Abstract
Propranolol has been widely used in treating infantile hemangiomas (IHs). But recurrence of IHs was found in some cases on cessation of propranolol treatment. The other is that Chinese individuals reacted to propranolol differently from American Whites. Whether the difference of sensitivity is due to the β adrenoceptor (β-AR) expression pattern of hemangioma initiating cells remains unclear. In the present study, we isolated hemangioma-derived stem cells (hemSCs) from proliferative IHs and analyzed the biological characteristics and β-AR expression pattern of hemSCs by immunostaining, Western blotting and multilineage differentiation assay as well. We also tested the effects of propranolol on hemSCs by evaluating VEGF expression, proliferation and apoptosis related parameters. Our results indicated that CD133+ hemSCs located pre-vascular in proiferative IH tissues. Both β1 and β2-AR were expressed, while β2-AR was dominant on hemSCs. Propranolol at 100-150 μM inhibited proliferation of hemSCs, not did 50 μM. Propranolol down-regulated VEGF expression of hemSCs, instead of inducing apoptosis. The adipogenic potential was enhanced by propranolol. Therefore, our current results suggested propranolol could not induce apoptosis of hemSCs, but played a curative role though suppressing VEGF synthesis and enhancement of adipogenesis of hemSCs. Our results might partially provide the insight of mechanism of relapse in some cases on cessation of propranolol treatment.
Keywords: Propranolol, hemangioma-stem cells, relapse, β adrenoceptor
Introduction
Infantile hemangiomas (IHs) are the most common vascular tumors of infancy, with an estimated worldwide prevalence of 1-10% in newborns and infants. The morbidity is preponderant in Caucasian (compared with blacks), female (compared with males) and premature (compared with full-term) infants [1]. The difference in morbidity was proposed to be relative with renin level between races. However, there was no accurate IHs morbidity in China until now. The nature pathological course of IH could be divided into three phases. The first phase is proliferative phase during the first half year of life, where IHs initiate and grow rapidly. Following proliferative phase is involuting phase, where tumor growth slows and vessels become prominent. Then IHs turn into involuted phase, where fibrofatty tissue finally replaces the tumor mass.
Although most IHs are benign and self-limiting in nature, a significant minority involve complications, which may be severe or life-threatening. In a prospective cohort study at seven US pediatric dermatology clinics, with a consecutive sample of 1,058 children, 24% of patients were found to experience complications related to IHs [2]. Since the initial successful report of treating IHs with propranolol [3], a non-selective β blocker used to treat hypertension, angina pectoris and myocardial infarction, numerous studies have confirmed this treatment as being superior to corticosteroids and recommend propranolol as the first-line treatment for problematic IHs. We started to treat IHs with low-dose propranolol since 2009 and acquired satisfied outcome [4].
Propranolol could inhibit proliferation, migration and induce apoptosis of hemangiomas endothelial cell (HemECs), mediated through suppression of VEGF expression, activation of caspase-9 and caspase-3, up-regulation or down-regulation of the pro-apoptotic or anti-apoptotic gene [5]. Even the mechanism of propranolol on HemECs is widely investigated and accepted, some phenomenon still puzzled clinicians in clinical work. The reasonable explanation for regrowth of IHs occurred in approximately 19% of cases on cessation of propranolol treatment has not been well answered [6,7].
Hemangioma stem cells (HemSCs) attract attention of many researchers and are sometimes recognized as the chief culprit of IHs. The CD133+ HemSCs underwent adipogenic differentiation during the involuting phase, gave rise to fibrofatty tissue [8-10]. In previous studies, propranolol was demonstrated to accelerate adipogenesis of HemSCs derived from a 6-month-old American child [11]. Since the morbidity differed between races and Chinese individuals reacted to propranolol differently from American Whites [12,13], we wondered whether the β AR expression of HemSCs from Chinese is similar to that of Caucasian and whether propranolol play the same role on HemSCs derived from Chinese individuals. However, it lacks related investigations. In the present study, we focused on the effect of propranolol on HemSCs and aimed to reveal whether propranolol induce apoptosis and enhance adipogenic differentiation in vitro.
Material and methods
Ethics statement
All parents whose infants participated in this study were informed of the research purpose and protocol, provided with written informed consent as well. The protocol to use discarded IHs tissue samples for subsequent cell isolation and culture was approved by ethics committee of Shanghai Jiaotong University School of Medicine.
Isolation and culture of hemSCs
The proliferating IHs tissues resected from patients were immediately immersed into 4°C growth medium {Dulbecco’s modified Eagle’s medium (DMEM)/high glucose, 10% Fetal Bovine Serum (FBS), 1% Glutamine, 1% Nonessential amino acid solution (NEAA) and 1% Penicillin-Streptomycin (all from Invitrogen)} and taken to our laboratory. Then resected the fat or skin tissue, rinsed the samples in phosphate buffered saline (PBS) three times and minced them. 0.05% Trypsin/0.53 mM EDTA (Invitrogen) was used to digest the sample at 37°C for 30 min and then transferred the tissues into DMEM with 1 mg/ml collagenase A for 1 hours. Stopped digestion by equal volume growth medium and filtered the suspension by 70 μm cell strainer (BD) to get single-cell suspension. CD133 MicroBead Kit (Miltenyi Biotec) was used to sort the CD133+ hemSCs according manufacturer’s instruction. The sorted CD133+ hemSCs were planted into Petri tissue culture dishes (Coring) and incubated in growth medium at 37°C in a 5% CO2 and 95% humidity chamber. Human umbilical vein endothelial cells (HUVECs), purchased from ATCC, cultured in the same condition and served as control.
Immunostaining
Cryosections or cells cultured on cover slips were washed with PBS and fixed with 4% paraformaldehyde for 20 min, followed by three washes with PBS for 5 min each. Then blocked the samples with 3% bovine serum albumin (BSA; Sigma-Aldrich) for 30 min at room temperature and washed again in PBS. The samples were then incubated with primary antibodies {rabbit anti-human CD133 (1:100; Abcam); mouse anti-human glucose transporter-1 (Glut-1,1:100; Abcam); mouse anti-human Vimentin (1:100; EMD Millipore); mouse anti-human VEGF (1:10; R&D Systems); mouse anti-human α-SMA (1:100; Abcam)} overnight at 4°C. After three washes, the samples were incubated with Cy3- or FITC-conjugated anti-mouse or rabbit IgG antibody (1:100; Jackson ImmunoResearch) for 2 h at room temperature. In the cryosections, tissues were double stained with CD133/Glut-1, CD133/Vimentin, CD133/VEGF and CD133/α-SMA. The nuclei were counterstained with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI, 5 mg/ml; Roche). Samples were observed using a Fluoview scanning confocal microscope (Olympus). Images were acquired and stored using FV1000 Viewer software.
Multilineage differentiation potential assays of hemSCs
HemSCs at 60-70% confluence were switched to osteogenic and adipogenic media, according to previously established methods [9]. Osteogenic and adipogenic differentiation potential was assessed by alizarin red S staining and Oil red O staining, using Osteogenesis Assay Kit (Millipore) and Adipogenesis Assay Kit (Chemicom), respectively.
Assay of proliferation and apoptosis effect of propranolol on hemSCs
MTT assay was performed to test viability of hemSCs. HemSCs were seeded at 5000/well in a 96-well plate and incubated in 100 μl growth medium for 24 h. Then propranolol (Sigma-Aldrich) was added with various concentrations (0, 50, 100 and 150 μM) for 24, 48 and 96 hours. The cells were used for determination of viability, using the MTT Cell Proliferation and Cytotoxicity Assay Kit (Beyotime) and the following calculation: Viability rate (%) = [(A570 for experimental group-A570 for blank control group)/(A570 for control group-A570 for blank control group-A570 for blank control group)] X 100. The MTT assay experiment was duplicated six times. Apoptosis effect of propranolol on hemSCs was assessed by Western blotting as described below.
Western blotting
Protein assay by Western blotting was used to determine β AR expression of HemSCs and detect the apoptosis effect of propranolol on hemSCs according the protocol previous reported [14]. The primary antibodies included rabbit anti-human β1 and β2 AR (Novus Biologicals), rabbit anti human Caspase 3 and Caspase 9, rabbit anti human Bcl-2 (Cell Signaling Technology), and rabbit anti human VEGF (EMD Millopore). Bands were visualized and analyzed by Bio-Rad Molecular Imager system and Image Lab 2.0 Software (Bio-Rad, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells with Trizol (Invitrogen). The first strand of cDNA was synthesized from 6 mg total RNA, using Reverse Transcriptase M-MLV, and PCR was performed with Ex Taq polymerase (Takara), according to the manufacturer’s instructions. For every reaction, 1.5 μl of the first strand cDNA was used as a template, and PCR was carried out using the following cycles: initial denaturation at 94°C for 4 min, denaturation at 94°C for 30 s, annealing at the primer-specific temperature for 30 s and extension at 72°C for 30 s. The final extension was performed at 72°C for 10 min. The level of GAPDH mRNA was quantified as an internal control. Primers for PCR were listed in Table 1. The experiment was duplicated three times.
Table 1.
RT-PCR primer sequences
| Gene (Accession No.) | Primer sequences (5’-3’) |
|---|---|
| Osteonectin (NM_003118.3) | Forward: GATGAGGACAACAACCTTCTGAC |
| Reverse: TTAGATCACAAGATCCTTGTCGAT | |
| RUNX2 (NM_006715232.1) | Forward: ACTGGGCCCTTTTTCAGA |
| Reverse: GCGCAAGCATTCTGGAA | |
| PPARγ (NM_015869.4) | Forward: GCTGTTATGGGTGAAACTCTG |
| Reverse: TCGCAGGCTCTTTAGAAACTC | |
| C/EBPβ (NM_005194.3) | Forward: TTTCGAAGTTGATGCAATCG |
| Reverse: CAACAAGCCCGTAGGAACAT | |
| RXRγ (NM_001256571.1) | Forward: TGTGGTCAACAGTGTCAGCA |
| Reverse: GTCTCCACAGATGGCACAGA | |
| GAPDH (NM_002046.3) | Forward: AGCCACATCGCTCAGACACC |
| Reverse: GTACTCAGCGCCAGCATCG |
Statistical analysis
All values are shown as mean ± sd. The differences were evaluated using Student’s t-tests with SPSS statistics software. A statistical threshold of P < 0.05 was used to detect whether there were statistical significances among different groups.
Results
HemSCs located per-vascular in proliferating IHs tissues
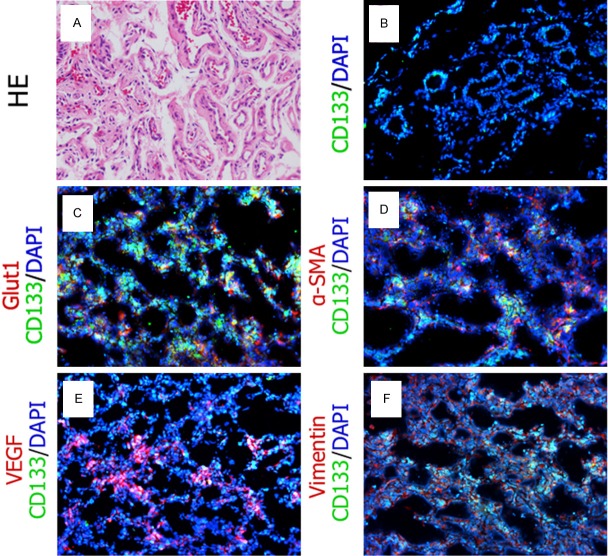
Eight proliferating IHs tissue samples were obtained from surgerical resection totally. HE staining demonstrated that IH tissue consisted of large amount of microvessels (Figure 1A). The CD133 positive hemSCs mostly located pre-vascular (Figure 1B). Double immunofluorescence staining indicated part of CD133+ hemSCs co-expressed Glut1, the diagnostic marker of proliferating IHs (Figure 1C). α-SMA, Vimentin and VEGF also co-expressed on CD133+ hemSCs (Figure 1D-F).
Figure 1.
HE staining and immunofluorescence staining of proliferating IHs. (A) Microvessels were enriched in the proliferating IHs. Immunofluorescence staining showed CD133 positive (Green) hemSCs located pre-vascular (B). Part of CD133 positive (Green) hemSCs co-expressed Glut1 (C, red), α-SMA (D, red), VEGF (E, red) and vimentin (F, red). The nuclei were counterstained with DAPI (Blue).
Biological characteristics and multilineage differentiation potential of hemSCs
The cultured sorted CD133+ hemSCs had spindle-shaped morphology (Figure 2A). HemSCs kept CD133 expressing despite in vitro culture, excluded lose of their stemness (Figure 2B). Glut1, the diagnostic marker of proliferating IHs, and Vimentin, mesenchymal stem cell marker, expressed on most cultured hemSCs, while α-SMA and VEGF could be detected on part of hemSCs (Figure 2C-F). The growth curve revealed that hemSCs proliferated somewhat sluggishly compared with HUVECs (Figure 2G). Since Chinese individuals reacted to propranolol differently from American Whites, we wondered whether the β-AR expression pattern is different from that of Whites. Thus, western blot was preformed to analysis the β-AR expression of HemSCs. The outcome showed that hemSCs expressed both β1-AR and β2-AR with a dominance of β2-AR. However, the two type AR expression was similar to each other in HUVECs. Multilineage differentiation potential was determined after 5 days’ lineage-specific induction. Calcium deposit and moderate cytoplasmic lipid vacuoles could be observed after Alizarin Red S staining and Oil Red O staining, respectively.
Figure 2.
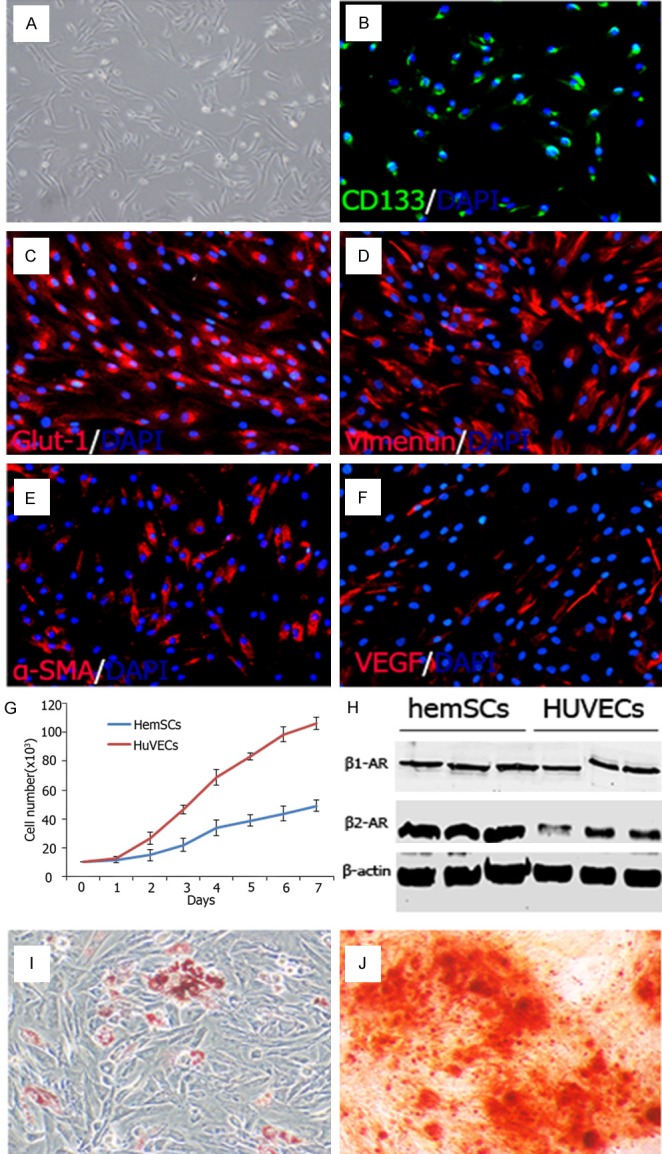
Biological characteristics of hemSCs. A: The morphology of CD133+ hemSCs. B-F: The CD133+ hemSCs expressed Glut-1, Vimentin, α-SMA and VEGF. G: The growth curve revealed that hemSCs proliferated sluggishly compared with HUVECs. H: Western blot showed hemSCs expressed both β1-AR and β2-AR with a dominance of β2-AR, while HUVECs expressed β1-AR and β2-AR equally. I, J: Adipogenic and osteogenic differentiation potential of hemSCs were confirmed by Oil red O staining and alizarin red S staining.
Propranolol inhibited hemSCs proliferation instead of inducing apoptosis
The propranolol concentration was selected based on the previous published studies from which propranolol had statistically different effect on hemECs [5,11]. As showed in Figure 3, propranolol at 50 μM didn’t suppress growth of hemSCs, but also accelerated proliferation rate during the 96 hours’ incubation, even there was no significant statistically difference compared with the control group (P > 0.05). The proliferation was inhibited by propranolol at 100 and 150 μM (P < 0.05). However, the total live cell number at each time point exceeded the number at seeding. The shortage cell number of 100 μM and 150 μM groups may due to apoptosis of hemSCs. For discovering whether apoptosis dominated the cell shortage, protein analysis was used to confirm apoptosis of hemSCs. In accordance with the outcome of MTT assay, no obvious apoptosis was found. Caspase 3 and caspase 9 did not activated 96 hours after gradient propranolol incubation (Figure 4). The anti-apoptosis protein Bcl-2 also expressed at a stable level (Figure 4).
Figure 3.
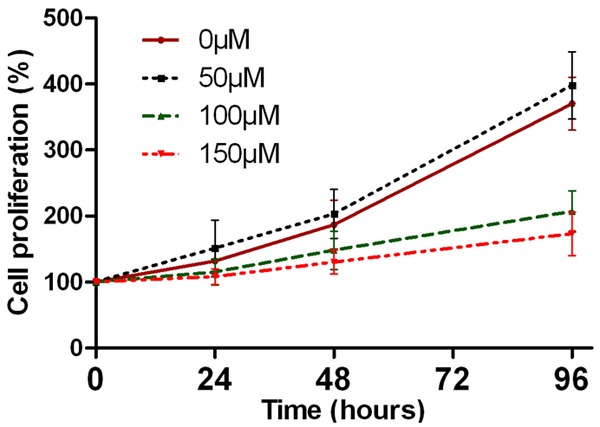
Proliferation of propranolol-treated HemSCs was analyzed by MTT. The cell proliferation was inhibited by 100-150 μM propranolol from 48 hours to 96 hours (P < 0.05). However, 50 μM propranolol stimulated the proliferation of hemSCs, even there was no statistically difference compared with control (P > 0.05).
Figure 4.
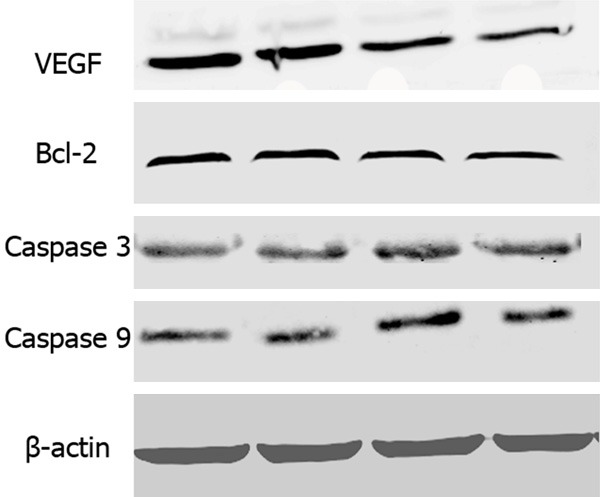
Western blotting showing expression of VEGF and apoptosis related proteins in HemSCs. Compared with the control groups, VEGF expression was down-regulated after 96 hours’ propranolol incubation. On the other hand, expression of anti-apoptotic protein (Bcl-2) and pro-apoptotic proteins (caspase 3 and caspase 9) was not significantly up-regulated or down-regulated.
Propranolol reduced VEGF expression and enhanced adipogenesis of hemSCs
After 96 hours’ propranolol incubation, VEGF expression was suppressed in a dose-dependent manner (Figure 4 Upper panel), in accordance with the result found in hemECs [5,15]. Osteogenic and adipogenic differentiation potential was assessed again in differentiation media with additional supplement of 100 μM propranolol. On the 5th day after differentiation, Oil Red O staining demonstrated robust lipid vacuoles in the cytoplasm of hemSCs (Figure 5A) compared with that without propranolol treatment (Figure 2I). The adipogenesis specific mRNA including PPRAγ, C/EBPβ and RXR γ transcribed at high level (Figure 5C). Osteogenic differentiation potential decreased compared with that without propranolol treatment (Figures 2J, 5B).
Figure 5.
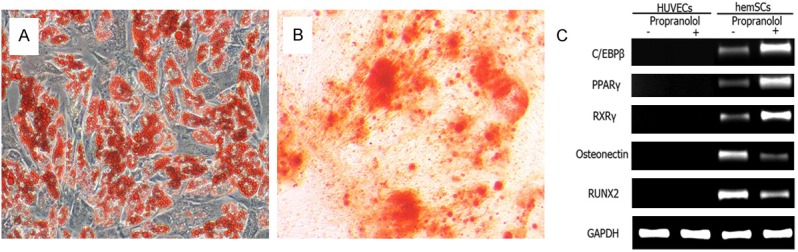
Adipogenic and osteogenic differentiation potential of hemSCs after propranolol treatment. A: On the 5th day after differentiation, robust lipid vacuoles were observed in the cytoplasm of hemSCs, compared with that of control groups. B: Calcium deposit was reduced after propranolol treatment, compared with that of control groups. C: RT-PCR also indicated the up-regulation of adipogenic genes and down-regulation of osteogenic genes.
Discussion
Propranolol has been used to treat infantile hemangiomas for 6 years and acquired satisfactory outcomes, especially in treating complicated or life-threatening cases, such as airway hemangiomas [16], ulcered [17] and visceral lesions [18,19]. Propranolol, the non-selective β blocker, played a role of treating IHs via vasoconstriction, inhibiting VEGF and b-FGF secretion and inducing apoptosis of HemECs. However, the sensitivity to β-blocking effects differed from races. Chinese individuals have a twofold greater sensitivity to effects of propranolol than Whites [12]. In Chinese individuals, less propranolol bound to plasma protein results in a greater proportion of pharmacologically active, unbound drug in the plasma, which may contribute to the increased sensitivity to this agent. In our previous work, we utilized low-dose propranolol in treating IHs and acquired satisfied outcome [4], further confirmed the increased sensitivity. The β-AR is another factor which may contribute to high sensitivity of propranolol. The subtypes of β-AR have controversial (opposing or synergetic) effects. Kum et al. revealed that hemSCs mainly expressed β2- and β3-AR (from Canada individuals) and concluded that the effect of propranolol on hemSCs differed from that on hemECs [20]. Ji et al. have identified the β-AR expression pattern of hemECs from Chinese individuals [21]. Whether the β-AR expression pattern of hemSCs differs from Western country individuals remains unknown. Thus, we analyzed β-AR expression pattern from Chinese infants. Our results indicated the expression of both β1- and β2-AR, while β2-AR was dominant on hemSCs. However, whether insufficiency of propranolol in inducing apoptosis of hemSCs is due to the subtype β-AR expression remains unknown.
The first mechanisms of propranolol in treating IHs supposed by Leaute-Labreze et al. have been widely accepted [3]. Besides inducing hemECs apoptosis, propranolol also suppressed angiogenesis via inhibition proliferation, migration and differentiation of hemECs [11,22]. However, with the progresses in IHs researches, new mechanisms have rose and assist to explain some phenomenon. The hemECs only hold one third of total apoptotic cells in involuted phase. Thus, the other two third cells must been explored. Studying the effect of any drug on hemSCs is the refreshing and pathbreaking work. Greenberger et al. revealed corticosteroid suppressed VEGF production by hemSCs, but not hemECs, thus inhibited vasculogenesis [23]. This explained the reason why corticosteroid had no effect on partial patients. Relapse is the hard nut to crack after dose de-escalation or cessation. The hemangiomas-initiating cells including endothelial progenitor cells (EPCs) and HemSCs may be the culprits. The main blood vessel formation of IHs was vasculogenesis, in which EPCs were recruited to the lesions by cytokines and chemokines [24]. The effect of propranolol on EPCs was inhibiting stromal-cell-derived factor 1α-induced EPCs homing by suppressing the expression of CXCR4 through the Akt and MAPK pathways. But propranolol failed to inhibit proliferation of EPCs [25]. Concerning about hemSCs, propranolol at conventional concentration for research failed to induce them undertake apoptosis. Escape of HemSCs from apoptosis might due to induction of anti-apoptotic pathway. Our results that production of VEGF was suppressed by propranolol in a dose-dependent pattern, but apoptosis was not obviously induced further confirmed the present theory. Our results combined with the results from other researchers might also partly discover the cause of rebound growth of IHs occurred on cessation of propranolol.
However, besides hemSCs, hemECs and EPCs, they also other cellular components engaged in the whole journey of IHs [26]. In previous reports, macrophages have been proven to contribute to the progression of IHs [27]. Macrophages were activated by in situ M-CSF in proliferative IHs. During the proliferating phase, hemECs were surrounded by macrophages, which promoted hemECs proliferation through VEGF secretion. Mast cells were also present in IHs [28]. The total number of mast cells was highest during the involuting phase, less in the involuted phase, and least in the proliferative phase. Although mast cells might have proangiogenic role on hemECs proliferation, they also played a crucial role in regression of his [29,30]. Mast cells expressed high and homogeneous β2-AR and heterogeneous β1-AR, which indicted that mast cells may be the possible targets of propranolol. Accordingly, the unique role of mast cells in IHs remains unknown. Pericytes located surrounding the nascent vessels in IHs. This cell population possessed the properties of increased VEGF, decrease angiopoietin-1, increased proliferation and vessel formation ability. They interacted with surrounding hemSCs and played a porangiogenic role [31,32]. Propranolol inhibited the contractility of pericytes, which might contribute to the vasoconstriction and reduced blood flow in IHs. In summary, all these indefinite factors may result to ineffective propranolol therapy or regrowth in some cases and desire to further investigate.
Adipogenesis accompanied with the progress of IH involuting. The origin of adipocytes in involuted IH is unclear. Cells possessed characteristics of stem cells including hemSCs, pericytes [33], hematopoietic stem cell, bone marrow mesenchymal stem or surrounding fat stem cells may be engaged in process of adipogenesis. The adipogenic potential of hemSCs has been proved [8,9]. In vitro studies indicated that propranolol could modulate global gene expression patterns with particular affect on genes involved in lipid/sterol metabolism [34]. Considering the existence of higher propranolol sensitivity than the Whites and latent difference β-AR expression pattern, we estimated adipogenic differentiation ability of hemSCs from Chinese individuals. As showed in Figures 2 and 5, propranolol enhanced adipogenesis of hemSCs. Despite the difference between races, these results were consistent with previous studies.
In summary, the present study demonstrated the hemSCs from Chinese individuals expressed β1- and β2-AR, with a dominance of β2-AR. Propranolol (100-150 μM) inhibited proliferation and VEGF production of hemSCs, but could not induce apoptosis of this population. Propranolol might suppress IHs proliferating via enhance hemSCs adipogenesis. Even though, the mechanisms of propranolol are complicated and there remains many unsolved mysteries. All studies focused on a single cellular target of propranolol. Whether propranolol performs researchers’ supposed pathways when all cellular components unite to be a combat alliance remains unanswerable. The non-selective block effect of propranolol may have unexpectable and unacceptable influence on subjects. We have noticed the signs of growth retardation in some cases. However, the first reported IH cases treated by propranolol were about 6-7 years old and the final problems may have not risen now. The other special problem is that in the stressful medical environment in China, off-label usage of propranolol may result conflict between doctors and patients.
Acknowledgements
This research was funded by the Research Project of Shanghai Municipal Health Bureau (20114020).
Disclosure of conflict of interest
None.
References
- 1.Itinteang T, Brasch HD, Tan ST, Day DJ. Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. J Plast Reconstr Aesthet Surg. 2011;64:759–765. doi: 10.1016/j.bjps.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 3.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Zhao T, Xiao Y, Yu J, Chen H, Huang Y, Liu J, Lin J, Ouyang T. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. Eur J Pediatr. 2013;172:653–659. doi: 10.1007/s00431-012-1928-9. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Li K, Xiao X, Zheng S, Xu T, Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg. 2012;47:2216–2223. doi: 10.1016/j.jpedsurg.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Q, Li Q, Zhang B, Yu W. Propranolol therapy of infantile hemangiomas: efficacy, adverse effects, and recurrence. Pediatr Surg Int. 2013;29:575–581. doi: 10.1007/s00383-013-3283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagazgoitia L, Hernandez-Martin A, Torrelo A. Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol. 2011;28:658–662. doi: 10.1111/j.1525-1470.2011.01644.x. [DOI] [PubMed] [Google Scholar]
- 8.Todorovich SM, Khan ZA. Elevated T-box 2 in infantile hemangioma stem cells maintains an adipogenic differentiation-competent state. Dermatoendocrinol. 2013;5:352–357. doi: 10.4161/derm.26739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S, Atala A, Mulliken JB, Bischoff J. Mesenchymal stem cells and adipogenesis in hemangioma involution. Stem Cells. 2006;24:1605–1612. doi: 10.1634/stemcells.2005-0298. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong A, Hardy KL, Kitajewski AM, Shawber CJ, Kitajewski JK, Wu JK. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg. 2012;130:1012–1021. doi: 10.1097/PRS.0b013e318267d3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou HH, Koshakji RP, Silberstein DJ, Wilkinson GR, Wood AJ. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N Engl J Med. 1989;320:565–570. doi: 10.1056/NEJM198903023200905. [DOI] [PubMed] [Google Scholar]
- 13.Zhou HH, Wood AJ. Increased suppression of exercise-induced increase in renin by propranolol in Chinese subjects. Clin Pharmacol Ther. 1991;50:150–156. doi: 10.1038/clpt.1991.119. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Mai HM, Zheng J, Zheng JW, Wang YA, Qin ZP, Li KL. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2014;7:48–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF-1alpha-mediated inhibition of VEGF-A. Ann Surg. 2012;256:146–156. doi: 10.1097/SLA.0b013e318254ce7a. [DOI] [PubMed] [Google Scholar]
- 16.Rosbe KW, Suh KY, Meyer AK, Maguiness SM, Frieden IJ. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg. 2010;136:658–665. doi: 10.1001/archoto.2010.92. [DOI] [PubMed] [Google Scholar]
- 17.Kim LH, Hogeling M, Wargon O, Jiwane A, Adams S. Propranolol: useful therapeutic agent for the treatment of ulcerated infantile hemangiomas. J Pediatr Surg. 2011;46:759–763. doi: 10.1016/j.jpedsurg.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for multiple hepatic and cutaneous hemangiomas with deranged liver function. Pediatrics. 2011;127:e772–776. doi: 10.1542/peds.2010-1703. [DOI] [PubMed] [Google Scholar]
- 19.Cavalli R, Novotna V, Buffon RB, Gelmetti C. Multiple cutaneous and hepatic infantile hemangiomas having a successful response to propranolol as monotherapy at neonatal period. G Ital Dermatol Venereol. 2013;148:525–530. [PubMed] [Google Scholar]
- 20.Kum JJ, Khan ZA. Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis. Pediatr Res. 2014;75:381–388. doi: 10.1038/pr.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Y, Chen S, Li K, Xiao X, Zheng S, Xu T. The role of beta-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div. 2013;8:1. doi: 10.1186/1747-1028-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamy S, Lachambre MP, Lord-Dufour S, Beliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197–207. doi: 10.1007/s10456-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou HX, Jia J, Zhang WF, Sun ZJ, Zhao YF. Propranolol inhibits endothelial progenitor cell homing: a possible treatment mechanism of infantile hemangioma. Cardiovasc Pathol. 2013;22:203–210. doi: 10.1016/j.carpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol. 2013;169:12–19. doi: 10.1111/bjd.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang FQ, Chen G, Zhu JY, Zhang W, Ren JG, Liu H, Sun ZJ, Jia J, Zhao YF. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J Clin Pathol. 2013;66:1058–1064. doi: 10.1136/jclinpath-2012-201286. [DOI] [PubMed] [Google Scholar]
- 28.Tan ST, Wallis RA, He Y, Davis PF. Mast cells and hemangioma. Plast Reconstr Surg. 2004;113:999–1011. doi: 10.1097/01.prs.0000105683.10752.a6. [DOI] [PubMed] [Google Scholar]
- 29.Prey S, Leaute-Labreze C, Pain C, Moisan F, Vergnes P, Loot M, Taieb A, Cario-Andre M. Mast cells as possible targets of propranolol therapy: an immunohistological study of beta-adrenergic receptors in infantile hemangiomas. Histopathology. 2014 doi: 10.1111/his.12421. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Itinteang T, Tan ST, Jia J, Steel R, Laing EL, Brasch HD, Day DJ. Mast cells in infantile haemangioma possess a primitive myeloid phenotype. J Clin Pathol. 2013;66:597–600. doi: 10.1136/jclinpath-2012-201096. [DOI] [PubMed] [Google Scholar]
- 31.Boscolo E, Mulliken JB, Bischoff J. Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1. Arterioscler Thromb Vasc Biol. 2013;33:501–509. doi: 10.1161/ATVBAHA.112.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D, Boscolo E, Durham JT, Mulliken JB, Herman IM, Bischoff J. Propranolol Targets Contractility of Infantile Hemangioma-derived Pericytes. Br J Dermatol. 2014 doi: 10.1111/bjd.13048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan SM, Chen RL, Shen WM, Chen HN, Zhou XJ. Mesenchymal stem cells in infantile hemangioma reside in the perivascular region. Pediatr Dev Pathol. 2012;15:5–12. doi: 10.2350/11-01-0959-OA.1. [DOI] [PubMed] [Google Scholar]
- 34.Stiles J, Amaya C, Pham R, Rowntree RK, Lacaze M, Mulne A, Bischoff J, Kokta V, Boucheron LE, Mitchell DC, Bryan BA. Propranolol treatment of infantile hemangioma endothelial cells: A molecular analysis. Exp Ther Med. 2012;4:594–604. doi: 10.3892/etm.2012.654. [DOI] [PMC free article] [PubMed] [Google Scholar]