Abstract
IL-22, one important inflammatory cytokine of the IL-10 family, exerts its functions via IL-22 receptor that is composed of IL-22R1 and IL-10R2 subunits. Although IL-22 expression is reported to be elevated in many cancers, and increased IL-22 expression correlates with tumor progression and poor prognosis, little is known about the role of IL-22 in gastric cancer. In our study, we found that IL-22 stimulation promoted the migration and invasion of SGC-7901 cells. Furthermore, IL-22 increased AKT activation and MMP-9 production in a time- and dose-dependent manner, while knockdown of IL-22R1 attenuated the effect of IL-22 on gastric cancer cells. In addition, blocking of AKT activation suppressed the expression and secretion of MMP-9. Taken together, this present study suggests that IL-22 stimulation enhances the migration and invasion of gastric cancer cells by regulating IL-22R1/AKT/MMP-9 signaling axis.
Keywords: IL-22, migration, invasion, IL-22R1, gastric cancer, MMP-9
Introduction
Gastric cancer is one of the most common malignancies worldwide [1]. Despite effective treatments have been developed, tumor invasion and metastasis remain the major causes of death for gastric cancer patients [2]. Tumor microenvironments are considered to be essential for the invasion and metastasis of cancers. Cytokines in the tumor microenvironments may have stimulation effects on tumor cell invasion and metastasis [3]. Therefore, determination of the functions of these cytokines in tumor microenvironments may help to provide novel perspectives for the treatment of gastric cancer.
As one of the cytokines mainly secreted by T helper 17 (Th17) cells, IL-22 belongs to the IL-10 family and exerts its biological actions via IL-22 receptor (IL-22R) [4]. IL-22R is a heterodimeric receptor consisted of two chains, IL-22R1 and IL-10R2. IL-22R1 is mainly expressed in non-immune tissues including the skin, liver and colon, whereas IL-10R2 is ubiquitously expressed in various cells [5]. Accumulating studies have found that IL-22 and IL-22R are involved in the development of cancer [6]. It is reported that the expression of IL-22 is elevated in many cancers such as colon and pancreatic cancers [7,8], and increased IL-22 expression is closely related with cancer progression and metastasis [9]. In addition, IL-22 stimulation promotes the tumorigenesis of breast cancer cells [10], and displays antiapoptotic activity to lung cancer [11]. However, the function of IL-22 in gastric cancer remains unclear. Our present study designed to analyze the role of IL-22 in gastric cancer cell migration and invasion. Thus, we investigated the effect of IL-22 stimulation on the migration and invasion of gastric cancer cells. We further elucidated the possible molecular mechanisms by which IL-22 stimulated the migration and invasion of gastric cancer cells.
Materials and methods
Antibodies and reagents
The antibodies of total AKT and phospho-AKT were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). The antibodies of IL-22R1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant human interleukin-22 (IL-22) was from Abacam (Cambridge, MA, USA). PI3K inhibitor LY294002 was from Sigma (St Louis, MO, USA).
Cell lines and growth conditions
Human gastric cancer SGC-7901 cell line was purchased from Cell Resource Center of the Chinese Academy of Medical Sciences (Beijing, China). Cells were cultured in RPMI 1640 media (Gibco, Maryland, USA) supplemented with 10% fetal bovine serum and incubated in a humidified atmosphere containing 5% CO2 at 37°C.
siRNA transfection
SGC-7901 cells were transfected with control siRNA (5’-UGGUUUACAUGUUUUCUGA-3’) or IL-22R1 siRNA (5’-GAGGGAAGCAGAGAGAAUA-3’) by using Lipofectamine 2000 (Invitrogen, Carlsbad, USA), according to the manufacturer’s instructions. Briefly, 2 × 105 cells were cultured in a 100-mm dish for 16 h. Then 15 μg of siRNA was mixed with 8 μg of Lipofectamine 2000 in 500 μl of serum-free media. The cell media was replaced with 4.5 ml of fresh media. Then the 500 μl of serum-free media mixed with siRNA and Lipofectamine 2000 was added to the cells. Knockdown efficiency was assessed by western blotting 48 h later.
Invasion assay
Invasion assay was performed using the Millipore Transwell inserts (Millipore Corp., Bedford, MA, USA). Briefly, the upper membrane was coated with matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and then the insert was incubated at 37°C for 1 h to form a thin layer. Cells were adjusted to the concentration of 5.0 × 105 cells/ml and suspended in the RPMI 1640 media. Then cells were treated with or without IL-22. Two hundred microliter cell suspensions were placed in the upper chamber, while 500 μl RPMI 1640 media containing 30% fetal bovine serum was added into the lower chamber. After 18 h, cells that invaded through the membrane were fixed with 4% neutral-buffered formalin and stained with crystal violet. A light microscope was used to count the number of invaded cells, and the average value was determined through seven random fields.
Migration assay
Cell migration was measured by using a 24-well Millipore Transwell plate (Millipore Corp., Bedford, MA, USA). 1.0 × 105 cells in 200 μl RPMI 1640 media were placed in the upper chamber. 500 μl RPMI 1640 media supplemented with 30% fetal bovine serum was added into the lower chamber. After incubation in the CO2 incubator for 18 h, the number of migrated cells in seven fields was counted, and the average value was calculated for each chamber.
Real-time PCR
Total RNA was isolated from cells using Trizol reagent (Invitrogen, Carlsbad, CA). Then 2 μg of RNA was reversely transcripted into cDNA using an oligo (dT) primer and M-MLV reverse transcriptase (Tiangen, Beijing, China). Real-time PCR was carried out with the primers, cDNA and real-time PCR master mix (TaKaRa Bio Inc., Shiga, Japan). The primers for MMP-9 gene were: sense 5’-AGACCTGGGCAGATTCCAAAC-3’, antisense 5’-CGGCAAGTCTTCCGAGTAGT-3’. The primers for β-actin gene were: sense 5’-CTTAGTTGCGTTACACCCTTTCTTG-3’, antisense 5’-CTGTCACCTTCACCGTTCCAGTTT-3’. The real-time PCR reaction was conducted as follows: denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 50 s. The housekeeping gene β-actin was used as an internal control and the expression of MMP-9 was quantified with the 2-ΔΔCt method.
MMP-9 ELISA assay
The protein level of MMP-9 in the supernatant of SGC-7901 cells was assessed by MMP-9 ELISA kit (Millipore Corp., Bedford, MA, USA). After incubation with or without IL-22 for 18 h, cell supernatant was collected and centrifuged at 10,000 g for 10 min at 4°C to remove the cell debris. Then the supernatant was added to the MMP-9 ELISA plate, following the manufacturer’s instructions. The relative protein level of MMP-9 in each sample was determined by quantifying to the total protein of the whole cell extract.
Gelatin zymography assay
MMP-9 activity was assessed by gelatin zymography assay. Briefly, after incubation with IL-22 (100 ng/ml) for 18 h, cell supernatant was collected and concentrated at 10,000 g for 20 min in a concentrator (Millipore, Billerica, MA, USA) at 4°C. Then the protein concentration in the collected media was measured by Bicinchoninic Acid (BCA) Assay kit (Applygen Technologies Inc., Beijing, China). Next, equal amounts of protein were mixed with Tris-Glycine SDS sample buffer and subjected to SDS-PAGE gel containing 0.1% gelatin. Further, gels were incubated in 2.5% Triton X-100 with gentle agitation for 30 minutes at room temperature and then incubated in Developing Buffer (50 mM TrisHCl, 5 mM CaCl2, 200 mM NaCl and 0.02% Brij35) at 37°C for 8 h. Finally, the gels were stained with Coomassie Blue R-250 for 30 minutes and destained with a Coomassie R-250 destaining solution (50% Methanol, 10% Acetic acid and 40% H2O).
Western blotting
Cells were collected and lysed in ice-cold RIPA lysis buffer (Beyotime, Wuhan, China) containing 1 mM sodium orthovanadate, 0.5 mM PMSF, 10 mg/ml aprotinin and 10 mg/ml leupeptin. Cell lysate was centrifuged at 10,000 g for 20 min at 4°C, and the protein concentration was determined by BCA assay. Total protein (50 μg) was separated by 10% SDS-PAGE gel and then was transferred to PVDF membranes. The membranes were blocked with 5% BSA diluted in TBST for 1 h and then were incubated with primary antibodies against IL-22R1 (1:500), AKT (1:1000), phospho-AKT (1:1000) or β-actin (1:1000) at 4°C overnight. Further, the membranes were washed for three times with TBST and incubated with the secondary antibodies for 1 h at room temperature. Next, the membranes were developed using Enhanced Chemiluminescence kit (Applygen Technologies Inc, Beijing, China) and exposed to X-ray film. Finally, the protein levels were assessed by densitometry with the software of Quantity One.
Data analysis
All experimental data were obtained from three independent experiments and analyzed with the software of SPSS. The data were presented as value ± SEM. Differences between two groups were assessed by Student’s t test, while differences between multiple groups were determined by one-way ANOVA. A P value less than 0.05 was considered statistically significant.
Results
IL-22 promotes the migration and invasion of gastric cancer cells
To examine the responsiveness of gastric cancer cells to IL-22, human gastric cancer SGC-7901 cells were pretreated with different dosages of IL-22 (0, 10, 50, 100 ng/ml), and then migration assay and invasion assay were carried out. The results showed that after IL-22 treatment, the migration and invasion abilities of SGC-7901 cells were increased in a concentration-dependent manner (*P < 0.05), suggesting that IL-22 can stimulate the migration and invasion of gastric cancer cells (Figure 1A and 1B).
Figure 1.
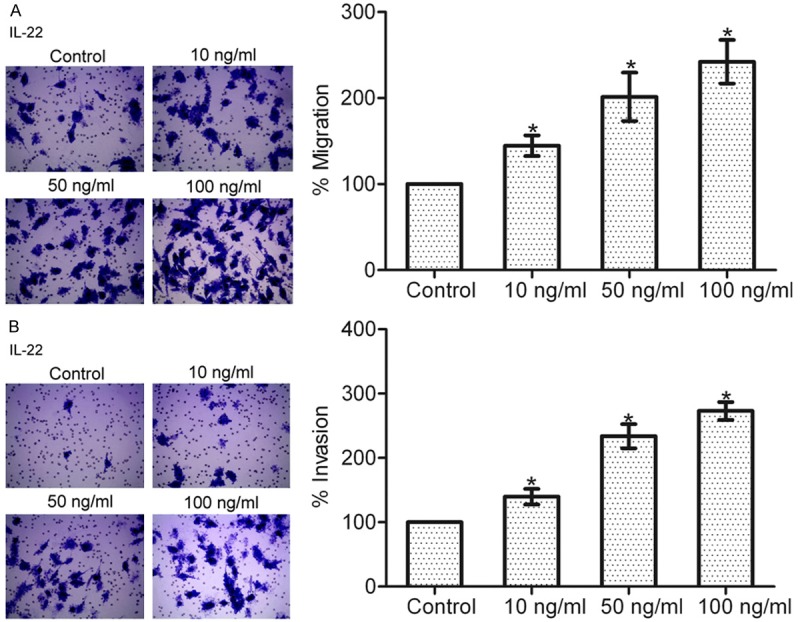
IL-22 dose-dependently increased gastric cancer cell migration and invasion. A: Effect of IL-22 stimulation on the migration of SGC-7901 cells. B: Effect of IL-22 stimulation on the invasion of SGC-7901 cells. Data are presented as the mean percentage ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
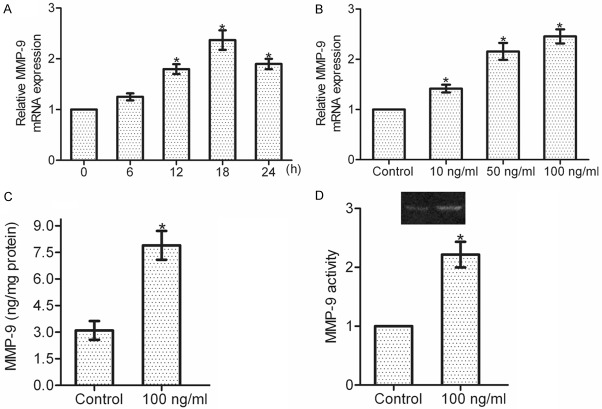
IL-22 upregulates MMP-9 expression and activity in gastric cancer cells
MMP-9 plays a crucial role in the dissemination of gastric cancer [12]. We here investigated whether IL-22 stimulation affected the expression and activity of MMP-9 in gastric cancer cells. Real-time PCR analysis showed that IL-22 upregulated the mRNA expression of MMP-9 in a time- and dose-dependent manner (*P < 0.05), and MMP-9 mRNA level reached the peak after 100 ng/ml IL-22 treatment for 18 h (Figure 2A and 2B). Further, ELISA assay showed that 18-h incubation with IL-22 (100 ng/ml) greatly increased MMP-9 secretion in SGC-7901 cells (*P < 0.05) (Figure 2C). Consistently, gelatin zymography assay proved that MMP-9 activity was also significantly enhanced after 18-h incubation with IL-22 (100 ng/ml) (*P < 0.05) (Figure 2D). Collectively, these data indicate that IL-22 is involved in the regulation of MMP-9 expression and activity in gastric cancer cells.
Figure 2.
MMP-9 production is upregulated by IL-22 stimulation. A: SGC-7901 cells were treated with or without IL-22 (100 ng/ml), and the mRNA expression of MMP-9 was detected within 24 h. B: SGC-7901 cells were incubated with different dosages of IL-22 (0, 10, 50, 100 ng/ml) for 18 h. Total RNA was extracted for real-time PCR analysis to detect the mRNA expression of MMP-9. C: Cell supernatant was collected for ELISA assay to observe the protein level of MMP-9. D: Cell supernatant was subjected to gelatin zymography assay to assess the activity of MMP-9. Data are presented as the mean ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
PI3K/AKT pathway is activated by IL-22 in gastric cancer cells
Multiple intracellular signaling pathways can be activated by IL-22 in cancer cells [13]. Here, we found that IL-22 stimulation resulted in a markedly increase activation of AKT in SGC-7901 cells. The IL-22-induced AKT activation was time- and dose-dependent (*P < 0.05), with peak activation was observed at 100 ng/ml in 10 min, indicating that IL-22 may activate PI3K/AKT pathway in gastric cancer cells (Figure 3A and 3B).
Figure 3.
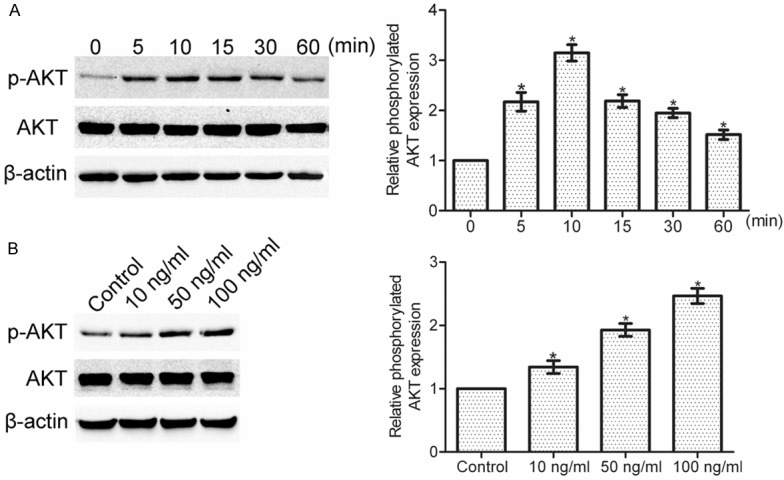
IL-22 stimulation time- and dose-dependently induced the activation of AKT. A: SGC-7901 cells were treated with or without IL-22 (100 ng/ml), and then the expression of phosphorylated AKT was observed by western blotting within 60 min. B: SGC-7901 cells were incubated with different dosages of IL-22 (0, 10, 50, 100 ng/ml) for 10 min, and then the expression of phosphorylated AKT was determined by Western blotting. Data are presented as the mean ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
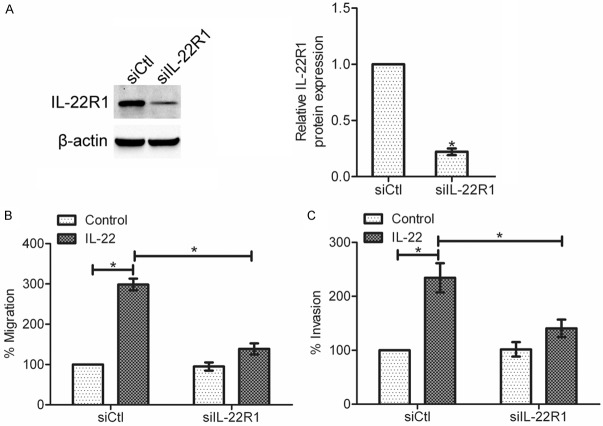
IL-22 effects on gastric cancer cells are mediated by IL-22R1
To determine whether IL-22R1 is required for gastric cancer cell migration and invasion stimulated by IL-22, an IL-22R1 siRNA was generated to silence IL-22R1 expression in SGC-7901 cells. Western blot analysis showed that up to 80% knockdown efficiency was achieved (*P < 0.05) (Figure 4A). Next, migration assay and invasion assay were performed with control siRNA cells and IL-22R1 siRNA cells. The results showed that the IL-22-enhanced migration and invasion was suppressed in IL-22R1 siRNA cells as compared to control siRNA cells (*P < 0.05) (Figure 4B and 4C). These data suggest that IL-22R1 is the major player in the regulation of the IL-22-induced gastric cancer cell migration and invasion.
Figure 4.
Knockdown of IL-22R1 suppressed gastric cancer cell migration and invasion that induced by IL-22. A: The expression of IL-22R1 was determined by western blotting after transfection of IL-22R1 siRNA. Data are presented as the mean ± SEM in relation to that of control group cells (n = 3). *P < 0.05. B: Effect of IL-22R1 knockdown on the IL-22-induced migration of SGC-7901 cells. C: Effect of IL-22R1 knockdown on the IL-22-induced invasion of SGC-7901 cells. Data are presented as the mean percentage ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
IL-22 regulates AKT activation and MMP-9 production via IL-22R1
To examine the effect of IL-22R1 knockdown on the IL-22-induced AKT activation, we transfected SGC-7901 cells with IL-22R1 siRNA or control siRNA, and incubated these cells with 100 ng/ml IL-22 for 10 min. As shown in Figure 5A, IL-22 increased the expression of phorsphorylated AKT in control siRNA cells (*P < 0.05). However, after knockdown of IL-22R1, the activation of AKT induced by IL-22 was significantly inhibited. These data indicate that IL-22 induces AKT activation through IL-22R1. To analyze the effect of IL-22R1 knockdown on the IL-22-mediated MMP-9 production in gastric cancer cells, SGC-7901 cells were transfected with IL-22R1 siRNA or control siRNA, and then were incubated with IL-22 (100 ng/ml) for 18 h. Real-time PCR and ELISA assay showed that knockdown of IL-22R1 attenuated the IL-22-mediated expression and secretion of MMP-9 (*P < 0.05), suggesting that IL-22R1 contributes to the IL-22-induced increase of MMP-9 production (Figure 5B and 5C).
Figure 5.
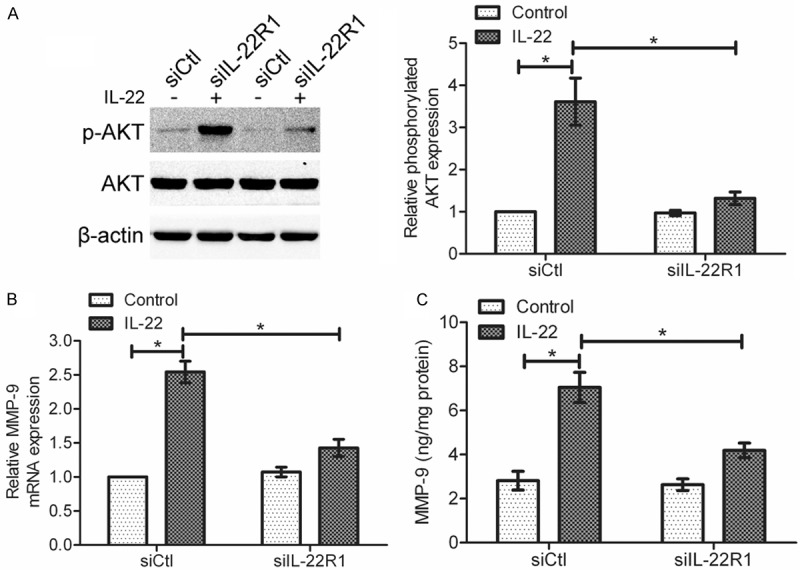
Knockdown of IL-22R1 modulated the IL-22-mediated AKT activation and MMP-9 production in gastric cancer cells. A: After transfection of IL-22R1 siRNA, cells were incubated with IL-22 (100 ng/ml) for 10 min. The difference in phosphorylated AKT expression between control siRNA cells and IL-22R1 siRNA cells was examined by Western blotting. B and C: After transfection of IL-22R1 siRNA, cells were incubated with IL-22 (100 ng/ml) for 18 h. B: MMP-9 mRNA expression was determined by real-time PCR. C: MMP-9 protein level in cell supernatant was determined by ELISA assay. Data are presented as the mean ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
Effect of AKT activation on the IL-22-mediated MMP-9 upregulation
In order to determine whether AKT activation is necessary for the IL-22-induced MMP-9 production, we pretreated SGC-7901 cells with 10 μM LY294002 (PI3K inhibitor) before IL-22 stimulation. Using real-time PCR and ELISA assay, we found that LY294002 reduced the IL-22-mediated expression and secretion of MMP-9 in SGC-7901 cells (*P < 0.05), suggesting the involvement of PI3K/AKT pathway in the IL-22-mediated MMP-9 upregulation (Figure 6A and 6B).
Figure 6.
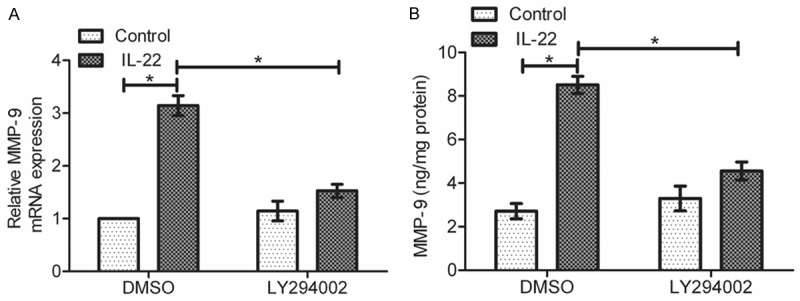
Effect of AKT activation on regulation of MMP-9 production. Cells were pretreated with LY294002 for 30 min, and then were incubated with or without IL-22 (100 ng/ml) for 18 h. A: Real-time PCR was performed to detect the mRNA expression of MMP-9. B: ELISA assay was performed to test the protein level of MMP-9 in cell supernatant. Data are presented as the mean ± SEM in relation to that of control group cells (n = 3). *P < 0.05.
Discussion
As one important member of IL-10 cytokine family, IL-22 is often upregulated in human cancers [14]. Clinical studies show that increased IL-22 expression correlates with tumor growth and metastasis of colon cancer [9]. Experimental data reveal that IL-22 stimulation promotes cell proliferation of lung cancer cells and mantle cell lymphoma cells in vitro [15,16]. In addition, IL-22 enhances the migration of intestinal epithelial cells and keratinocytes [17,18], and contributes to the metastasis of human hepatocellular carcinoma cells [19]. However, the role of IL-22 in gastric cancer, to our knowledge, is not elucidated. Here, we found that IL-22 could promote the migration and invasion of gastric cancer cells via IL-22R1/AKT/MMP-9 signaling, implying that IL-22 may be a potent stimulator of gastric cancer cell migration and invasion.
IL22R is a heterodimeric complex that is consisted of IL22R1 and IL10R2 subunits [20]. IL-22 initially binds to the IL22R1 subunit, leading to a formation of IL22–IL22R1 complex, which in turn the IL-22–IL-22R1 complex binds to IL-10R2 to activate multiply intracellular signals [21]. Published studies show that IL-22R expression is closely related with the invasion and metastasis of pancreatic ductal adenocarcinoma [22]. Some reports have suggested a positive role of IL-22R1 in the IL-22-mediated growth and tumorigenicity [16,23]. We here found that knockdown of IL-22R1 attenuated the IL-22-mediated migration and invasion of gastric cancer cells. Thus, our findings indicate that IL-22R1 is required for the IL-22-promoted migration and invasion of gastric cancer cells.
Several important signaling pathways can be regulated downstream of IL-22R1 activation. For instance, IL-22 can activate the JAK/STAT signaling in human hepatocellular carcinoma cells [19], and induce the activation of ERK1/2, p38 and JNK in H4IIE cells (rat hepatoma cells) [13]. Besides, IL-22 can stimulate PI3K/AKT pathway in epidermal keratinocytes [24] and endometrial stromal cells [25]. However, little is known regarding the effect of IL-22 on AKT activation in gastric cancer cells. Our study determined that IL-22 could time- and dose-dependently induce the activation of AKT, and the IL-22-induced AKT activation was mediated, at least partially, by IL-22R1.
IL-22 can induce the secretion of many cytokines in cancer cells, such as VEGF, IL-10 and TGF-β1 [26]. In addition, IL-22 stimulation increases the expression of MMP-1 in synovial fibroblasts [27]. Matrix metalloproteinases (MMPs) are a family of zinc-dependent enzymes that are essential for the progression of cancers [28]. Recent work reveals that IL-22 increases the expression of MMP-9 in pancreatic cancer cells [22]. Similarly, we showed that IL-22 upregulated MMP-9 production in a time- and dose-dependent manner in SGC-7901 cells. As one prominent member of MMPs, MMP-9 is responsible for the degradation of extracellular matrix and plays a pivotal role in tumor invasion and metastasis [29]. In our study, we found that IL-22 induced MMP-9 production via activation of IL-22R1 and AKT, further confirming the involvement of IL-22R1/AKT signaling in the IL-22-mediated migration and invasion of gastric cancer cells.
In summary, our study demonstrated that IL-22, acting via IL-22R1, induced AKT activation and MMP-9 production, consequently enhancing gastric cancer cell migration and invasion. Further investigations are still needed to determine the functions of IL-22 and IL-22R1 in the metastasis of gastric cancer cells in vivo.
Acknowledgements
This work was supported in part by the Natural Science Foundation (No. 2011A310005), the Science and Technique Foundation (No. 112102310206) and a University Key Teacher grant from the Ministry of Education (No. 2012GGJS-136) of Henan province.
Disclosure of conflict of interest
None.
References
- 1.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng L, Wang L, Ajani J, Xie K. Molecular basis of gastric cancer development and progression. Gastric Cancer. 2004;7:61–77. doi: 10.1007/s10120-004-0277-4. [DOI] [PubMed] [Google Scholar]
- 3.Chung HW, Lim JB. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J Gastroenterol. 2014;20:1667–1680. doi: 10.3748/wjg.v20.i7.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 7.Petanidis S, Anestakis D, Argyraki M, Hadzopoulou-Cladaras M, Salifoglou A. Differential expression of IL-17, 22 and 23 in the progression of colorectal cancer in patients with K-ras mutation: Ras signal inhibition and crosstalk with GM-CSF and IFN-gamma. PLoS One. 2013;8:e73616. doi: 10.1371/journal.pone.0073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B, Wang H. Increased Intratumoral Interleukin 22 Levels and Frequencies of Interleukin 22-Producing CD4+ T Cells Correlate With Pancreatic Cancer Progression. Pancreas. 2014;43:470–477. doi: 10.1097/MPA.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 9.Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, Sun B. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. doi: 10.1186/1471-2407-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Kim G, Kim JY, Yun HJ, Lim SC, Choi HS. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis. 2014;35:1352–61. doi: 10.1093/carcin/bgu044. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, Tian Z. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–6439. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 12.Zhao ZS, Zhou JL, Yao GY, Ru GQ, Ma J, Ruan J. Correlative studies on bFGF mRNA and MMP-9 mRNA expressions with microvascular density, progression, and prognosis of gastric carcinomas. World J Gastroenterol. 2005;11:3227–3233. doi: 10.3748/wjg.v11.i21.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 14.Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY, Sun C, Shi RH. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol. 2013;19:2638–2649. doi: 10.3748/wjg.v19.i17.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelebart P, Zak Z, Dien-Bard J, Anand M, Lai R. Interleukin 22 signaling promotes cell growth in mantle cell lymphoma. Transl Oncol. 2011;4:9–19. doi: 10.1593/tlo.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobold S, Volk S, Clauditz T, Kupper NJ, Minner S, Tufman A, Duwell P, Lindner M, Koch I, Heidegger S, Rothenfuer S, Schnurr M, Huber RM, Wilczak W, Endres S. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J Thorac Oncol. 2013;8:1032–1042. doi: 10.1097/JTO.0b013e31829923c8. [DOI] [PubMed] [Google Scholar]
- 17.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Li Z, Pan W, Qin L, Zhu G, Ke Y, Wu J, Bo P, Meng S. Participation of Gab1 and Gab2 in IL-22-mediated keratinocyte proliferation, migration, and differentiation. Mol Cell Biochem. 2012;369:255–266. doi: 10.1007/s11010-012-1389-5. [DOI] [PubMed] [Google Scholar]
- 19.Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 20.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 21.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 22.Wen Z, Liao Q, Zhao J, Hu Y, You L, Lu Z, Jia C, Wei Y, Zhao Y. High expression of interleukin-22 and its receptor predicts poor prognosis in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21:125–132. doi: 10.1245/s10434-013-3322-x. [DOI] [PubMed] [Google Scholar]
- 23.Bard JD, Gelebart P, Anand M, Amin HM, Lai R. Aberrant expression of IL-22 receptor 1 and autocrine IL-22 stimulation contribute to tumorigenicity in ALK+ anaplastic large cell lymphoma. Leukemia. 2008;22:1595–1603. doi: 10.1038/leu.2008.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Chen Y, Liu LB, Chang KK, Li H, Li MQ, Shao J. IL-22 in the endometriotic milieu promotes the proliferation of endometrial stromal cells via stimulating the secretion of CCL2 and IL-8. Int J Clin Exp Pathol. 2013;6:2011–2020. [PMC free article] [PubMed] [Google Scholar]
- 26.Curd LM, Favors SE, Gregg RK. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol. 2012;168:192–199. doi: 10.1111/j.1365-2249.2012.04570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrion M, Juarranz Y, Seoane IV, Martinez C, Gonzalez-Alvaro I, Pablos JL, Gutierrez-Canas I, Gomariz RP. VIP modulates IL-22R1 expression and prevents the contribution of rheumatoid synovial fibroblasts to IL-22-mediated joint destruction. J Mol Neurosci. 2014;52:10–17. doi: 10.1007/s12031-013-0177-3. [DOI] [PubMed] [Google Scholar]
- 28.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 29.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]