Abstract
Objective: This study examined the mechanism of bone marrow mesenchymal stem cells (BMSCs) up-regulating the expression of 14-3-3 protein, blocking the myocardial apoptosis in diabetic cardiomyopathy and thereby improving cardiac function. Methods and Results: (1) Rat model of diabetic cardiomyopathy was made by feeding rats with high fat/high sugar diet and intraperitoneal injection of small dose of streptozocin (STZ). The model was successfully established as confirmed by the detection of blood sugar, lipid profile, ultrasonographic and hemodynamic examinations. (2) Bone marrow (BM) liquid was taken from the rat femur and tibia bones. The BMSCs were obtained by culture and were confirmed by phase-contrast microscopy and flow cytometry. The BMSCs were transplanted into the rats and fluorescent microscopy showed that transplantation was successful. (3) TUNNEL, Western blotting revealed that in rats of DCM group, myocardial apoptosis was more severe and expression of capase-3 was significantly up-regulated while in rats receiving transplantation of BMSCs showed opposite changes, with the differences being statistically significant (P < 0.05). (4) Western blotting exhibited that, compared with DCM group, 14-3-3 and p-Ask1 protein was significantly increased while Ask1 was obviously decreased. Conclusion: Our findings suggested that transplantation of bone marrow mesenchymal stem cells could inhibit the myocardial apoptosis in diabetic cardiomyopathy, possibly by up-regulating the expression of 14-3-3 protein and inhibiting the phosphorylation of Ask1.
Keywords: Diabetic cardiomyopathy, bone marrow mesenchymal stem cells, 14-3-3 protein, Ask1, caspase-3
Introduction
In 1974, Hamby et al [1], for the first time, proposed diabetic cardiomyopathy (DCM) as a separate disease entity. This condition is characterized by disordered microcirculation of cardiac muscles, apoptosis and necrosis of myocardial cells and progressive interstitial fibrosis, which eventually lead to aberrant perfusion of heart muscles [2]. The development and progression of DCM are believed to be associated with hyperglycemia, insulin resistance, abnormal metabolism of fatty acids, impaired autonomic nerve system of heart [3]. Clinically, the early signs of DCM included heart failure with normal ejection fraction (EF), which is possibly followed by impaired systolic function, as manifested by heart failure with reduced EF, and poor prognosis. In the pathogenesis of DCM, cardiac remodeling plays a critical role [4,5].
Bone marrow mesenchymal stem cells (BMSCs) are a kind of pluripotent stem cells and can differentiate into myocardial cells and vascular endothelial cells. In the process of the differentiation, the stems cells secrete vascular endothelial growth factor (VEGF) and factors that promote angiogenesis and inhibit apoptosis, thereby enhancing myocardial contractility, reducing hypertrophy of myocardial cells, inhibiting the synthesis of extracellualr matrix, mitigating matrix remodeling, promoting the formation of capillaries and proliferation of exiting vascular system and eventually inhibiting ventricular remodeling and improving cardiac functions [6].
Apoptosis is a programmed cell death, which is believed to be a gene-controlled autonomic death [7]. Hyperglycemia-induced apoptosis of myocardial cells is, at least, partially mediated by activating caspase pathway.
It has been well established that caspase plays an essential role in the apoptosis. Caspase family includes a series of proteolytic enzymes [8,9]. Caspase-3 is an important member of caspase family and is capapble of degrade cellular protein and serves as an executor of apoptosis. It is currently believed that caspase is the common pathway of all apoptosis signals. Caspase-3 takes the center stage, i.e., caspases-3 is activated by its upstream signals, then the activated caspase-3 further works on its substrates and triggers the caspase cascade, which and eventually leads to apoptosis [10,11].
Recently, it has been confirmed that 14-3-3 protein possesses the anti-apoptotic effect [12]. In 1967, Moore and Perez, for the first time, found a soluble acidic heterogeneous dimmer protein (molecular weight: 30 KD) and named it 14-3-3 on the basis of its positions during eletrophoresis. 14-3-3 proteins are a kind of highly conserved proteins and are involved in the regulation of cellular apoptosis, adhesion, proliferation and signal transduction [13,14]. Recent studies showed that 14-3-3 proteins have anti-apoptotic and anti-fibrotic effects on muscular muscles and can protect cardiac functions [15-17]. Thandavarayan et al [18], used dominant negative 14-3-3η mutant (DN14-3-3η) to induced experimental diabetics mellitus in transgenic mice (transgenic DN 14-3-3η mice) and found that, in transgenic DN14-3-3η mice, Ask1 activity was increased and cardiac function was evidently impaired, with myocardial cells suffering from apoptosis and cardiac muscles experiencing hypertrophy and fibrosis. Ask1 is an important signal-regulating protein when myocardial cells go through apoptosis during diabetes mellitus and belongs to the family of mitogen-activated protein kinases (MAPKs), which are located upstream of JNK and p38 in the MAPK pathway. After activation, Ask1 activates JNK and p38 to induce apoptosis. Current studies revealed that 14-3-3 protein is an important regulator of Ask1 activity. In a model of diabetics mellitus, 14-3-3 was believed to bind to phosphorylated serine-967 of ASK1 [19]. Li et al found that binding between 14-3-3 protein and Ask1 could protect the c-terminal of Ask1 kinase, thereby inhibiting the de-phosphorylation of Ask1 and, as a result, restricting its activity and blocking myocardial apoptosis. It is generally believed that p-Ask1 is the non-activated form of Ask1 and binding of 14-3-3 with Ask1 by phosphorylation limits the activity of Ask1 [20,21].
It was reported that bone marrow mesenchymal stem cells could inhibit the myocardial apoptosis during diabetes mellitus by regulating 14-3-3 and elevated 14-3-3 protein can retard the progression of diabetic cardiomyopathy via ask1 signal regulation and ameliorate hyperglycemia-induced dysfunction of left ventricle [22-24].
Since diabetic cardiomyopathy is intimately associated with myocardial fibrosis and apoptosis [25,26], this study examined the effect of transplanted bone marrow mesenchymal stem cells on the apoptosis of myocardial cells in diabetic cardiomyopathy and explored the possible molecular mechanism.
Materials and methods
Animals
Male SD rats of 7-8 weeks old, weighing 160-180 g were procured from the Center of Experimental Animals, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (Permit No. SCXK [E] 2010-0009). The animals were kept in the center and raised by following two guidelines: Guide for the Care and Use of Laboratory Animals formulated by NIH, USA and the Guideline for the Management of Experimental Animals introduced by the Administration Bureau of Science and Technology of Hubei Province, China.
Experimental design
The rat model of diabetic cardiomyopathy was first established. Newly purchased rats were randomly divided into two groups: a control group and a model group. Rats in the model group were raised by following the method for making model of diabetic cardiomyopathy [27]. Rats in the controls group were routinely fed with conventional feed for 12 weeks.
To examine the effect of hematopoietic stem cells on rat model of diabetic cardiomyopathy, we set up three groups: MS group, DCM group and PBS group. In MSC group, model rats were injected with bone marrow mesenchymal stem cells at 5×105/rat via tail vein four times over a period of four weeks (1 time per week). In DCM group, model rats were given PBS via tail vein four times (1 time per week). Rats in PBS group were raised with average chow for 12 weeks and then administered PBS through tail vein four times (1 time per week).
Establishment of diabetic cardiomyopathy model
SD rats of 7-8 weeks old, weighing 160-180 g were fed with high-fat/high sugar diet and streptozocin (STZ) was intraperitoneally injected in the 2nd, 4th and 6th weeks at a dosage of 20 mg/kg. Success of model establishment was assessed on the basis of blood sugar, lipid profile and amount of urine at the 8th week. After feeding for another four weeks, the success of the model making was further evaluated on the basis of ultrosonographic and hemodynamic findings and EF.
Ultrasonographic evaluation of cardiac assessment
LVDD, LVPW, LVFS, IVSD, which reflect the diastolic function of heart, and EF, indicative of systolic function, were ultrasonographically detected. Especially, we used ketamine in combination with xylazine (100 mg:40 mg/1000 g) to control the heart rate in the range of 180-250 beats/minute.
Hemodynamic detection
Rats were anesthetized with 3% pentobarbital sodium at 30 mg/kg by intraperitoneal injection. The right common carotid artery (CCA) was isolated and ligated with silk thread at distal end (from heart) and the proximal end was clipped with an arterial clip. A small opening was made on the artery and a micro-cannula was inserted into the opening. Then the clip was released to introduce a lead wire into left ventricle via CCA. The other end of the lead wire was interfaced with a MPA-2000 polygraph system to record LVSP, LVEDV, and ± LVdp/dtmax.
Cardiac index
The rat hearts were removed of the muscles of atria and right ventricle. The remaining left ventricular muscles were dried with filter paper and weighed. The cardiac index was the ratio between the weight of left ventricular muscles (VW) and body weight (BW) and ratio (VW/BW) was used for the quantitative evaluation of cardiac hypertrophy.
Collection and transplantation of bone marrow mesenchymal stem cells
SD rats were sacrificed by decapitation and femoral and tibia bones were aseptically taken for harvesting bone marrow. Ficoll-Paque density gradient was employed for isolation of mononuclear cells. The cells were cultured and passaged. The cells of the 3rd to 4th passages were used for transplantation. The cells were observed under phase-contrast microscope and flow cytometrically analyzed.
Flow cytometry
The rat bone marrow mesenchymal stem cells of the 3rd to 4th passages were examined for markers of cell-surface molecules, including CD44 and CD34.
Pathological examination of cardiac muscles
The samples were Masson-Goldner trichrome stained to observe the pathological changes of cardiac muscles after MMSC transplantation, cardiac fibrosis in particular. TUNEL staining (Roch kit, Catalog No. 11684817910) was employed for observing myocardiac apoptosis.
Detection of expression of 14-3-3 protein, Ask1 and p-Ask-1 in myocardiac tissues
The antibody against 14-3-3 recognized all subtypes of 14-3-3 protein. Pan 14-3-3 antibody was bought from Santa Cruz (Batch No. sc-629; concentration: 1:1000). Caspase-3 antibody was from Abcam Co. (Batch No. 3764; concentration: 1:1000) and could target the active segment of Caspase-3. Caspase-3 was from Abcam Company (Batch No. Ab4051, size of active segment: 17 kDa, Concentration: 1:1000). Phospho-ASK1 antibody was purchased from Cell Signalling Co. and was able to specifically recognize the Ser967 phosphorylation site (Batch No. 3764; concentration: 1:1000).
Slow virus infection
The rat bone marrow mesenchymal stem cells of the 3rd to 4th passages were used for the test. Three days before the transplantation, the cells were infected green fluorescent protein (GFP)-tagged slow virus (Shanghai GeneChem Co., Ltd., China; MOI = 100). GFP expression was confirmed under a fluorescent microscope before the transplantation.
Statistical analysis
The data were statistically analyzed by using the SPSS software package (Ver. 17.0). ANOVA with Newman-Keuls test was employed for comparison among different groups. A P < 0.05 was considered to be statistically significant.
Results
Evaluation of diabetes mellitus model
At the end of the 8th week, blood sugar (FBG, HbAIc) and blood lipids (TCH, TG) were all significantly higher in model group than in control group (P < 0.001) (Figure 1) and the amount of urine and water-intake was obviously increased, suggesting that the model of diabetes mellitus was successfully established.
Figure 1.
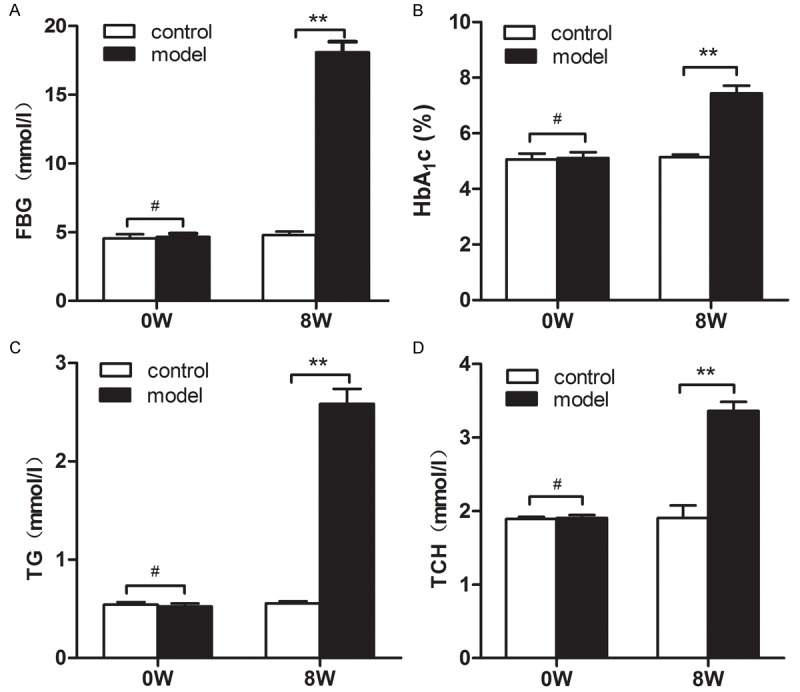
Assessment of the model of diabetes mellitus. Before model establishment (0W), there were no siginificant differences in blood sugar (FBG, HbAIc) and blood lipids (TCH, TG) between the two groups. At the end of the 8th week, blood sugar (FBG, HbAIc) and blood lipids (TCH, TG) were all significantly higher in model group than in control group (P < 0.001).
Evaluation of diabetic cardiomyopathy model
After rats were raised with high sugar/high lipid chow for another 4 weeks, ultrasonographic examination showed there existed significant differences in LVDD, LVPW, LVFS, IVSD (the measures of heart diastolic function) and EF (an indicator of systolic function) between model group and controls (Figure 2A, 2B) suggesting that the cardiac muscles in the model group became hypertrophic and both diastolic and systolic functions were impaired in the model group.
Figure 2.
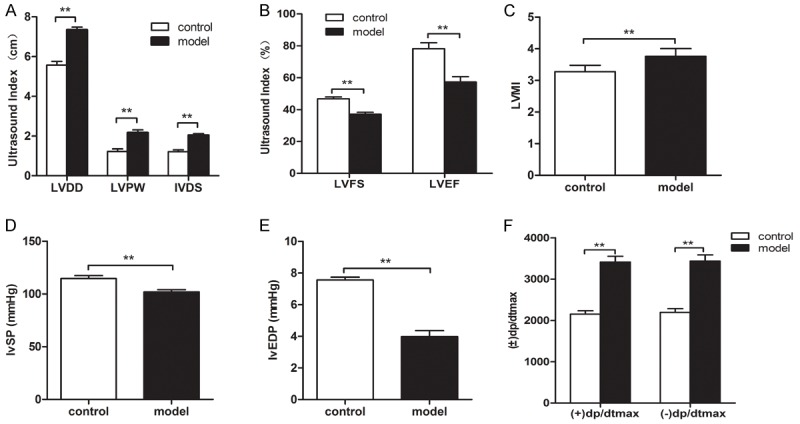
Identification of bone marrow mesenchymal stem cells and differentiation to vessels. After rats were raised with high sugar/high lipid chow for another 4 weeks, ultrasonographic examination showed there were significant differences in LVDD, LVPW, LVFS, IVSD and EF between model group and controls (A, B) suggesting that the cardiac muscles in the model group became hypertrophic and both diastolic and systolic functions were impaired in the model group. Hemodynamic tests exhibited that there were significant differences in LVSP, LVEDV and ± LVdp/dtmax between the two groups (P < 0.05), suggesting that the cardiac functions in model group was impaired (D, E, F). The cardiac index was significantly higher in the model group than that in controls (P < 0.01) (C).
Hemodynamic tests exhibited that there were significant differences in LVSP, LVEDV and ± LVdp/dtmax between the two groups (P < 0.05), suggesting that the cardiac functions in model group was impaired (Figure 2D-F).
The cardiac index was significantly higher in the model group than that in controls (P < 0.01) (Figure 2C).
On the basis of aforementioned findings, we were led to conclude the model of diabetic cardiomyopathy was successfully established in this study.
Identification of bone marrow mesenchymal stem cells and differentiation to vessels
Bone marrow mesenchymal stem cells were obtained form rat femur and tibia bones. Cultured primary bone marrow mesenchymal stem cells attached to the walls and became spindle-shaped, some becoming polygonal, and grew in clusters. Single cells were occasionally observed. At day 5, obvious colonies formed, and at day 10 virtually all cells attached to the walls, being flat and narrow and assuming vortex pattern (Figure 3Aa). Three days after infection with slow virus, green fluorescent signals were observed under fluorescent microscope (Figure 3Ab). Flow cytometry showed that 0.19% of the cultured cells were CD34-negative and 25.25% of the cells were CD44-positive. The findings confirmed that the cultured cells of 3-4 passages were bone marrow mesenchymal stem cells (Figure 3C).
Figure 3.
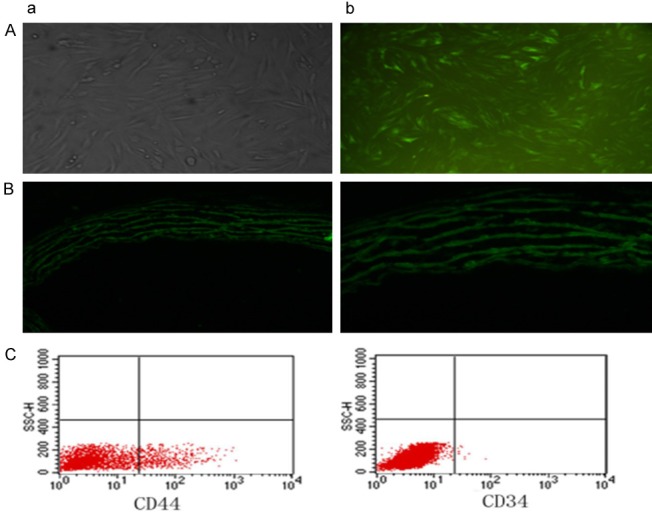
Identification of bone marrow mesenchymal stem cells and differentiation to vessels. Cultured primary bone marrow mesenchymal stem cells attached to the walls and became spindle-shaped, some assuming polygonal pattern, and grew in clusters. Single cells were occasionally observed. At day 5, obvious colonies formed, and at day 10 almost all cells attached to the walls, being flat and narrow and assuming vortex pattern (Aa). Three days after infection with slow virus, green fluorescent signals were observed under fluorescent microscope (Ab). Flow cytometry showed that 0.19% of the cultured cells were CD34-negative and 25.25% of the cells were CD44-positive. The findings confirmed that the cultured cells of 3-4 passages were bone marrow mesenchymal stem cells (C). After transplantation of the stem cells, in MSC group, MSCs were found to have migrated to and settled in cardiac tissues, eventually differentiating into blood vessels (B: a: 200× b: 400×).
After transplantation of the stem cells, in MSC group, MSCs were found to have migrated to and settled in cardiac tissues, eventually differentiating into blood vessels (Figure 3B).
Comparison in the parameters of cardiac function between DCM and SMC groups
Four weeks after transplantation of BMSCs, ultrasonography showed that, compared with SMC group, heart muscles in DCM group became significantly thickened and the diastolic and systolic functions were apparently impaired. Statistical analysis showed that there were significant differences in LVDD, LVPW, LVFS, IVSD, EF between the two groups (Figure 4A, 4B). Hemodynamic studies revealed that there were statistically significant differences in the parameters of cardiac function between the two groups (P < 0.05). In DCM group, the cardiac function continue to worsen while the heart function in the DCM group was improved (Figure 4D-F). Analysis also exhibited that cardiac index was significantly higher in DCM group than in SMC group (Figure 4C). Moreover, hearts in DCM group were grossly hypertrophic.
Figure 4.
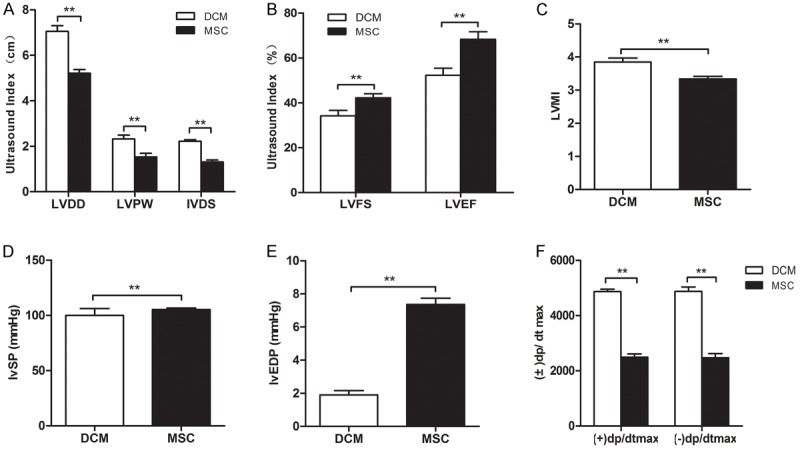
Comparison in the parameters of cardiac function between DCM and SMC groups. Four weeks after transplantation of BMSCs, ultrasonography showed that, compared with SMC group, heart muscles in DCM group became significantly thickened and the diastolic and systolic functions were apparently impaired. Statistical analysis showed that there were significant differences in LVDD, LVPW, LVFS, IVSD, EF between the two groups (A, B). Hemodynamic studies revealed that there were statistically significant differences in the parameters of cardiac function between the two groups (*P < 0.05). In DCM group, the cardiac function continue to worsen while the heart function in the DCM group was improved (D-F) (**P < 0.01). Analysis also exhibited that cardiac index was significantly higher in DCM group than in SMC group (*P < 0.001) (C).
Apoptotic and fibrotic changes after transplantation of bone marrow mesenchymal stem cells
Four weeks after transplantation, myocardial fibrosis (Figure 5A) and apoptosis (Figure 5B) were much more serious in DCM group than in MSC group, with the difference being statistically significant (P < 0.05). Caspase-3 expression was significantly higher in DCM group than in MSC group (P < 0.001) (Figure 6E). These results showed that transplantation of the mesenchymal stem cells could relieve myocardial apoptosis and fibrosis in diabetic myocardiopathy (Figures 4, 5 and 6).
Figure 5.
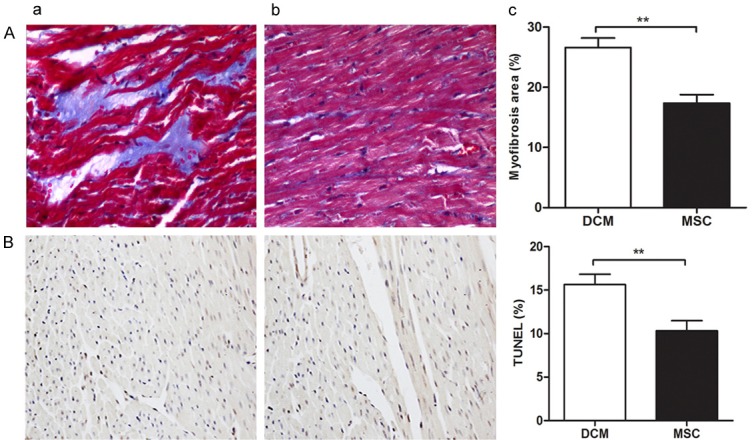
Apoptotic and fibrotic changes after transplantation of bone marrow mesenchymal stem cells. Four weeks after transplantation, myocardial fibrosis (A) and apoptosis (B) were much more serious in DCM group than in MSC group, with the difference being statistically significant (*P < 0.05).
Figure 6.

Changes in the expression of related proteins after transplantation. After the transplantation of stem cells 1, the expression of 14-3-3 and p-Ask1 was significantly higher in SMC group and PBS group (A, B, F) and Ask1 showed opposite changes (A, C, E). (D) The result of capase-3 was consistent with ASK.
Changes in the expression of 14-3-3, Ask1 and p-Ask1 after transplantation
After the transplantation of stem cells1, we detected the expression of 14-3-3, Ask1 and p-Ask1 in MSC, DCM and PBS groups and found that the expression of 14-3-3 and p-Ask1 was significantly higher in MSC group and PBS group (Figure 6A, 6B and 6F) and Ask1 showed opposite changes (*P < 0.05;**P < 0.001) (Figure 6A, 6C and 6E).
Discussion
A recent study [28] showed that transplantation of bone marrow mesenchymal stem cells could, to some extent, ameliorate the diabetic cardiomyopathy. Transplanted bone marrow mesenchymal stem cells have multiple differentiation potential and have been induced to differentiate to myocardial cells, vascular endothelial cells, smooth muscle cells. Newly generated vascular endothelial cells and smooth muscle cells can form new vessels and collateral circulation, increase local blood perfusion, relieve myocardial apoptosis and fibrosis and thereby improve cardiac function. But the exact mechanism of the transplanted stem cells improving cardiac function remains poorly understood. This study examined the effect of bone marrow mesenchymal stem cells on heart function and explored its possible mechanisms.
We first established rat models of diabetes mellitus type 2 and then models of diabetic cardiomyopathy (Our detection revealed that the blood sugar and lipids were significantly higher in model rats than in control rats (P < 0.001) and the amount of urine and water intake were obviously increased, suggesting that the model of diabetes mellitus (type 2) was successfully made. Then, after raising for four weeks, we found that there existed significant differences in the ultrasound measures of cardiac function, i.e., LVDD, LVPW, LVFS, IVSD, EF and hemodynamic indicators of heart function, such as LVSP, LVEDV, ± LVdp/dtmax (P < 0.001), suggesting that the heart muscles of model rats became hypertrophic and heart function was impaired. Moreover, the cardiac index was significantly higher in model rats than in control rats. These findings led us to conclude that the model of diabetic cardiomyopathy was successfully established.
After successful establishment of the model of diabetic cardiomyopathy, the rats received transplantation of BMSCs for 4 weeks. Before the transplantation, the cultured cells of 3-4 passages had been identified to be bone marrow mesenchymal stem cells by flow cytometry and phase-contrast microscopy. Histological examination showed that MSCs migrated and settled in cardiac tissues and differentiated into vessels, which was consistent with previously reported findings [29-32]. In rats receiving MSC transplantation, the measures of cardiac function, i.e., LVDD, LVPW, LVFS, IVSD, EF were significantly improved as compared with diabetic cardiomyopathy rats that were not transplanted with MSCs. These findings suggested that MSC transplantation could improve heart function.
Since bone marrow mesenchymal stems cells secrete not only vascular endothelial growth factor (VEGF) but also factors that inhibit apoptosis and fibrosis, the development of diabetic cardiomyopathy is closely linked with the myocardial fibrosis and apoptosis and hyperglycemia can directly lead to myocardial apoptosis in vivo.
This study, by dynamically observing the change in the expression of Ask1 and its regulator 14-3-3 protein, explored the mechanism of bone marrow mesenchymal stems cells working on diabetic cardiomyopathy. 14-3-3 protein is a kind of highly conserved protein and is involved in the regulation of apoptosis, adhesion, differentiation, proliferation and signal transduction of cells [13,14]. Recent studies showed that 14-3-3 protein possesses anti-apoptotic, anti-fibrotic and heart-protective effects [15-17]. Ask1 is an important signal regulator of cellular apoptosis in diabetes mellitus. It belongs to the family of mitogen-activated protein kinases (MAPKs), which are situated upstream of JNK and p38 in the MAPK pathway. After activation, Ask1 activates JNK and p38 to induce apoptosis. Current studies demonstrated that 14-3-3 protein is an important regulator of Ask1 activity. In a model of diabetics mellitus, 14-3-3 was believed to bind to phosphorylated serine-967 of ASK1 [19]. Li et al found that binding between 14-3-3 protein and Ask1 could protect the c-terminal of Ask1 kinase, thereby inhibiting the de-phosphorylation of Ask1 and, as a consequence, restricting its activity and blocking myocardial apoptosis. It is generally believed that p-Ask1 is the non-activated form of Ask1 and binding of 14-3-3 with phosphorylated Ask1 limits the activity of Ask1 [19,20]. To further understand the mechanism by which bone marrow mesenchymal stem cells inhibit the myocardial apoptosis in diabetic myocardiopathy, we detected the expression of 14-3-3, Ask1andp-Ask1 in SMC, DCM and PBS groups by using Western blotting and found that expression of 14-3-3 and p-Ask1 was significantly higher in SMC and PBS group than in DCM group and Ask1 expression showed opposite changes.
To sum up, our study demonstrated that bone marrow mesenchymal stem cells could improve the cardiac function of rats with diabetic cardiomyopathy, possibly by their ability to up-regulate the expression of 14-3-3 protein and to inhibit Ask1 pathway.
Disclosure of conflict of interest
None.
References
- 1.Hamby RI, Zonezaich S, Sherman L. Diabetic cardiomyopathy. JAMA. 1974;229:1749–1754. [PubMed] [Google Scholar]
- 2.Adameova A, Dhalla NS. Role of microangiopathy in diabetic cardiomyopathy. Heart Fail Rev. 2014;19:25–33. doi: 10.1007/s10741-013-9378-7. [DOI] [PubMed] [Google Scholar]
- 3.Isfort M, Stevens SW, Schaffer S. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acar E, Ural D, Bildirici U, Sahin T, Yılmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg. 2011;11:732–7. doi: 10.5152/akd.2011.196. [DOI] [PubMed] [Google Scholar]
- 5.Marangoni MN, Brady ST, Chowdhury SA, Piano MR. The co-occurrence of myocardial dysfunction and peripheral insensate neuropathy in a streptozotocin-induced rat model of diabetes. Cardiovasc Diabetol. 2014;13:11. doi: 10.1186/1475-2840-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SH, Guo JX, Zhang P. Long-term effects of bone marrow mononuclear cell transplantation on left ventricular function and remodeling in rats. Life Sci. 2004;74:2853–2864. doi: 10.1016/j.lfs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scatena R, Bottoni P, Botta G. The role of mitochondria in pharmacotoxicology: a reevaluation of an old,newly emerging topic. Am J Physiol Cell Physiol. 2007;293:C12–21. doi: 10.1152/ajpcell.00314.2006. [DOI] [PubMed] [Google Scholar]
- 9.Morales CD, Calvino E, Rubio V. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp Toxicol Pathol. 2013;65:1101–1108. doi: 10.1016/j.etp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Cho JH, Lee PY, Son WC. Identification of the novel substrates for caspase-6 in apoptosis using proteomic approaches. BMB Rep. 2013;46:588–593. doi: 10.5483/BMBRep.2013.46.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurtl B, Kratky D, Guelly C. Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int J Exp Pathol. 2009;9:338–346. doi: 10.1111/j.1365-2613.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurusamy N, Watanabe K, Ma M. Glycogen synthase kinase 3beta together with 14-3-3 protein regulates diabetic cardiomyopathy: Effect of losartan and tempol. FEBS Lett. 2006;580:1932–1940. doi: 10.1016/j.febslet.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Thandavarayan RA, Gurusamy N, Zhang S, Muslin AJ, Suzuki K, Tachikawa H, Kodama M, Aizawa Y. Role of 14-3-3 protein and oxidative stress in diabetic cardiomyoathy. Acta Physiol Hung. 2009;96:277–287. doi: 10.1556/APhysiol.96.2009.3.3. [DOI] [PubMed] [Google Scholar]
- 14.Sari FR, Watanabe K, Thandavarayan RA, Harima M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. 14-3-3 Protein Protects Against Cardiac Endoplasmic Reticulum Stress (ERS) and ERS-Initiated Apoptosis in Experimental Diabetes. J Pharmacol Sci. 2010;113:325–334. doi: 10.1254/jphs.10047fp. [DOI] [PubMed] [Google Scholar]
- 15.Gurusamy N, Watanabe K, Ma M, Prakash P, Hirabayashi K, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Glycogen synthase kinase 3beta together with 14-3-3 protein regulates diabetic cardiomyopathy: effect of losartan and tempol. FEBS Lett. 2006;580:1932–1940. doi: 10.1016/j.febslet.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Gurusamy N, Watanabe K, Aizawa Y. Inactivation of 14-3-3 protein exacerbates cardiac hypertrophy and fibrosis through enhanced expression of protein kinase C beta 2 in experimental diabetes. Biol Pharm Bull. 2005;28:957–962. doi: 10.1248/bpb.28.957. [DOI] [PubMed] [Google Scholar]
- 17.Thandavarayan RA, Giridharan VV, Sari FR. Depletion of 14-3-3 protein exacerbates cardiac oxidative stress, inflammation and remodeling process via modulation of MAPK/NF-κB signaling pathways after streptozotocin-induced diabetes mellitus. Cell Physiol Biochem. 2011;28:911–922. doi: 10.1159/000335805. [DOI] [PubMed] [Google Scholar]
- 18.Thandavarayan RA, Watanabe K, Ma M. 14-3-3 protein regulates Ask1 signaling and protects against iabetic cardiomyoathy. Biochem Pharmacol. 2008;75:1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Goldman EH, Chen L, Fu H. Activation of apoptosis signalregulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem. 2004;279:10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zhang R, Luo D, Park SJ, Wang Q, Kim Y. Tumor necrosis factor alpha-induced desumoylation and cytoplasmic translocation of homeodomain-interacting protein kinase 1 are critical for apoptosis signal-regulating kinase 1-JNK/p38 activation. J Biol Chem. 2005;280:15061–15070. doi: 10.1074/jbc.M414262200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SS, Ren J, Zhang CE. Role of 14-3-3–mediated p38 mitogen-activated protein kinase inhibition in cardiac myocyte survival. Circ Res. 2003;93:1026–1028. doi: 10.1161/01.RES.0000104084.88317.91. [DOI] [PubMed] [Google Scholar]
- 22.He A, Jiang Y, Gui C, Sun Y, Li J, Wang JA. The antiapoptotic effect of mesenehymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can J Cardiol. 2009;25:353–358. doi: 10.1016/s0828-282x(09)70094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban VS, Kiss J, Kovacs J. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 24.Thandavarayan RA, Watanabe K, Ma M. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol. 2008;75:1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Frustaci A, Kajstura J, Chimenti C. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 26.Grimm D, Jabusch HC, Kossmehl P. Experimental diabetes and left ventricular hypertrophy: effects of beta-receptor blockade. Cardiovasc Pathol. 2002;11:229–237. doi: 10.1016/s1054-8807(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YX, Guo SF, Liu GB. Transplantation of bone marrow-derived endothelial progenitor cells attenuates myocardial interstitial fibrosis and cardiac dysfunction in streptozotocin-induced diabetic rats. Int J Mol Med. 2012;30:1083. doi: 10.3892/ijmm.2012.1083. [DOI] [PubMed] [Google Scholar]
- 28.Dong S, Ma W, Hao B, Hu F, Yan L, Yan X, Wang Y, Chen Z, Wang Z. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol. 2014;7:565–74. [PMC free article] [PubMed] [Google Scholar]
- 29.Maciver DH, Townsend MA. Novel mechanism of heart failure with normal ejection fraction. Heart. 2008;94:446–449. doi: 10.1136/hrt.2006.114082. [DOI] [PubMed] [Google Scholar]
- 30.Ozasa N, Furukawa Y, Morimoto T. Relation among left ventricular mass, insulin resistance, and hemodynamic parameters in type 2 diabetes. Hypertens Res. 2008;31:425–432. doi: 10.1291/hypres.31.425. [DOI] [PubMed] [Google Scholar]
- 31.Van Heerebeek L, Hamdani N, handoko ML. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 32.Uemura R, Xu M, Ahmad N. Bone marrow stem cells prevent left ventricular remodeling of ischemie heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]


