0.0001 and I2 = 64.5, random-effect) and lymph node metastasis (N1-3, 3.52, 95% CI: 2.08-5.93, P = 0.0001 and I2 = 78.5, random-effect) in patients with NSCLC. However, there was no significant association between CD44-V6 and tumor size (T category, OR = 1.42, 95% CI: 0.73-2.78, P = 0.0001 and I2 = 77.5, random-effect).
Figure 4.
Forest plot of OR was assessed for association between CD44-v6 and clinicopathological features such as (A), tumor size (B), tumor differentiation (C), tumor histological type (D), clinical TMN stage (E), lymph node metastasis.
Next, we performed analyses to investigate clinicopathological parameters if there were differences in results with respect to panCD44 expression. However, because of the limited number of studies that we could not get the statistically significant results mostly. Thus, more studies about panCD44 and NSCLC should be conducted in the future.
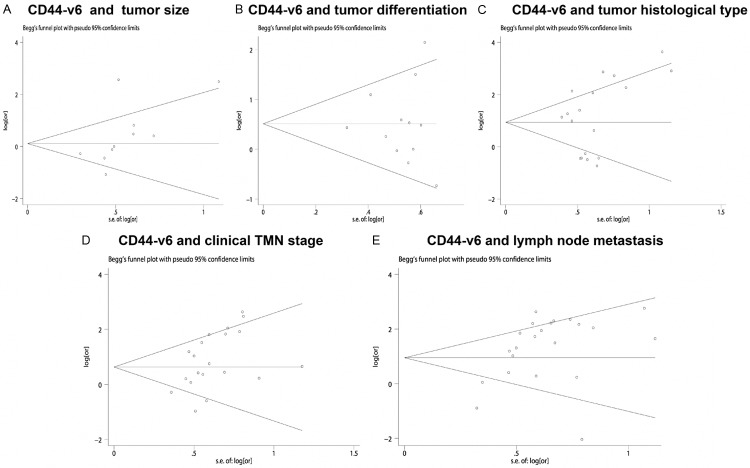
Publication bias statistics were determined using the method of Begg’s test (Figure 5) between CD44 (including CD44-v6 and panCD44) expression and clinicopathological features. In all included studies, no funnel plot asymmetry was found Sensitively analysis was performed to investigate the effect of every study on the overall meta-analysis by omitting one study each time, and the omission of any study made no significant difference, demonstrating that our results were statistically reliable.
Figure 5.
Begg’s test results of CD44-v6 and clinicopathological features such as tumor category (A), tumor size (B), tumor differentiation (C), tumor histological type (D), clinical TMN stage (E), lymph node metastasis.
Discussion
The correlation between CD44 expression and NSCLC has been widely studied, and most of them showed that over-expression of CD44 was associated with some clinicopathologic features and a poor prognosis, but a great deal of controversy results of the prognostic implications of CD44 in NSCLC remains. To get a better understanding of the relationship between CD44 expression and NSCLC, this meta-analysis was carried out including 1772 patients from 23 evaluable studies for Prognostic Value and 2167 patients from 28 evaluable studies for clinicopathological. The pooled data revealed the CD44 expression in whole tissue sections, revealed a poor prognostic outcome in patients expressing high levels of CD44v6. The results indicate that CD44v6 expression is significantly associated with tumor differentiation, tumor histological type, clinical TMN stage, lymph node metastasis and OS. We also have collected the information about the relationship between the pan-CD44 expression and OS and clinicopathological features at the same time. Although we also observed statistically significant effects of panCD44 expression and OS rate in three studies (including a total of 291 patients) from multivariate analysis, with a combined HR of 3.00 (95% CI: 1.53-5.87, P = 0.623, I2 = 0.0%). Furthermore, because of the limited number of studies that we could not get the statistically significant results about pan-CD44 expression and clinicopathological parameters. Thus, more studies about panCD44 and NSCLC should be conducted in the future.
Extensive evidence showed that CD44 played important roles in tumor progression, especially with cancer stem cell related characteristics.What makes CD44 account for the poor prognosis in NSCLC? On the one hand, recently several studies have reported the specific role of CD44v6 and CD44s. CD44 could participate in a important biological event in the invasion process, epithelial mesenchymal transition (EMT) [58,59]. In some epithelial cells the EMT process was accompanied by a transition in CD44 isoforms from CD44v6 to CD44s, and CD44s has been proved to promote the EMT process [60]. In tumor progression CD44 was related to cancer cell invasion and metastasis. when CD44 interacted with hyaluronan, by virtue of their ability to binded to the actin cytoskeleton, CD44 could move to the leading edge of the migrating cells. Thereafter migrating cells moving to the endothelial cells as the initial step of the extravasation relying on CD44 could bind to CD62 on endothelial cells [61,62]. Effects of HA and CD44 interaction could activate the ROK pathway, which induced the phosphorylation of the Na+/H+ exchange [63] an decreased of tumor extracellular matrix and invasion. Many articles have proved that CD44 was closely related with lymph node metastasis, which were well supported by our meta-analysis. The results of our meta-analysis supported that the function of the lymph node metastasis might be dependent on CD44v6.
The heterogeneity could be explained by the fact that the technique of detecting CD44 is not comparable among the studies. These differing results in NSCLC may be partially attributed to differences in antibodies used for staining, different criteria for defining stain positivity, and different patient demographics such as tumor stage,histological subtype, adjuvant treatment, and insufficient SCC immunoreactivity. However, obviously, CD44 is a membrane-bound glycoprotein and mediates a complex range of functions. Invasive and metastatic growth can be mediated through the interaction of cell surface CD44 with ECM components such as hyaluronan or cell-cell interactions [64]. CD44-hyaluronan aggregates are essential for activation of metalloproteinase, which induces tissue invasion and tumor growth factor-β [65].
This contradiction could be explained by the relative small burden of some NSCLC cancers that could not change the systemic CD44 level. However, when stratified analysis was conducted about different stages of NSCLC, the association was also found in stage I-III, but not stage I-II, indicating that CD44-v6 could probably predict worse prognosis in advanced-stage NSCLC. Overexpression of CD44-v6 was a negative prognostic marker with significance in patients of with squamous cell carcinomas (SCC). This tendency was reported in previous reports [66,67]. SCC derives from dysplastic or metaplastic stratified epithelia. In a recent study by Leung et al, evaluating non-cancerous lung tissue, membrane expression of CD44 was confined to the surface of bronchial basal cells, alveolar macrophages, and regenerating cuboidal pneumocytes of injured lung, whereas no CD44 expression was observed in terminally differentiated epithelial cells such as ciliated or nonciliated columnar cells of bronchial epithelium or type I flat pneumocytes lining alveolar spaces [13]. However, squamous metaplasia showed strong CD44 immunoreactivity in the proliferating basal layers, while in premalignant dysplasia, the entire thickness displayed aberrant CD44 expression, indicating that squamous malignant transformation is closely associated with CD44 expression.
Although we performed a comprehensive analysis of the association between CD44 expression and overall survival and patient clinicopathological parameters, there were some limitations to this meta-analysis. The risks calculated in our meta-analysis may be an overestimate due to publication and reporting bias. We did not include unpublished papers and abstracts into meta-analysis because the required data was available only in full publications. Positive results tend to be more acceptable by journals, whereas negative results often are rejected or are not even submitted for review. Furthermore, another potential source of bias is related to the method used to extrapolate the HR. HR was extracted from the data included in the article directly or calculated from the survival curves. Actually, the method of extrapolating HR from survival curves seems to be less reliable because this strategy did not completely eliminate inaccuracy in the extracted survival rates. Moreover, we also think different objects included in these studies have different impact on overall survival, so this factor should be taken into consideration. Therefore, more meticulous research should be conducted. Nevertheless, no publication bias was detected using Begg’s test (P > 0.05), indicating that the statistics obtained approximate the actual results. Sensitivity analysis was also conducted to investigate the influence of a single study on the overall meta-analysis by omitting one study at a time, and the omission of any study made no significant difference, suggesting that our results were statistically reliable.
In summary, overexpression of CD44v6 was associated with poor overall survival in patients with NSCLC on univariate analysis but not multivariate analysis. However, more prospective clinical studies are needed to explore the prognostic value of panCD44 in NSCLC. Overexpression of CD44-V6 is associated with tumor differentiation, tumor histological type, clinical TMN stage and lymph node metastasis in patients with NSCLC. However, there was no significant association between expression of CD44-V6 and tumor size. Large prospective studies are now needed to confirm the clinical utility of CD44 as an independent prognostic marker.
Disclosure of conflict of interest
None.
References
- 1.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Wang B, Chen X, Bi J. The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis. 2011;32:411–416. doi: 10.1093/carcin/bgq266. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Kim SH, Lee ES, Kim YS. CD24 overexpression in cancer development and progression: a meta-analysis. Oncol Rep. 2009;22:1149–1156. doi: 10.3892/or_00000548. [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Xu J, Zhang J, Huang J. Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer. 2012;12:573. doi: 10.1186/1471-2407-12-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni C, Zhang Z, Zhu X, Liu Y, Qu D, Wu P, Huang J, Xu AX. Prognostic value of CD166 expression in cancers of the digestive system: a systematic review and meta-analysis. PLoS One. 2013;8:e70958. doi: 10.1371/journal.pone.0070958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv Exp Med Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Klaveren RJ, van’t Westeinde SC, de Hoop BJ, Hoogsteden HC. Stem cells and the natural history of lung cancer: implications for lung cancer screening. Clin Cancer Res. 2009;15:2215–2218. doi: 10.1158/1078-0432.CCR-08-1920. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010;29:61–72. doi: 10.1007/s10555-010-9216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 11.van der Windt GJ, Schouten M, Zeerleder S, Florquin S, van der Poll T. CD44 is protective during hyperoxia-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:377–383. doi: 10.1165/rcmb.2010-0158OC. [DOI] [PubMed] [Google Scholar]
- 12.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Wang XQ, Zhou B, Zhang L. The prognostic value of MGMT promoter methylation in Glioblastoma multiforme: a meta-analysis. Fam Cancer. 2013;12:449–458. doi: 10.1007/s10689-013-9607-1. [DOI] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke MR, Landreneau RJ, Resnick NM, Crowley R, Dougherty GJ, Cooper DL, Yousem SA. Prognostic significance of CD44 expression in adenocarcinoma of the lung. Clin Mol Pathol. 1995;48:M200–204. doi: 10.1136/mp.48.4.m200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata T, Fukuse T, Naiki H, Hitomi S, Wada H. Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res. 1998;58:1108–1110. [PubMed] [Google Scholar]
- 22.Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Expression of proliferating cell nuclear antigen and CD44 variant isoforms in the primary and metastatic sites of nonsmall cell lung carcinoma with intrapulmonary metastases. Cancer. 1999;86:1174–1181. doi: 10.1002/(sici)1097-0142(19991001)86:7<1174::aid-cncr11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Pirinen R, Hirvikoski P, Bohm J, Kellokoski J, Moisio K, Viren M, Johansson R, Hollmen S, Kosma VM. Reduced expression of CD44v3 variant isoform is associated with unfavorable outcome in non-small cell lung carcinoma. Hum Pathol. 2000;31:1088–1095. doi: 10.1053/hupa.2000.16277. [DOI] [PubMed] [Google Scholar]
- 24.Ramasami S, Kerr KM, Chapman AD, King G, Cockburn JS, Jeffrey RR. Expression of CD44v6 but not E-cadherin or beta-catenin influences prognosis in primary pulmonary adenocarcinoma. J Pathol. 2000;192:427–432. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH741>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HZ, Li HG, Zhang H. Expression of CD44v6 and CEA in non-small cell lung cancer tissue and relationship with prognosis. Guang dong Medical Journal. 2001;22:911–912. [Google Scholar]
- 26.GR Z. The expressions of VEGF, CD44v6 and Ki67 in non-small cell lung carcinoma. Journal of Chongqing Medical University. 2003;28:473–476. [Google Scholar]
- 27.Zhang YC, QY K. Expression of CD44v6 in patients with lung carcinoma and its relationship with lymph node metastasis and prognosis. Actaacademiae Medicine Xuzhou. 2003;23:325–327. [Google Scholar]
- 28.Chen C, Wu YL, Zhou ZM, LJ G. The impact of the expression of multiple genes on the metastasis and survival in resected stage IIIA non2small cell lung cancer (NSCLC) patients. Tumor. 2004;24:62–64. [Google Scholar]
- 29.Zhang L, Meng L, Wang L, Peng Z, Chen J, Wang Z. The clinical significance of detection of vascular endothelial growth factor and CD44v6 expression in human non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2004;7:427–430. doi: 10.3779/j.issn.1009-3419.2004.05.12. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Wang Y, JQ , et al. Expression of Combined Detection on CD44V6 and nm23H1 in Non- small Cell Lung Cancer. Chinese Journal of Clinical Oncology. 2005;32:784–787. [Google Scholar]
- 31.Wu Q, Jiang Y, Min J, Xiang M. Expression of CD44v6 and its prognostic significance in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2005;8:215–218. doi: 10.3779/j.issn.1009-3419.2005.03.12. [DOI] [PubMed] [Google Scholar]
- 32.Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, Donington J, Cannon W, Wakelee H, Tibshirani R, Mitchell JD, Richardson D, O’Byrne KJ, Koong AC, Giaccia AJ. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 33.Li GH, Wu Y, T F. Expression of CD44 gene in non-small cell lung cancer tissue and relationship with prognosis. Journal of Jilin University (Medicine Edition) 2007;33:330–334. [Google Scholar]
- 34.Weng MX, Wu CH, Yang XP. Expression and Significance of E-Cadherin, CD44v6 and Proliferating Cell Nuclear Antigen in Non-small Cell Lung Cancer. Ai Zheng. 2008;27:191–195. [PubMed] [Google Scholar]
- 35.Xie ZM, Zhu ZH, LJ Z. Expression of proteins related to invasion and metastasis in stage I NSCLC and their prognostic impact. Journal of The Fourth Military Medica. 2008;29:74–745. [Google Scholar]
- 36.Y W. Muc21 mRNA and CD44 mRNA expression and prognosis in non-small cell lung carcinoma. Chinese Journal of Gerontology. 2009;10:1239–1242. [Google Scholar]
- 37.Zhang XH, Luo LP, XL Z. Correlative study among CT signs, prognosis and the expression ofCD44v3 and CD44v6 in peripheral non small cell lung cancer. Chin J Med Imaging Technology. 2009;25:1195–1198. [Google Scholar]
- 38.Meng L, Zhang YD, Wang YB. Clinical significance of vascular endothelial growth factor C and CD44v6 expressions in human non-small cell lung cancer. Journal of Shandong (Health Sciences) 2010;48:83–86. [Google Scholar]
- 39.Situ D, Long H, Lin P, Zhu Z, Wang J, Zhang X, Xie Z, Rong T. Expression and prognostic relevance of CD44v6 in stage I non-small cell lung carcinoma. J Cancer Res Clin Oncol. 2010;136:1213–1219. doi: 10.1007/s00432-010-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko YH, Won HS, Jeon EK, Hong SH, Roh SY, Hong YS, Byun JH, Jung CK, Kang JH. Prognostic significance of CD44s expression in resected non-small cell lung cancer. BMC Cancer. 2011;11:340. doi: 10.1186/1471-2407-11-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and b-catenin, in primary lung adenocarcinoma—their prognostic significance. Pathol Int. 2012;62:792–801. doi: 10.1111/pin.12019. [DOI] [PubMed] [Google Scholar]
- 42.Wei MC, Yue SC, QM W. Relation of NM23-H1 and CD44v6 expression to patient’s prognosis in the non-small cell lung cancer without lymph node metastasis. Journal of Dalian Medical University. 2011;33:437–440. [Google Scholar]
- 43.Miyoshi T, Kondo K, Hino N, Uyama T, Monden Y. The expression of the CD44 variant exon 6 is associated with lymph node metastasis in non-small cell lung cancer. Clin Cancer Res. 1997;3:1289–1297. [PubMed] [Google Scholar]
- 44.Dai BJ, ZS Z. Expression of CD44V6 in non-small cell lung carcinoma and its significance in prognosis. Journal of Practical Oncology. 1998;4:214–216. [Google Scholar]
- 45.Zhao H, Fang J, Du G. Expression of standard and variant CD44 in human lung cancer. Zhonghua Jie He He Hu Xi Za Zhi. 1998;9:541–543. [PubMed] [Google Scholar]
- 46.Zhao ZS, Ru GQ, Xu WJ. Expressions of CD44V3 and CD44V6 in non small cell lung carcinoma and their significance in prognosis. Chinese Journal of Clinical Oncology and Rehabilitation. 1999;4:24–27. [Google Scholar]
- 47.Wang Z, Zhang H, Li S. Expression and clinical significance of nm232H1 and CD44v6 in non2small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2002;4:278–281. doi: 10.3779/j.issn.1009-3419.2002.04.11. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Han J, Yao J, Yang S, Ren Y. Expression and clinicopathological significance of CD44V6 expression in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2000;1:48–50. doi: 10.3779/j.issn.1009-3419.2002.01.14. [DOI] [PubMed] [Google Scholar]
- 49.Zuo W, Li H. Relationship of the Expression of CD44v6 and Paxllin to the Prognos is of Non-small Cell Lung Carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:484–485. [PubMed] [Google Scholar]
- 50.Gao K, Song GY, Ma Q. Study on Expression of CD44v6, p53, and PCNA Protein in Non small Cell Lung Cancer. Cancer Research on Prevention and Treatment. 2003:93–95. [Google Scholar]
- 51.Zhang Q, Wu ES, Chen Q, HZ Y. Expession and prognostic signif icance of CD44v6 and C-erbB-2 in lung carcinomas. China Journal of Modern Medicine. 2004;3:57–61. [Google Scholar]
- 52.Lin H, YQ Y. Expression and significance of CD44v6 in non-small cell lung cancer (NSCLC) specimens. He Long Jiang Medicine and Pharmacy. 2005;4:13–414. [Google Scholar]
- 53.Wu GZ, Zheng XZ, N Z, L G. Expression and significance of PTEN and CD44V6 in human non small cell lung cancer of 50 patients aged over 50 years. Practical Geriatrics. 2005;6:312–314. [Google Scholar]
- 54.Yu J, TC P. Expression and clinicopathologic signif icance of OPN, CD44v6 and MMP22 in squamous cell carcinoma and adenocarcinoma of the lung. Chin J Lung Cancer. 2006;4:325–328. doi: 10.3779/j.issn.1009-3419.2006.04.05. [DOI] [PubMed] [Google Scholar]
- 55.Yj Y, CD Expression and clinicopathological signif icance of OPN and CD44v6 in lung cancer. Chin J Lung Cancer. 2007;2:98–101. doi: 10.3779/j.issn.1009-3419.2007.02.04. [DOI] [PubMed] [Google Scholar]
- 56.Eren B, Sar M, Oz B, Dincbas FH. MMP-2, TIMP-2 and CD44v6 Expression in Non-small-cell Lung Carcinomas. Ann Acad Med Singapore. 2008;37:32–39. [PubMed] [Google Scholar]
- 57.H Y, F Y. The expression and clinical significance of CD44 in non-small cell lung cancer. Journal of Mudanjian Medical University. 2010;4:16–19. [Google Scholar]
- 58.Zhang Y, Wei J, Wang H, Xue X, An Y, Tang D, Yuan Z, Wang F, Wu J, Zhang J, Miao Y. Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer. Oncol Rep. 2012;27:1599–1605. doi: 10.3892/or.2012.1681. [DOI] [PubMed] [Google Scholar]
- 59.Cho SH, Park YS, Kim HJ, Kim CH, Lim SW, Huh JW, Lee JH, Kim HR. CD44 enhances the epithelial-mesenchymal transition in association with colon cancer invasion. Int J Oncol. 2012;4:211–218. doi: 10.3892/ijo.2012.1453. [DOI] [PubMed] [Google Scholar]
- 60.Biddle A, Gammon L, Fazil B, Mackenzie IC. CD44 staining of cancer stem-like cells is influenced by down-regulation of CD44 variant isoforms and up-regulation of the standard CD44 isoform in the population of cells that have undergone epithelial-to-mesenchymal transition. PLoS One. 2013;8:e57314. doi: 10.1371/journal.pone.0057314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 63.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 64.Kim HR, Wheeler MA, Wilson CM, Iida J, Eng D, Simpson MA, McCarthy JB, Bullard KM. Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res. 2004;64:4569–4576. doi: 10.1158/0008-5472.CAN-04-0202. [DOI] [PubMed] [Google Scholar]
- 65.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen VN, Mirejovsky T, Melinova L, Mandys V. CD44 and its v6 spliced variant in lung carcinomas: relation to NCAM, CEA, EMA and UP1 and prognostic significance. Neoplasma. 2000;47:400–408. [PubMed] [Google Scholar]
- 67.Penno MB, August JT, Baylin SB, Mabry M, Linnoila RI, Lee VS, Croteau D, Yang XL, Rosada C. Expression of CD44 in human lung tumors. Cancer Res. 1994;54:1381–1387. [PubMed] [Google Scholar]