Abstract
Leucine aminopeptidases (LAPs) were associated with tumor cell proliferation, invasion and/or angiogenesis. LAP3 is one important member of this family. However, its clinical significance and biological function in hepatocellular carcinoma (HCC) remains unknown. In the present study, we demonstrated that LAP3 expression was significantly up-regulated in HCC tissues as well as cells and was closely correlated with lower differentiation, positive lymph node metastasis and high Ki-67 expression, indicating a poor prognosis. Then cell viability assays, flow cytometry assays, wound-healing assays and matrigel invasion assays were performed to demonstrate that LAP3 promoted HCC cells proliferation by regulating G1/S checkpoint in cell cycle and advanced HCC cells migration. Furthermore, we discovered that knockdown LAP3 will enhance the sensitivity of HCC cells to cisplatin, thus promoting the cell death of HCC cells. Collectively, our results indicated that up-regulated expression of LAP3 might contribute to the proliferation and metastasis of HCC. Our data gains greater insight into the cancer-promoting role of LAP3 and its functions in HCC cells, possibly providing potential therapeutic strategies for clinical trials.
Keywords: Hepatocellular carcinoma (HCC), LAP3, prognosis, proliferation, migration
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death in the world [1]. Being the fifth most prevalent lethal neoplasia, HCC is associated with a poor prognosis largely due to high incidence of tumor recurrence and intrahepatic metastasis after surgical resection [2,3]. Hepatocarcinogenesis is thought to be an extensive process associated with dysfuction of various oncogenes and tumor suppressors, ultimatly leading to the malignant transformation of normal liver cells [4,5]. Despite great advancement in both diagnosis and treatment of HCC, the exact molecular mechanisms underlying the development of HCC remains poorly understood. Thus, the identification of the molecular mechanism during HCC progression may provide patients with HCC novel diagnostic and therapeutic strategies.
Leucine aminopeptidases (LAPs) are a family of proteins implicated in the progress of various pathological disorders, and play a role in tumor cell proliferation, invasion and angiogenesis [6]. Being one important member of this family, Leucine aminopeptidase 3 (LAP3) is originally identified as exopeptidase that catalyzes the hydrolysis of leucine residues from the amino termini of protein or peptide substrates [7]. Alteration of LAP3 expression pattern and/or catalytic function lead to peptide activation changing, thus contributing to the changes of cell cycle progression in gynecologic malignancy such as ovarian epithelial malignancy, breast cancer and endometrial carcinoma [8-10]. LAP3 is also involved in the malignant development of human esophageal squamous cell carcinoma [11] and plays a significant role in diabetic patients [12,13]. Therefore, it is of interest to see whether LAP3 could also modulate cell cycle progress and cell proliferation of HCC.
Although studies have showed the important role of aberrant expression of LAP3 in tumorigenesis such as human breast cancer and human esophageal squamous cell carcinoma, reports investigating the functions of LAP3 in human HCC progression are still lacking. In the present study, for the first time, we demonstrated that LAP3 was significantly over-expressed in human HCCs pecimens and HCC cell lines. Then, we also investigated its associations with clinical and pathologic factors. To investigate possible tumor activity of LAP3 in HCC, LAP3 was transfected into HCC cells. The functions of LAP3, as well as its clinical significance, were also investigated. This study was conducted to gain greater insight into the cancer-promoting role of LAP3 and its functions in HCC cells, possibly providing potential therapies for clinical trials.
Materials and methods
Tissue collection
115 samples of HCC samples with their corresponding adjacent non-tumor liver tissues were obtained with informed consent in accordance with the requirements of the Research Ethics Committee in the Cancer Hospital of Nantong University, from May 2006 to May 2013. The 115 HCC cases comprised 24 males and 91 females. Their ages ranged from 25 to 77 years, with an average age of 43.25 years. According to the 2002 International Union against Cancer TNM classification system and the Edmondson grading system, histological grades were classified to well (grade I; n = 47), moderately (grade II; n = 53) and poorly differentiated (grade III; n = 15). The main clinicopathologic characteristics of the patients are summarized in Table 1.
Table 1.
LAP3 Expression and clinicopathological features in 115 HCC specimens
| Clinicopathological Features | Total | LAP3 | P value | X2 value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Low expression n=41 | High expression n=74 | |||||
| Ages (years) | <45 | 50 | 18 | 32 | 0.55 | 0.005 |
| ≥45 | 65 | 23 | 42 | |||
| sex | Male | 24 | 10 | 14 | 0.322 | 0.478 |
| Female | 91 | 31 | 60 | |||
| Serum AFP Level (ng/ml) | <50 | 45 | 16 | 29 | 0.574 | 0.000 |
| ≥50 | 70 | 25 | 45 | |||
| Cirrhosis | Absent | 23 | 8 | 15 | 0.563 | 0.009 |
| Present | 92 | 33 | 59 | |||
| Maximal Tumor Size (cm) | <4.5 | 68 | 24 | 44 | 0.539 | 0.009 |
| ≥4.5 | 57 | 17 | 30 | |||
| Tumor Metastasis | Absent | 43 | 20 | 23 | 0.047* | 3.530 |
| Present | 72 | 21 | 51 | |||
| HBs Ag | (-) | 25 | 6 | 19 | 0.126 | 1.891 |
| (+) | 90 | 35 | 55 | |||
| Histological Grade | Poor | 47 | 23 | 24 | 0.039* | 6.465 |
| Moderate | 53 | 15 | 38 | |||
| Well | 15 | 3 | 12 | |||
| Ki-67 | Low | 45 | 22 | 23 | 0.015* | 5.646 |
| High | 70 | 19 | 51 | |||
Statistical analyses were carried out usting Pearson X2 test.
P<0.05 was considered significant.
Immunohistochemical staining
All tissue samples were fixed in phosphate-buffered neutral formalin and routinely embedded in paraffin, and then cut into 5-μm-thick sections. Tissue sections were incubated with 0.3% hydrogen peroxide/phosphate-buffered saline for 30 minutes and blocked with 10% BSA (Sangon, Shanghai, China), then were detected with primary polyclonal antibody for LAP3 (Abcam, Cambridge, UK) and Ki-67 (Abcam, Cambridge, UK) overnight at 4°C in a moist chamber. After incubated with the second antibody (Thermo Scientific, US) labeled by HRP (rabbit) for 1 hour at room temperature, the sections were treated with diaminobenzidine and counterstained with hematoxylin. All the sections were observed and photographed with a micro-scope (Carl Zeiss) and scored was conducted according to the ratio and intensity of positive-staining cells as followed: strongly stained (score 1) designated as high expressionand weakly stained (score 0) designated as low expression [14]. All the LAP3 and Ki-67 expression level was quantified two-blindly by two independent pathologists.
Western blotting
Whole cell lysates were prepared by lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton-X 100, 1 mM each MgCl2, MnCl2 and CaCl2, 1 mM PMSF and 10 mM sodium fluoride). Proteins were separated by SDS-PAGE and were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Then the electroblotted membranes were blocked in phosphate-buffered saline/Tween-20 containing 5% BSA. The primary antibodies for LAP3 (Abcam Cambridge, UK), proliferating cell nuclear antigen (PCNA, Abcam, Cambridge, UK), cyclin A (Abcam, Cambridge, UK), CDK2 (Abcam, Cambridge, UK), CDK6 (Abcam, Cambridge, UK), E-cadherin (Cell Signaling Technology, Beverly, MA), β-actin (Abcam, Cambridge, UK) and GAPDH (Proteintech, US) were used. After incubating with the IRDye 680 anti-mouse (LI-COR, Lincoln, NE) or IRDye 800 anti-rabbit (LI-COR, Lincoln, NE) secondary antibodies for 2 hour at room temperature, the bands were detected by an Odyssey infrared imaging system (LI-COR, Lincoln, NE).
Cell lines and cell culture
Normal hepatocyte cell line LO2 and human hepatocarcinoma cell lines, including BEL-7404, HuH7, HepG2 and MHCC-97H, were all obtained from the Institute of Cell Biology, Academic of China (Shanghai, China), and cultured in Dulbecco-modified Eagle medium (DMEM, Sigma) supplemented with 10% fetal bovine serum (FBS, Hyclone), penicillin in 100 U/mL, and streptomycin 100 μg/mL in 5% CO2 at 37°C.
Plasmid construction
The full-length LAP3 (Genbank Accession No.NM_015907.2) was isolated from the human cDNA library and connected to pcDNA3.1-myc. The primers used for LAP3 were as follows: 5’-CCGCTCGAGGGATGTTCTT-GCTGCCTTA-3’ (sense) and 5’-GGGGTACCAGCATTGTCTTGACTTA-3’ (anti-sense). Primer pairs for the LAP3-shRNA expression vector was target the sequence: 5’-GCCCATTAATATTATAGGT-3’ [11].
LAP3 overexpression or LAP3 shRNA plasmids transfection
Cells were grown in culture medium with 10% FBS. When cells had reached confluency, Lipofectamine 2000 (Invitrogen; USA), OPTI-MEM (Gibco, USA) and plasmid pCDNA3.1-LAP3 or LAP3 shRNA were mixed and incubated for 20 min and added to HCC cells at room temperature respectively. After 6 h of incubation, these cells were then incubated in DMEM containing 10% FBS in 5% CO2 at 37°C. After 48 h transfection, cells were subjected to western blotting analysis. Cells transfected with pcDNA3.1 (empty vector) and control shRNA were considered as negative control.
Cell viability assay
Cells were seeded into a 96-well plate at 2×104 cells per well with 100 μl culture medium supplemented with 10% FBS and cultured at 37°C. 10 μl Cell Counting Kit-8 (CCK-8, WST-8, Dojindo, Japan) was added to each well after 24 h, 48 h and 72 h, respectively. The absorbance values were measured at 450 nm wave-length using a microplate reader (Spectra Max M5, Molecular device), representing the rate of DNA synthesis and corresponding to the number of proliferating cells. The experiment was performed in triplicate and repeated twice.
Flow cytometry assay
For cell cycle analysis, 2×105 cells were plated in 60 mm plates and grown for 24 h. Cells were then incubated with 1 mM thymidine (Sigma-Aldrich) for 24 h to synchronize cells at the G1/S boundary. The cells were then treated with serum-deprived culture medium for another 48 h. Next, the cells were trypsinized, washed twice with cold PBS and fixed with cold 70% ethanol at -20°C overnight. The cells were then washed twice with PBS and incubated with 10 mg/ml RNase A and 400 mg/ml propidium iodide (PI) in PBS at room temperature for 30 mins. Cells were subsequently analyzed by flow cytometry (Becton, Dickinson and Company).
Wound-healing assay
Tumor cells (5×105) were seeded in 6-well plates and grown to confluence overnight. After twenty-four hours, a line was scratched within the confluent cell layer using the fine end of a 10 ml pipette tip (time 0). Images of migrating cells were sequentially taken during closure of the wounded region.
Matrigel invasion assay
Cell invasion assay was performed using transwell chamber (8 μm pore size, 6.5 mm diameter, Corning, USA). BD MatrigelTM Basement Membrane Matrix was added to the upper membrane and allowed to gel for 1 h at 37°C. Then, 500 μL medium containing 1% FBS was placed in the lower chamber. Cells were plated in the upper chamber of quadruplicate wells at a density of 2×104/mL and incubated at 37°C for 24 h. Cells were then fixed with paraformaldehyde, stained with 5% crystal violet, and counted immediately after staining. Experiments were repeated five times. The results were expressed as a percentage of the controls.
Statistical analysis
Data were presented as the means ± standard error of the mean (SEM). Statistical analyses were done using SPSS 16.0 for windows (IBM). The chi-square test was used for comparison between groups. Values of P<0.05 were considered statistically significant.
Results
LAP3 is expressed in HCC tissues and cells
In order to reveal the relevance of LAP3 with HCC, we detected LAP3 expression in a tissue microarray (TMA) containing 115 HCC samples and 15 normal liver samples by immunohistochemical staining. Representative sections of LAP3 expression in all tissues were shown in Figure 1A. While the clinicopathological characteristics of HCC patients are summarized in Table 1. Analysis showed that LAP3, predominantly localized in the cytoplasm of HCC cells, wassignificantly upregulated in HCC tissues compared with normal liver samples. Thereafter we investigated the expression of LAP3 in normal hepatocyte cell line LO2 as well as human hepatocarcinoma cell lines, including BEL-7404, HuH7, HepG2 and MHCC-97H cells (Figure 1B). We found that LAP3 was expressed at a high level in HCC cells compared with normal hepatocyte cell line LO2 cells. These findings showed that LAP3 was expressed highly in HCC tissues andcells.
Figure 1.
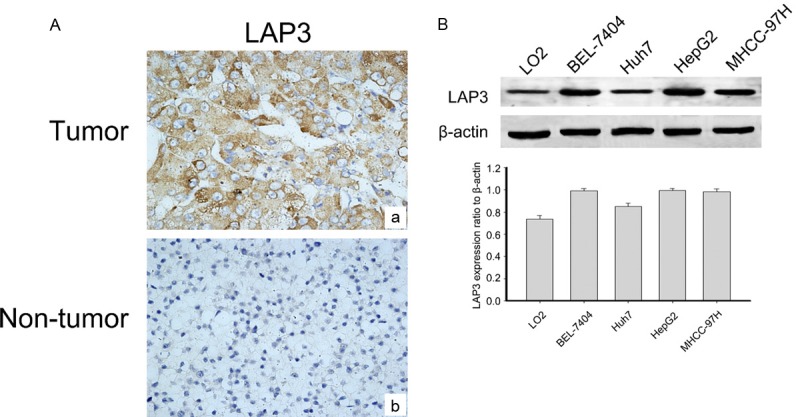
LAP3 is expressed in HCC tissues and cells. A. Representative images of LAP3 immunohistochemical staining in a tissue microarray that contains containing 115 HCC samples and 15 normal liver samples, Scale bar: 50 μm. B. Western blot analysis shows that LAP3 protein expression is increased in HCC cells compared with the normal liver cell lines (LO2). GAPDH was used as a loading control. The bar chart demonstrates the ratio of LAP3 protein to GAPDH by densitometry. The data are means ± SEM.
LAP3 is associated with adverse clinical characteristics and poor outcome of HCC patients
Clinical association study was performed to analyze the correlation of LAP3 upregulation with HCC clinicopathological features using the SPSS 16.0 for windows (IBM). Notably, we found that highly expression of LAP3 was significantly associated with adverse clinicopathological features such as histological grade (P = 0.039), tumor metastasis (P = 0.047) and Ki-67 (P = 0.015) (Table 1). Kaplan-Meier analysis were also applied to further investigate the prognostic significance of LAP3 in HCC. The result revealed that upregulation of LAP3 was significantly associated with poor overall survival rate in HCC (Figure 2). Furthermore, a multivariate Cox proportional hazard model was constructed and showed that LAP3 was the strongest independent predictor of survival (P = 0.001) (Table 2).
Figure 2.
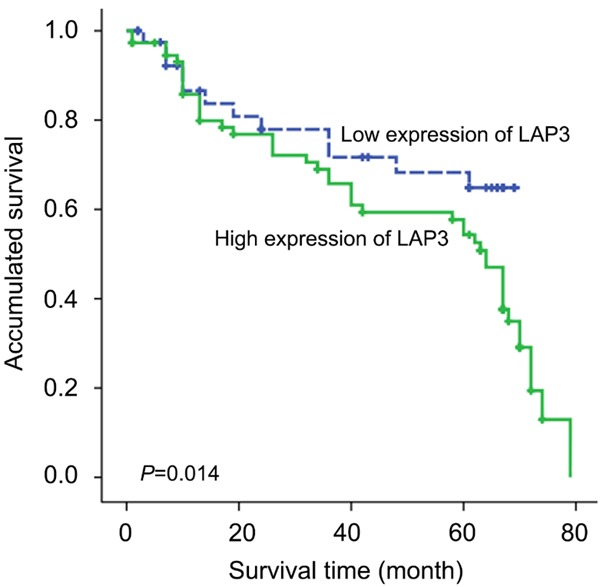
Kaplan-Meier survival curves for low versus high LAP3 expression in 115 patients with HCC show a highly significant separation between curves (P<0.05, log-rank test).
Table 2.
Contribution of various potential prognostic factors to survival by Cox regression analysis in 115 HCC specimens
| Parameters | Hazard ratio | 95% Confidenece interval | P value |
|---|---|---|---|
| Age (years) | 0.321 | 0.309-0.970 | 0.548 |
| Gender | 0.497 | 0.404-2.479 | 0.413 |
| Serum AFP Leve (ng/ml) | 0.294 | 0.560-1.727 | 0.953 |
| Cirrhosis | 1.430 | 1.198-5.176 | 0.373 |
| Maximal Tumor Size (cm) | 0.845 | 0.988-3.120 | 0.055 |
| Tumor Metastasis | 2.608 | 0.066-1.296 | 0.033* |
| HBs Ag | 0.365 | 0.421-1.712 | 0.646 |
| Histological Grade | 3.762 | 0.084-1.428 | 0.018* |
| LAP3 | 4.745 | 1.416-9.084 | 0.001* |
| Ki-67 | 2.589 | 0.009-1.200 | 0.048* |
Statistical analyses were performed using Cox regression analysis.
P<0.05 was considered significant.
Knockdown of LAP3 inhibited HCC cells viability and migration in vitro
To investigate whether LAP3 could influence the tumorigenesis of HCC, shRNA-LAP3 vector was stably transfected into HCC cells. HCC cell line HepG2, which expresses a high level of endogenous LAP3 (Figure 1B), was used in the shRNA experiment. Firstly, we examined the efficiency of LAP3 gene silencing (Figure 3A). Since LAP3 was reported to promote G1/S transition and enhance cell motility and invasiveness in esophageal squamous cell carcinoma (ESCC) [11], we also wonder whether LAP3 has a tumorigenesis potential via promoting cell cycle and/or cell motility. Thus a common proliferation marker PCNA [15], several key cell cycle regulators including CDK2, CDK6 and CyclinA as well as E-cadherin which is a retrorse marker of metastasis [16] were tested. Decreased expression of PCNA, CDK2, CDK6 and Cyclin A as well as increased expression of E-cadherin were detected in LAP3-silencing cells (Figure 3A). Furthermore, the tumorigenic function of LAP3 was assessed by cell viability assays and flow cytometry assays. Figure 3B showed that the cell growth rates in LAP3-silencing cells were significantly attenuated compared with control cells, while the percentage of LAP3-silencing cells in G1 phase was obviously increased in comparison with control cells (Figure 3C). These data all confirmed that knockdown of LAP3 could inhibit HCC cells viability via restrain G1/S transition.
Figure 3.
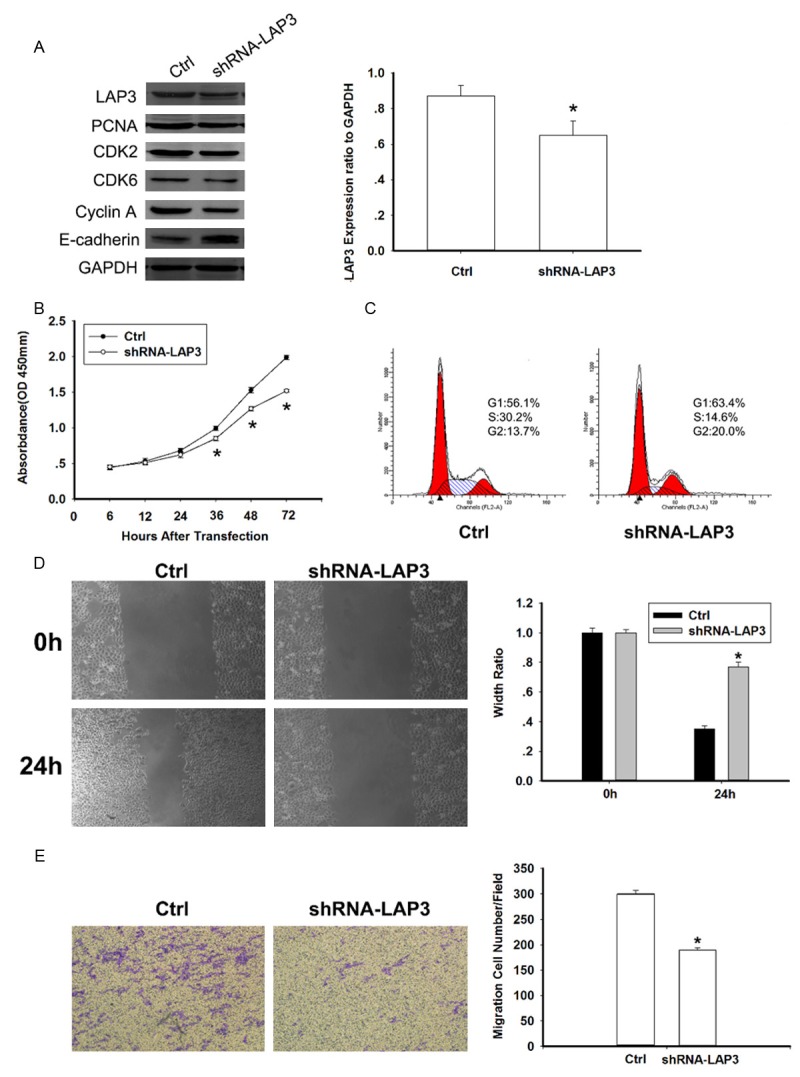
Knockdown of LAP3 inhibited HepG2 cells viability and migration in vitro. A. Western blot analysis of LAP3, PCNA, CDK2, CDK6, Cyclin A, E-cadherin, GAPDH (loading control) in control and shRNA-LAP3 HepG2 cells. The bar chart showed LAP3 expression ratio to GAPDH. The data are mean ± SEM (n = 3, *P<0.01, compared with control). B. Effect of LAP3 knock-down on proliferation of HepG2 cells, analyzed by the cell viability assay. Data are representative of three independent experiments. *P<0.05. C. Knockdown of LAP3 could restrain G1/S transition in HepG2 cells. The cell cycle distribution of HepG2 cells detected by flow cytometric analysis. D. Wound healing assays with negative control and shRNA-LAP3 HepG2 cells. Migration of the cells to the wound was visualized at 0, 48 h with an inverted Leica phase-contrast microscope. The bar chart showed the relative migration distance of cells. The data are mean ± SEM (n = 3, *P<0.05, compared with 0 h). E. Knockdown of LAP3 suppressed cell migration by transwell assays. For transwell assays, control and shRNA-LAP3 HepG2 cells were seeded into the upper chamber of the transwell and the cells that migrated through the pores to the lower surface of the filter were counted in 10 fields under 20× objective lens. The bar chart showed the percentage of migrant cells. The data are mean ± SEM (n = 3, *P<0.01, compared with control).
Since overexpression of LAP3 was closely associated with HCC metastasis by IHC analysis and E-cadherin expression was increased in the LAP3-silencing cells (Figure 3A), wound-healing (Figure 3D) and matrigel invasion (Figure 3E) assays were performed to explore the effects of LAP3 on HCC cell migration and invasion. As expect, the two experiments both showed that knockdown of LAP3 could attenuated the migration and invasiveness of HCC cells.
Overexpression of LAP3 promotes HCC cell proliferation and migration abilities in vitro
Based on the above data, we suspected whether overexpression of LAP3 expression could promote HCC cell proliferation and migration abilities. The Huh7 cell line, which had a low level of LAP3, was transfected with Myc-LAP3 (Figure 4A). Being consistent with Figure 3A, PCNA expression was increased in cells transfected with Myc-LAP3 compared with controlled cells while E-cadherin abundance was attenuated (Figure 4A). Overexpression of LAP3 could significantly promote the proliferative ability in Huh7 cells (Figure 4B) and promote G1/S transition in comparison with control cells (Figure 4C). Moreover, the migration ability was markedly advanced in Huh7 cells transfected with Myc-LAP3 (Figure 4D and 4E).
Figure 4.

Overexpression of LAP3 promotes Huh7 cell proliferation and migration abilities in vitro. A. Western blot analysis of LAP3, PCNA, E-cadherin, GAPDH (loading control) in control and myc-LAP3 Huh7 cells. The bar chart showed LAP3 expression ratio to GAPDH. The data are mean ± SEM (n = 3, *P<0.01, compared with control). B. Effect of LAP3 up-regulation on proliferation of Huh7 cells, analyzed by the cell viability assay. Data are representative of three independent experiments. *, P<0.05. C. Up-regulation of LAP3 could promote G1/S transition in Huh7 cells. The cell cycle distribution of Huh7 cells detected by flow cytometric analysis. D. Wound healing assays with negative control and myc-LAP3 Huh7 cells. Migration of the cells to the wound was visualized at 0, 48 h with an inverted Leica phase-contrast microscope. The bar chart showed the relative migration distance of cells. The data are mean ± SEM (n = 3, *P<0.05, compared with 0 h). E. Up-regulation of LAP3 promoted cell migration by transwell assays. For transwell assays, control and myc-LAP3 Huh7 cells were seeded into the upper chamber of the transwell and the cells that migrated through the pores to the lower surface of the filter were counted in 10 fields under 20× objective lens. The bar chart showed the percentage of migrant cells. The data are mean ± SEM (n = 3, *P<0.01, compared with control).
Downregulation of LAP3 increased the sensitivity of HCC cells to cisplatin
Chemotherapy plays a crucial role in HCC treatment especially for advanced HCC patients [17]. Cisplatin is one of the most commonly used chemotherapeutic drugs for the treatment of HCC [18]. Thus, we examined whether cisplatincould influence the expression of LAP3 in HCC cells. Exposure of HepG2 cells to cisplatin for 48 h induced a dose-dependent decrease in the LAP3 protein level in HepG2 cells, detected by western blot, while the apoptosis marker, cleaved-caspase 3 was showed a reverse trend (Figure 5A). Furthermore, the down-regulation of the LAP3 expression induced by cisplatin was enhanced in LAP3-silencing HepG2 cells (Figure 5B). After cisplatin treatment, the cell death of LAP3-silencing cells was increased in comparison with that of control cells (Figure 5C). Our data strongly indicated that downregulation of LAP3 increased the sensitivity of HCC cells to cisplatin, thus promoting thecell death of HCC cells.
Figure 5.
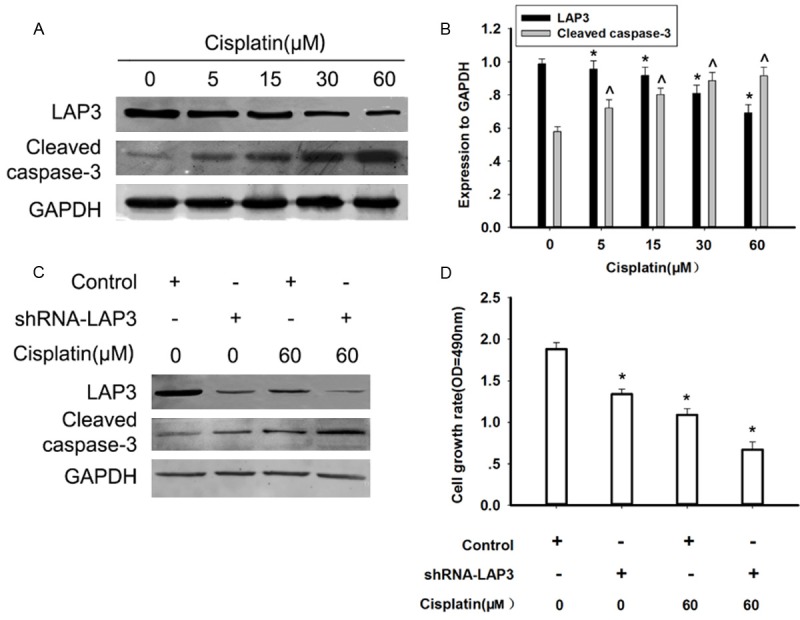
Downregulation of LAP3 increased the sensitivity of HCC cells to cisplatin. A. Western blot analysis indicated the dose-dependent effect of cisplatin on the LAP3 expressions in HepG2 cells, which were treated by cisplatin at different concentrations for 48 h. GAPDH was used as a control for protein load and integrity. B. The bar chart showed the expression ratio to GAPDH. The data are mean ± SEM (n = 3, *P<0.01, compared with 0h). C. Western blot analysis indicated the effects of cisplatin on the LAP3 expression in HepG2 cells, which were transfected with control or shRNA-LAP3 for 24 h, respectively, and then subjected to treatment with or without cisplatin for 48 h, respectively. GAPDH was used as a control for protein load and integrity. D. A bar chart showed that the comparison in the cell growth rate in HepG2 cells, which were transfected with control or shRNA-LAP3 for 24 h, respectively, and then subjected to treatment with or without cisplatin for 48 h, respectively, as measured by cell viability assays. Data were presented as mean ± SEM. *P<0.05, versus controls (non-treated with cisplatin), respectively.
Discussion
Being the fifth most common malignancy and the third leading cause of cancer-related mortality globally, hepatocellular carcinoma (HCC) is a prevalent problem worldwide [19]. Although the identification of many prognostic markers of HCC has been made, the available markers are still unable to predict the survival of patients accurately [20,21]. Furthermore, effective treatments for HCC are essentially absent [22]. These all emphasize both the potential for novel prognostic markers of HCC and the need to develop new therapies [23].
In current study, we proved that LAP3 was upregulated in human HCC tissues relative to adjacent noncancerous tissues by IHC analysis. Overexpression of LAP3 was siginificantly correlated with histological grade, lymph node metastasis and Ki-67, a proliferating cell marker [24]. And LAP3 expression was an independent risk factor for survival of HCC patients. Our data firstly demonstrated that LAP3 contributed to the malignant progression of HCC and might be a novel meaningful prognostic biomarker.
Several reports showed that increased expression of LAP3 protein could enhance malignancy. LAP3 promotes the cell cycle progression of endothelial cells and angiogenesis [25]. Upregulation of LAP3 plays a crucial role in the malignant development of human ESCC [11]. Placental LAP3 (P-LAP3) is also involved in progression of breast cancer [10] and ovarian epithelial malignancy [8] as well as diabetes [12,13]. In addition, adipocyte-derived LAP3 (A-LAP3) is a biomarker for the evaluation of endometrial carcinoma [9]. These reports all suggested that the over-expression of LAP3 protein might affect tumor development. Our clinical data forcefully implied that LAP3 over-expression was significantly associated with the histological grade and tumor metastasis in HCC patients. Hence, these results suggested that LAP3 might contribute to the malignant development of HCC.
To further investigate the role of LAP3 in the development and progression of HCC, we silence the LAP3 expression in HepG2 cells and found that growth rate of cells transfected with shRNA-LAP3 was significantly decelerated compared with control cells. Further study revealed that knockdown of LAP3 in HCC cells HepG2 induced the cell cycle arrest at G1/S checkpoint. While overexpression of LAP3 promoted tumorigenesis in Huh7 cells via accelerating G1/S phase transition. G1/S phase transition is a major checkpoint for cell cycle progression [26]. CDK2 and CDK6 are two of the critical positive regulators during this transition [27]. Moreover, PCNA is a nuclear polypeptide essential for DNA synthesis and replication of mammalian cells, which is commonly used as a proliferation marker [15]. Alteration of LAP3 expression in HCC cells gives rise to the changes of PCNA, CDK2 and CDK6 expression in accord with LAP3. This phenomenon reconfirmed our hypothesis that LAP3 could contribute to HCC malignancy via promoting G1/S phase transition. And the flow cytometry assays also authenticated this.
Meanwhile, our results also revealed that LAP3 could furtherance cell motility and invasiveness of HCC cells in vitro. IHC analysis showed that LAP3 was markedly associated with HCC metastasis and knockdown LAP3 increased expression of E-cadherin. E-cadherin is a cardinal regulator of epithelial-to-mesenchymal transition (EMT) and plays a critical role in tumour invasion [11]. Low E-cadherin expression correlate with an aggressive, malignant phenotype in HCC [28]. Upregulation of LAP3 induced low expression of E-cadherin and advanced the mobility of HCC cells. Conversely, when we silenced the endogenous LAP3 by shRNA in HCC cells, the metastatic potential of HCC cells was significantly depressed, suggesting that LAP3 might alter HCC cells invasion and metastasis.
Chemotherapy is the best option for advanced liver cancer [17] and cisplatin is a potent chemotherapeutic agent used to treat a wide range of human malignancies, including HCC [18]. The cytotoxicity of cisplatin is mediated mainly through the apoptotic pathways [29]. Caspase family plays a significant role in apoptosis and caspase-3 is one of the most important member [30]. Our data showed that shRNA-LAP3 markedly promoted the cell death in HCC cells treated with cisplatin, suggesting knockdown LAP3 will enhance the sensitivity of HCC cells to cisplatin.
In summary, at the first time, our study demonstrate that LAP3 is expressed in HCC tissues and cells and provide clear evidence for the importance role of LAP3 in HCC development and metastasis. Targeting this protein may be of great value in the treatment of HCC.
Disclosure of conflict of interest
None.
References
- 1.Zhou P, Wu LL, Wu KM, Jiang W, Li JD, Zhou LD, Li XY, Chang S, Huang Y, Tan H, Zhang GW, He F, Wang ZM. Overexpression of MMSET is correlation with poor prognosis in hepatocellular carcinoma. Pathol Oncol Res. 2013;19:303–309. doi: 10.1007/s12253-012-9583-z. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 4.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 5.Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573–588. doi: 10.2174/1566524033479546. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Tanaka H, Hammock BD, Morisseau C. Novel and highly sensitive fluorescent assay for leucine aminopeptidases. Anal Biochem. 2009;391:11–16. doi: 10.1016/j.ab.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujimoto M, Goto Y, Maruyama M, Hattori A. Biochemical and enzymatic properties of the M1 family of aminopeptidases involved in the regulation of blood pressure. Heart Fail Rev. 2008;13:285–291. doi: 10.1007/s10741-007-9064-8. [DOI] [PubMed] [Google Scholar]
- 8.Mizutani S, Shibata K, Kikkawa F, Hattori A, Tsujimoto M, Ishii M, Kobayashi H. Essential role of placental leucine aminopeptidase in gynecologic malignancy. Expert Opin Ther Targets. 2007;11:453–461. doi: 10.1517/14728222.11.4.453. [DOI] [PubMed] [Google Scholar]
- 9.Shibata K, Kikkawa F, Mizokami Y, Kajiyama H, Ino K, Nomura S, Mizutani S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumour Biol. 2005;26:9–16. doi: 10.1159/000084181. [DOI] [PubMed] [Google Scholar]
- 10.Pilar Carrera M, Ramirez-Exposito MJ, Duenas B, Dolores Mayas M, Jesus Garcia M, De la Chica S, Cortes P, Ruiz-Sanjuan M, Martinez-Martos JM. Insulin-regulated aminopeptidase/placental leucil Aminopeptidase (IRAP/P-lAP) and angiotensin IV-forming activities are modified in serum of rats with breast cancer induced by N-methyl-nitrosourea. Anticancer Res. 2006;26:1011–1014. [PubMed] [Google Scholar]
- 11.Zhang S, Yang X, Shi H, Li M, Xue Q, Ren H, Yao L, Chen X, Zhang J, Wang H. Overexpression of leucine aminopeptidase 3 contributes to malignant development of human esophageal squamous cell carcinoma. J Mol Histol. 2014;45:283–292. doi: 10.1007/s10735-014-9566-3. [DOI] [PubMed] [Google Scholar]
- 12.Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- 13.Keller SR. Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes. Biol Pharm Bull. 2004;27:761–764. doi: 10.1248/bpb.27.761. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Jiang F, Bi X, Zhang YQ. Drosophila FMRP participates in the DNA damage response by regulating G2/M cell cycle checkpoint and apoptosis. Hum Mol Genet. 2012;21:4655–4668. doi: 10.1093/hmg/dds307. [DOI] [PubMed] [Google Scholar]
- 15.Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, Vermeulen W. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol. 2005;25:9350–9359. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka H, Shiozaki H, Kobayashi K, Tahara H, Kobayashi T, Takatsuka Y, Mori T. [Correlation between E-cadherin expression and metastasis in human breast cancer: preliminary report] . Nihon Geka Gakkai Zasshi. 1992;93:105. [PubMed] [Google Scholar]
- 17.Li Z, Tu M, Han B, Gu Y, Xue X, Sun J, Ge Q, Miao Y, Qian Z, Gao W. Vasohibin 2 decreases the cisplatin sensitivity of hepatocarcinoma cell line by downregulating p53. PLoS One. 2014;9:e90358. doi: 10.1371/journal.pone.0090358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin J, Luo M, Qian H, Chen W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene. 2014;538:342–347. doi: 10.1016/j.gene.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Cui X, Hu B, Lu C, Huang X, Cai J, He S, Lv L, Cong X, Liu G, Zhang Y, Ni R. Upregulated expression of CAP1 is associated with tumor migration and metastasis in hepatocellular carcinoma. Pathol Res Pract. 2014;210:169–175. doi: 10.1016/j.prp.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Wang XM, Yang LY, Guo L, Fan C, Wu F. p53-induced RING-H2 protein, a novel marker for poor survival in hepatocellular carcinoma after hepatic resection. Cancer. 2009;115:4554–4563. doi: 10.1002/cncr.24494. [DOI] [PubMed] [Google Scholar]
- 21.Makuuchi M, Imamura H, Sugawara Y, Takayama T. Progress in surgical treatment of hepatocellular carcinoma. Oncology. 2002;62(Suppl 1):74–81. doi: 10.1159/000048280. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Qian X, Cheng C, He S, Sun L, Ke Q, Zhang L, Pan X, He F, Wang Q, Meng J, Ni R, Shen A. Expression of Pirh2, a p27 (Kip1) ubiquitin ligase, in hepatocellular carcinoma: correlation with p27 (Kip1) and cell proliferation. Hum Pathol. 2011;42:507–515. doi: 10.1016/j.humpath.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, Muthusamy N, Byrd JC, Cheng AL, Chen CS. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204–1212. doi: 10.1158/0008-5472.CAN-07-2621. [DOI] [PubMed] [Google Scholar]
- 24.Vijayachandra K, Higgins W, Lee J, Glick A. Induction of p16ink4a and p19ARF by TGFbeta1 contributes to growth arrest and senescence response in mouse keratinocytes. Mol Carcinog. 2009;48:181–186. doi: 10.1002/mc.20472. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T, Akada T, Niizeki O, Suzuki T, Miyashita H, Sato Y. Puromycin-insensitive leucyl-specific aminopeptidase (PILSAP) binds and catalyzes PDK1, allowing VEGF-stimulated activation of S6K for endothelial cell proliferation and angiogenesis. Blood. 2004;104:2345–2352. doi: 10.1182/blood-2003-12-4260. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Heuvel S. Cell-cycle regulation. WormBook. 2005:1–16. doi: 10.1895/wormbook.1.28.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai X, Zhu H, Wang W, Zhang S, Zhang Y, Mao G. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31:970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]
- 29.Hong JY, Kim GH, Kim JW, Kwon SS, Sato EF, Cho KH, Shim EB. Computational modeling of apoptotic signaling pathways induced by cisplatin. BMC Syst Biol. 2012;6:122. doi: 10.1186/1752-0509-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]


