Abstract
Monoacylglycerol lipase (MAGL) is a serine hydrolase that hydrolyzes monoacylglycerides into free fatty acids and glycerol. It has recently been found to be involved in cancer progression through the free fatty acid or endocannabinoid network after studies on its function in the endocannabinoid system. Here, we determined a role for MAGL in nasopharyngeal carcinoma (NPC), which is known for its high metastatic potential. Among the different NPC cells we tested, MAGL was highly expressed in high metastatic NPC cells, whereas low metastatic potential NPC cells exhibited lower expression of MAGL. Overexpression of MAGL in low metastatic NPC cells enhanced their motile behavior and metastatic capacity in vivo. Conversely, knockdown of MAGL reduced the motility of highly metastatic cells, reducing their metastatic capacity in vivo. Growth rate was not influenced by MAGL in either high or low metastatic cells. MAGL expression was associated with the epithelial-mesenchymal transition (EMT) proteins, such as E-cadherin, vimentin and Snail. It was also related to the sidepopulation (SP) of NPC cells. Our findings establish that MAGL promotes metastases in NPC through EMT, and it may serve as a target for the prevention of NPC metastases.
Keywords: MAGL, metastases, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a disease with remarkable racial and geographic distribution. It has a high incidence in southern China and southeast Asia, with an incidence rate ranging from 25 to 50/100,000; however, it is rarely found in most of the rest of the world [1-3]. However, the molecular basis of NPC is far from being fully understood. Previous studies revealed that genetic susceptibility, Epstein-Barr virus (EBV) infection and environmental factors play roles in the development of NPC [4,5]. Moreover, NPC has the highest metastasis rate among the head and neck cancers [6-8]. Patients with metastases to regional lymph nodes (LN) or even distant organs exhibited poor prognosis, with disease relapse rates as high as 82% [9].
Cancer cells differ from normal cells, and deregulation of metabolism is a hallmark of cancer [10]. The transformation from a noncancerous to a cancerous state is associated with alterations in metabolic pathways, which include aerobic glycolysis (“Weinberg effect”) [11-13], glutamine-dependent anaplerosis [14,15], and de novo lipid biosynthesis [16,17].
A well-studied lipid metabolism gene is fatty acid synthase (FASN). It is a key lipogenic enzyme in the synthesis of long-chain fatty acids from acetyl-coenzyme A (CoA) and malonyl CoA and catalyzes one of the terminal steps in the de novo biogenesis of fatty acids [18-21]. Its high expression is observed in a wide variety of human cancers . Increased FASN has been linked to short-term survival in colorectal, ovarian and melanoma cancers [22-24], and the inhibition of FASN resulted in decreased cell proliferation, loss of cell viability, and decreased tumor growth in vivo [20,25-27]. Its selective expression in cancer cells makes it a good target for cancer therapy. The development of several FASN inhibitors both by academic labs and in industry has been reported; these inhibitors include Cerulenin, C75, orlistat, C93, C247, and GSK837149A [28].
High FASN expression in NPC correlates with advanced stage and shorter survival [29]. Previous studies have reported that the concentration of some lipids and unsaturated fatty acids were increased in the serum of NPC patients. Recently, a familial study from a research group at Sun-Yat Sen University Cancer Center indicated that NPC patients exhibit a high concentration of high density lipoprotein (HDL) compared with normal controls. These studies indicate that fatty acid metabolism may also play an important role in NPC pathogenesis.
Monoacylglycerol lipase (MAGL) is a serine hydrolase that hydrolyzes monoacylglycerides into free fatty acids and glycerol. MAGL studies over the last few decades have focused on its participation in the breakdown of adipose triacylglyceride (TCA) stores and its role in the endogenous cannabinoid system [30-32]. Endocannabinoids are lipid molecules that modulate a diverse set of physiological processes such as pain, cognition, appetite, and emotional states, and their levels and functions are tightly regulated by enzymatic biosynthesis and degradation [33]. 2-Arachidonoylglycerol (2-AG) is the most abundant endocannabinoid in the brain and is believed to be hydrolyzed primarily by the serine hydrolase monoacylglycerol lipase (MAGL). In 2011, Nomura’s group found that MAGL plays a protumorigenic role in prostate cancer [34]. Later, MAGL’s function in colon cancer was studied.
Therefore we sought to determine how MAGL is involved in NPC carcinogenesis and development. Does it play an important role in NPC as well as in prostate cancer?
Materials and methods
Cell culture
The human NPC cell line CNE-2 and its clones (S18 and S26, cultured in less than 50 passages) and SUNE-1 and its clone 5-8F were kindly provided by Dr. Chao-Nan Qian, Sun Yat-Sen University Cancer Center (SYSUCC), China. The other NPC cell lines, NP69 and CNE1, were obtained from SYSUCC. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) at 37°C in 5% CO2.
Human tissues
All of the human tissue samples were obtained from the Department of Pathology, Sun Yat-Sen University Cancer Center (SYSUCC) with prior patient consent and the approval of the Institutional Clinical Ethics Review Board at SYSUCC.
MTT assay
The MTT assay was performed as previously described [35]. Briefly, cells were seeded in 96-well plates at a density of 3000 cells per well. At the same time on Days 1, 2, 3, 4 and 5, 20 μl MTT (5 mg/ml) solution was added to each well, followed by the addition of 150 μl DMSO. Cell viability was measured with a microtiter plate reader (Bio-Tek, VT, USA).
Transwell assay
Migration and invasion assays were performed as previously described [37]. Migration assays were performed in transwell chambers (8 μm pore size; Costar). Invasion assays were conducted using the BD Matrigel Invasion Chambers. The numbers of migrated and invaded cells in five random optical fields (200 × magnification) from triplicate filters were averaged.
Quantitative PCR
Total RNA was extracted from malignant and non-malignant nasopharyngeal tissues or from NPC cells using Qiagen RNeasy mini-columns according to the manufacturer’s instructions. RNA was reverse transcribed using random hexamers (Invitrogen) and reverse transcriptase (Superscript II, Invitrogen). Real-time PCR was performed using TaqMan gene expression pre-synthesized reagents and master mix (Applied Biosystems, Warrington, UK). Reactions were prepared following the manufacturer’s instructions. All reactions were performed in triplicate on 96-well PCR plates (ABIPRISM, Applied Biosystems) using an ABI PRISM 7000 sequence detection system (Applied Biosystems). Standard thermal cycling conditions included a hot start of 5 min at 50°C and 10 min at 95°C followed by up to 40 cycles of the following: 95°C for 15 seconds, 60°C for 1 min. The sequences of PCR primers used for amplification of MAGL and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: MAGL forward, 5’-GTGACAGCCGCAACCCT-3’; MAGL reverse, 5’-GAGCCACAGTGACATAGCAAAC-3’; GAPDH forward, 5’-TCACTGCCACCCAGAAGA-3’; and GAPDH reverse, 5’-TACCAGGAAATGAGCTTGAC-3’. The experiments were performed in triplicate.
Overexpression studies in human cancer cell lines
Stable expression of MAGL was achieved by retroviral infection. CNE-2 and SUNE-1 cells were transduced for 4 h with supernatants obtained from 293T cells that were previously transfected with a retroviral vector carrying flag-tagged MAGL or the corresponding empty construction (pBABE). Infected cells were selected with puromycin.
Knockdown studies in human cancer cell lines
Mission Sh RNA (Cat. SHCLND-NM_007283)were purchased from Sigma-Aldrich, and cell lines stably expressing either MAGL short hairpin RNA (shRNA) or a scrambled nontarget shRNA were established according to the manufacturer’s instructions. The targets of human MAGL shRNAs are 5’-CCGGCAACTCCTTCCATGAAATCTCGAGATTTCATGGAAGACGGAGTTGTTTTTG-3’ and 5’-CCGGCCAATCCTGAATCTGCAACAACTCGAGTTGTTGCAGATTCAGGATTGTTTTTG-3’.
Side population (SP) assay by flow cytometry
SP assay was performed as previously described [36]. The cells were digested with trypsin (Sigma-Aldrich, St. Louis, MO), and resuspended at a concentration of 1 × 106 cells/ml. The DNA binding dye, Hoechst 33342, was then added to a final concentration of 5 ug/ml, and the samples were incubated for 90 min in the dark with periodic mixing. After washing twice with PBS, the cells were kept at 4°C in the dark before fl ow cytometry (Mofl o XDP, Beckman Coulter) using dual-wavelength analysis. On the other hand, a subset of the cells was incubated with 10 M fumitremorgin C (FTC, a specifi c inhibitor ABCG2) for 5 min at 37°C prior to adding Hoechst 33342 to determine whether this would block the fl uorescent effl ux of SP cells.
Western blotting
Western blotting was conducted using standard procedures. Briefly, cells were lysed with RIPA lysis buffer (100 mM Tris-HCl, 300 mM NaCl, 2% NP40, 0.5% sodium deoxycholate) supplemented with proteinase inhibitors, and the concentration of total protein in lysate was measured using the Bradford method. An equal amount of protein in each sample was mixed with loading buffer and subjected to SDS-PAGE separation followed by blotting onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes probed with specific primary antibodies were washed in PBS with Tween-20 (PBST) for three times and then probed with secondary peroxidase conjugated antibodies (1:5000). Protein bands were detected on X-ray film using a chemiluminescence method.
Antibodies and reagents
An MAGL polyclonal antibody (Cat. 011994) was purchased from Sigma-Aldrich. E-cadherin (Cat. 610181) and vimentin (Cat. 550513) antibodies were obtained from BD Biosciences (Franklin Lakes, NJ). Snail (Cat. 3879) was purchased from Cell Signaling (Beverly, MA). Antibodies against MMP2 (Cat. 104577) and GAPDH (Cat. 48166) were obtained from GeneTex (Irvine, CA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Animals and spontaneous lymph node metastasis assay
Female nude mice aged 5 and 6 weeks were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). All of the animal studies were conducted in accordance with the principles and procedures outlined in the guidelines of Institutional Animal Care and Use Committee at Sun Yat-Sen University. The spontaneous LN metastasis model was performed as previously described [37]. Briefly, a total of 1 × 105 cells were injected into the left hind footpad of each mouse to generate a primary tumor. After one and a half months, the popliteal LN of the left hind foot was collected on the last day for routine tissue processing.
Statistical analysis
The data were presented as the means ± standard deviation (SD) and were analyzed with Student’s t-test or χ2 test. All statistical tests were two-tailed. The results were considered statistically significant when the p values were less than 0.05.
Results
MAGL expression is elevated in NPC cells with high metastasis potential
First, we examined the expression of the MAGL in different NPC cell lines. Quantitative PCR results (Figure 1A) showed a particularly low mRNA expression of MAGL in NP 69, an immortalized nasopharyngeal epithelial cell line. S18 originated from the CNE-2 cell, but S18 had a higher metastatic ability. Interestingly, S18, with a relatively high metastatic potential, showed a relatively high expression of MAGL. CNE-2, the origin cell line of S18, with a relative low metastatic ability, exhibited low expression of MAGL. The same results were obtained with the SUNE-1 and 5-8F groups. 5-8F was a single clone from SUNE-1, and 5-8F exhibited a higher metastatic ability than SUNE-1; correspondingly, the expression of MAGL in 5-8F was higher than that in SUNE-1 cells.
Figure 1.

MAGL expression in different NPC cells and tissues. A. The mRNA level of MAGL (normalized to GAPDH) in the 6 NPC cell lines were measured by quantitative real–time PCR, showing that the expression of MAGL was significantly higher in high metastatic potential cells, S18, than in its parental low metastasis cell line, CNE-2. Moreover, high levels of mRNA were also observed in another high-metastatic clone, 5-8F, in contrast to its parental low metastatic NPC cell line SUNE-1. NP 69, an immortalized nasopharyngeal epithelial cell line exhibited a relatively low expression of MAGL. Column, mean; error bar, ± SD (from triplicates). B. The MAGL protein levels in these cell lines were confirmed by western blotting. C. The mRNA level of MAGL (normalized to GAPDH) in 10 NPC patients was significant higher than that in 10 non-cancer patients with rhinitis (***, p < 0.001).
The western blot results confirmed the expression levels in the different NPC cell lines (Figure 1B); high potential metastatic cells exhibited a higher expression of MAGL. In addition, we obtained nasopharyngeal samples from the NPC patients as well as patients with rhinitis (Figure 1C). The cancer patients exhibited higher expression of MAGL, whereas the non-cancer patients exhibited a relatively low expression of MAGL at the mRNA level. These interesting findings led us to determine whether MAGL plays a protumorigenic role in NPC development or in NPC metastases.
Overexpression of MAGL promotes the motility of low metastatic NPC cells
We selected the two relatively low metastatic cells, CNE-2 and SUNE-1, for further study. Stable overexpression of MAGL in CNE-2 and SUNE-1 cells was achieved (Figure 2A).
Figure 2.
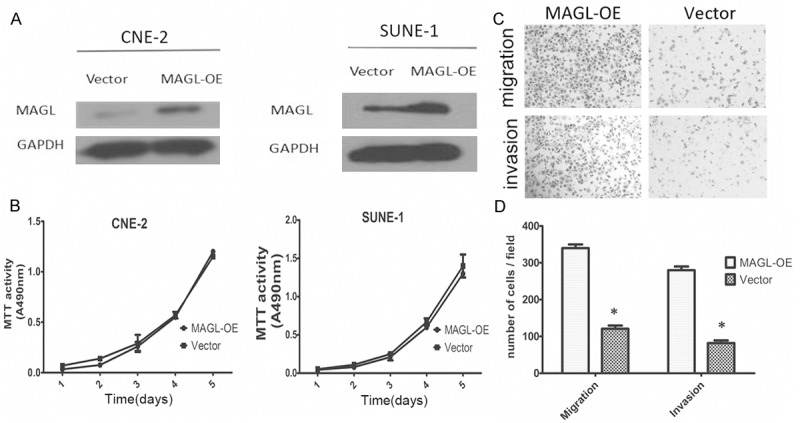
Overexpression of MAGL enhanced the metastasis ability in NPC cells. A. The stable overexpression of MAGL was achieved in CNE-2 cells and SUNE-1 cells. B. Overexpression of MAGL did not affect the growth rate of both CNE-2 and SUNE-1 cells. C. Transwell assay with and without Matrigel was used to evaluate the migration and invasion abilities, respectively. MAGL-OE cells displayed more migration or invasion through the chamber. D. Numbers of cells obtained through the chamber per field. Column, mean; bars, ± SD (from triplicates). *, P < 0.01 with Student’s t test.
The MTT assay showed there was not a significant difference between the MAGL overexpressing cells and the vector cells (Figure 2B). Using the transwell assay, we compared the metastatic ability of both the MAGL overexpressing cells and the vector cells, as shown in the Figure 2C; the overexpressing cells showed greater metastatic ability than the control cells (Figure 2D).
Knockdown of MAGL suppresses the migration and invasion of NPC cells without influencing growth rate
To examine the causal role of MAGL in the motility of NPC cells, we engineered cell lines from S18 and 5-8F that stably expressed either shRNAs targeting MAGL expression (SH MAGL1 and SH MAGL2) or a scrambled nontarget shRNA (NC). The suppression of MAGL expression at the mRNA level was confirmed by quantitative real-time PCR (Figure 3A). Immunoblotting confirmed the decreased MAGL expression in SH 1 and SH 2 cells (Figure 3B).
Figure 3.

Suppression of MAGL inhibits the migration and invasion abilities of S18 cells and 5-8F cells. S18 and 5-8F cells were transduced with lentiviruses expressing scrambled shRNA (NC) or shRNAs targeting MAGL (SH 1 and SH 2). A. MAGL mRNA levels (normalized to GAPDH) determined by quantitative real-time PCR in the MAGL knockdown cells were significantly reduced. B. MAGL protein levels were determined by immunoblotting. Expression of MAGL in SH 1 and SH 2 cells were decreased both in S18 and 5-8F cells. C, D. Transwell assay was used for further evaluation of the invasion/migration ability of the S18 cells. Suppression of MAGL significantly reduced the migration and invasion abilities of the cells. Column, mean; bars, ± SD (from triplicates). Student’s t test was used for the statistical analyses. **, P < 0.001 relative to that of scrambled shRNA (S18-NC); #, P < 0.05 for SH 1 and SH 2 when compared with that of scrambled shRNA (5-8F NC).
Consistent with the overexpression group, sh MAGL S18 and 5-8F cells had a weaker metastatic ability than the scramble groups, which could influence the migration ability and invasion ability of the cells (Figure 3C and 3D).
Expression of MAGL mediates the metastasis rate of NPC in vivo
After confirming the role of MAGL role in vivo, we next examined its functionin vitro. To determine whether it can mediate the metastasis rate of NPC in vivo, we used a lymph node metastasis model. Cancer cells were injected into the foot pad after one and a half months to induce the formation of carcinoma in situ; then, the mice were sacrificed, and the lymph nodes were removed. RNA was extracted, and the metastases were examined using quantitative PCR of the HPRT, which is a homo gene. As expected, the MAGL overexpression group showed an increased metastasis rate: 60% versus 16.67% in the CNE-2 cells (Figure 4A) and 63.33% versus 20% in the SUNE-1 cells (Figure 4B); MAGL knockdown produced a significant reduction in the metastasis rate from 76.67% to 40% in the S18 group and 73.33% to 40% in the 5-8F group (Figure 4C, 4D). These results confirm that MAGL is involved in the control of NPC metastasis in vivo.
Figure 4.
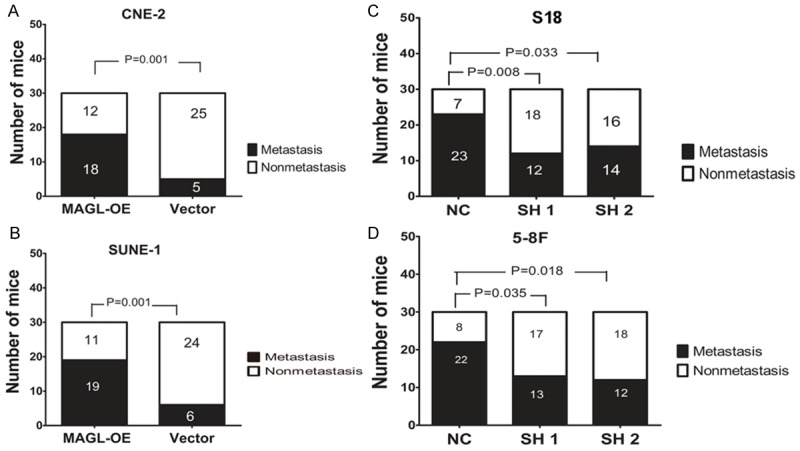
In vivo metastasis rates of MAGL-overexpressing cells and MAGL knockdown cells. For overexpressing cells, CNE-2-MAGL-OE and vector cells were subcutaneously injected into the left hind footpad of nude mice as well as SUNE-1-MAGL-OE and its vector cells, which led to metastases to the left popliteal LN in some animals. For the knockdown cells, S18 and 5-8F cells stably expressing MAGL shRNAs (SH 1 and SH 2) or scrambled shRNA (NC) were used. A. The proportion of popliteal LN metastasis after inoculation of CNE-2 cells. Overexpression of MAGL in CNE-2 cells significantly increased the metastasis rate from 16.67% (5 of 30) to 60% (18 of 30). B. The proportion of popliteal LN metastasis after inoculation of SUNE-1 cells. Overexpression of MAGL in SUNE-1 cells significantly increased the metastasis rate from 20% (6 of 30) to 63.33% (19 of 30). C. Knockdown of MAGL in S18 cells significantly reduced the metastasis rate from 76.67% (23 of 30) to 40% (12 of 30). D. The proportion of popliteal LN metastases after inoculation of 5-8F cells. Knockdown of MAGL in 5-8F cells significantly reduced the metastasis rate from 73.33% (22 of 30) to 40% (12 of 30). χ2 test was used.
MAGL expression is associated with the epithelial-mesenchymal transition
Metastatic ability was found to be related to the epithelial-to-mesenchymal transition, EMT. We sought to determine whether MAGL expression was related to EMT. Western blotting was conducted to investigate the EMT markers in the overexpression and knockdown of MAGL.
As the figure shows (Figure 5A, 5B), the overexpression of MAGL increased the expression of Snail and vimentin, but decreased the expression of E-cadherin.
Figure 5.
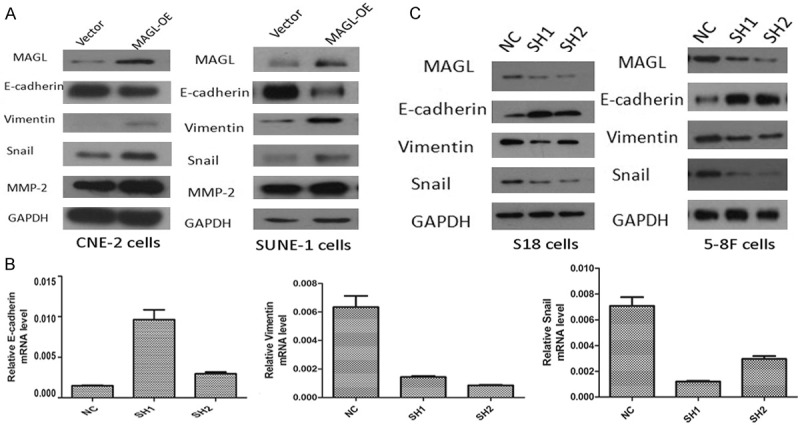
MAGL expression is associated with the epithelial-mesenchymal transition. A. Western blotting was performed to determine the expression of EMT markers of MAGL-OE cells. In CNE-2 cells, vimentin, Snail and MMP-2 were increased in MAGL-overexpression group, when E-cadherin expression was decreased (left). The same situation was found in SUNE-1 cells (right). B. The quantitative real-time PCR was conducted to check the mRNA level of several EMT markers. In SH MAGL cells, the mRNA of vimentin and Snail were decreased, as accompanied by the increased mRNA expression of E-cadherin. C. The protein levels of relative EMT markers were checked by western blotting. In SH 1 and SH 2 cells, with the decreased expression of MAGL, the expression of vimentin and Snail were also decreased, while the expression of E-cadherin obtained a higher level.
Degradation of the extracellular matrix is a crucial step in the metastatic process, especially during tumor cell intravasation and extravasation [38]. Matrixmetallo-proteinases (MMPs) have long been associated with this process due to their ability to degrade the components of the extracellular matrix [39]. We found that overexpression of MAGL increased the expression of MMP-2, which also supported the relationship between MAGL and EMT.
In sh MAGLS18 and 5-8F cells, the mRNA levels of vimentin and Snail were decreased along with an increase in the mRNA levels of E-cadherin (Figure 5C). These results showed that MAGL was relevant to the EMT.
MAGL expression is associated with the percentile of cancer stem cells
EMT was shown to be correlated with cancer stem cells. We determined the sidepopulation (SP) percentile of the overexpression of MAGL in CNE-2 and SUNE-1 cells, and as expected, the MAGL-OE cells had a high SP percentile compared to the control CNE-2 cells (Figure 6A). The same result was obtained for the SUNE-1 cells.
Figure 6.
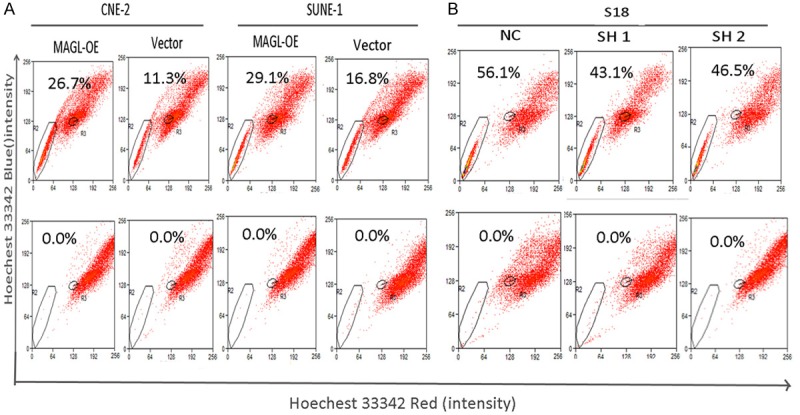
MAGL expression is associated with the percentile of cancer stem cells. A. The percentages of sidepopulation (SP) cells in CNE-2 and SUNE-1 cells analyzed by FACS. The percentage of SP cells was increased in MAGL-overexpression cells, 11.3% to 26.7% in CNE-2 cells and 16.8% to 29.1% in SUNE-1 cells. The bottom panels show samples in the presence of FTC. B. The percentages of sidepopulation (SP) cells in SH 1 (43.1%) and SH-2 cells (46.5%) were decreased when compared to the scrambled group NC (56.14%).
Accordingly, the shMAGL in the S18 and 5-8F cells had a lower percentile of the SP cells (Figure 6B). These results were consistent with the expression of EMT markers.
Discussion
Cancer cells undergo heighten lipogenesis to meet the dysregulated need for biosynthetic and bioenergetic requirements, which include membrane building blocks, signaling lipid molecules, posttranslational modifications of proteins as well as energy supply [40]. Dysregulated lipid metabolism enzymes have been implicated in cancer; these enzymes include FASN, ATP citrate lyase (ACL) and acetyl-CoA carboxylase (ACC).
Monoacylglycerol lipase (MAGL) was investigated in the current study. It catalyzed the conversion of monoacylglycerol (MAG) to glycerol, with the release of a free fatty acid molecule. Previous studies on MAGL were mainly focused on its role in endocannabinoid signaling in the nervous system and some peripheral tissues, where it can degrade the endocannabinoid receptors (CB1 and CB2), 2-arachidonoylglycerol (2-AG; C20:4 monoacylglycerol). Studies have shown that cannabinoids inhibit cancer cell proliferation, induce cancer cell apoptosis, and impair tumor angiogenesis [41-44]. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition.
In addition, the free fatty acid is another product of MAGL. Normura found that MAGL regulates a fatty acid network that feeds into a number of protumorigenic signaling pathways [34], which include LPA and PGE2, that have been shown to stimulate the motility of cancer cells [45].
This study is the first to show that MAGL is involved in NPC cancer cells. MAGL was highly expressed in high metastatic cells but lowly expressed in NPC cells with low metastatic potential. MAGL may promote NPC metastases both in vivo and in vitro. However, MAGL did not affect the growth rate of NPC cells, which is not the same as its effect in prostate cancer cells. Overexpression of MAGL increases vimentin expression as well as the stem cancer cell percentile but decreases the expression of E-cadherin.
In conclusion, our study showed that MAGL plays an important role in regulating NPC metastasis by enhancing cellular migration, cellular invasiveness, vimentin and Snail expression, and the in vivo spread of cancer cells. Those findings suggest that MAGL may be a potential target for preventing NPC metastases. The metabolic pathway of cancer is important, and the precise mechanism by which MAGL influences the metastases in NPC requires further study.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program; 2011CB504302 and 2012CB967002) and the National High Technology Research and Development Program of China (863 Program; 2012AA020307 and 2012AA020818).
Disclosure of conflict of interest
None.
References
- 1.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29:517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 2.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 3.Razak AR, Siu LL, Liu FF, Ito E, O’Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46:1967–1978. doi: 10.1016/j.ejca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 5.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 6.Geara FB, Sanguineti G, Tucker SL, Garden AS, Ang KK, Morrison WH, Peters LJ. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol. 1997;43:53–61. doi: 10.1016/s0167-8140(97)01914-2. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad A, Stefani S. Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J Surg Oncol. 1986;33:194–197. doi: 10.1002/jso.2930330310. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Law SC, Foo W, Poon YF, Cheung FK, Chan DK, Tung SY, Thaw M, Ho JH. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976-1985: survival after local recurrence. Int J Radiat Oncol Biol Phys. 1993;26:773–782. doi: 10.1016/0360-3016(93)90491-d. [DOI] [PubMed] [Google Scholar]
- 9.Guigay J. Advances in nasopharyngeal carcinoma. Curr Opin Oncol. 2008;20:264–269. doi: 10.1097/CCO.0b013e3282fad846. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 12.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacevic Z, McGivan JD. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983;63:547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- 15.De Berardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 17.Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta. 2010;1801:381–391. doi: 10.1016/j.bbalip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 19.Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay S, Pai SK, Watabe M, Gross SC, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Markwell SJ, Wang Y, Huggenvik J, Pauza ME, Iiizumi M, Watabe K. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24:5389–5395. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 21.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 22.Gansler TS, Hardman W 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 23.Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 24.Innocenzi D, Alo PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30:23–28. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- 25.Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun. 2001;285:217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- 26.Barger JF, Plas DR. Balancing biosynthesis and bioenergetics: metabolic programs in oncogenesis. Endocr Relat Cancer. 2010;17:R287–304. doi: 10.1677/ERC-10-0106. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 28.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011;223:283–294. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao YC, Lee SW, Lin LC, Chen LT, Hsing CH, Hsu HP, Huang HY, Shiue YL, Chen TJ, Li CF. Fatty acid synthase overexpression confers an independent prognosticator and associates with radiation resistance in nasopharyngeal carcinoma. Tumour Biol. 2013;34:759–768. doi: 10.1007/s13277-012-0605-y. [DOI] [PubMed] [Google Scholar]
- 30.Tornqvist H, Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem. 1976;251:813–819. [PubMed] [Google Scholar]
- 31.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, Piesla MJ, Zhang MY, Bingham B, Uveges A, Kowal D, Garbe D, Kouranova EV, Ring RH, Bates B, Pangalos MN, Kennedy JD, Whiteside GT, Samad TA. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- 34.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou CY, Wang J, Shen JN, Huang G, Jin S, Yin JQ, Guo QC, Li HM, Luo L, Zhang M, Zhang LJ. Establishment and characteristics of two syngeneic human osteosarcoma cell lines from primary tumor and skip metastases. Acta Pharmacol Sin. 2008;29:325–332. doi: 10.1111/j.1745-7254.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, Liu Q, Pei D, Kang T, Zeng YX. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, Chen J, Resau JH, Teh BT. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 38.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 39.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3:167–174. doi: 10.4331/wjbc.v3.i8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafsson SB, Palmqvist R, Henriksson ML, Dahlin AM, Edin S, Jacobsson SO, Oberg A, Fowler CJ. High tumour cannabinoid CB1 receptor immunoreactivity negatively impacts disease-specific survival in stage II microsatellite stable colorectal cancer. PLoS One. 2011;6:e23003. doi: 10.1371/journal.pone.0023003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blazquez C, Gonzalez-Feria L, Alvarez L, Haro A, Casanova ML, Guzman M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–5623. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- 43.Casanova ML, Blazquez C, Martinez-Palacio J, Villanueva C, Fernandez-Acenero MJ, Huffman JW, Jorcano JL, Guzman M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 45.Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, Kimura T, Kuwabara A, Yanagita Y, Ikeya T, Tanahashi Y, Ogawa T, Ohwada S, Morishita Y, Ohta H, Im DS, Tamoto K, Tomura H, Okajima F. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem. 2004;279:6595–6605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]


