Abstract
S100 calcium binding protein B (S100B) is recently known as the markers for inflammatory diseases. However, its roles and underlying mechanism on multiple-traumas remain unclearly. In this study, total 123 patients (87 male and 36 female) were enrolled and divided into two group: Injury severity score (ISS) ≥ 16 (n = 69); ISS < 16 (n = 54). ELISA assay confirmed that the circulating S100B levels in multi-trauma were obviously higher than that in healthy volunteers. Additionally, S100B concentrations was associated with injury severity as an obviously higher levels of S100B (2.18 μg/L) in severe trauma group (ISS ≥ 16) than 1.26 μg/L in moderate trauma group (ISS < 16). Furthermore, the average concentration of S100B was 2.91 μg/L (n = 14) in fatal patients and 2.21 μg/L in survivors, suggesting an obvious correlation between S100B and the severity degree of multi-injury. Further analysis confirmed an obvious correlation between S100B levels and sE-selectin, and Von willebrand factor (vWF), both of these are the marker of endothelial cell injury. After transfection with pcDNA3.1-S100B, human umbilical vein endothelial cells (HUVECs) cell apoptotic ratio was dramatically up-regulated, concomitant with the increase in IL-6 and IL-8 levels, suggesting that S100B might regulate the development of polytrauma by mediating endothelial cell dysfunction. Together, these results suggest a potential predictive value of S100B and its underlying mechanism in the pathological process of polytrauma. Therefore, this study will support the potential clinical aspect for the diagnostic and treatment of polytrauma and its complications.
Keywords: Polytrauma, S100B, injury severity, endothelial cell dysfunction, inflammatory cytokine
Introduction
Traumatic injuries rank as the leading cause of disability and are responsible for more than five million life years lost per year [1]. Its serious complications remain the fourth major cause of mortality after the first 24 hours post-injury in the United States, such as severe sepsis, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndromes (MODS) [2,3]. It has been reported that multiple trauma results in about 2.3 million hospitalizations and more than 156,000 American deaths each year.
Patients with multiple traumatic injuries often suffer from infection, organ failure and death, which have spurred on the development of trauma-specific scoring systems. Currently, physiological, anatomical, and clinical laboratory analytic scoring systems (Acute Physiology and Chronic Health Evaluation [APACHE], Injury Severity Score [ISS]) are commonly used to quantify the extent of multiple injury and to help predict clinical trajectories [4,5]. However, these methods have had a modest success to evaluate outcome following multiple injuries. Recently, some specific molecules have exhibited potential as agents for the development of an injury scoring system based on their expression levels [6,7]. However, controversy remains over which is the best predictive factor. Therefore, there is an urgent need to identify a new molecule that can effectively evaluate trauma outcome.
With advances in molecular medicine, the theory of neutrophil-mediated tissue injury has gained wide acceptance, and analysis of the inflammatory markers has been undertaken to assess patients who have sustained trauma [6]. As a common inflammation molecule, the S100 calcium binding protein B (S100B) is a low-molecular-weight Ca2+-binding protein composed of two isomeric subunits [8,9]. It has been reported that the up-regulated S100B was confirmed in ischemia, suggesting a potential function as a marker of the degree and severity of cellular injury in acute ischemic stroke [10]. A similar role of S100B is also demonstrated as a potential marker of brain trauma [11-13]. Furthermore, recent investigations suggest that higher S100B levels are detected in patients with Central Nervous System Infections and can be used as a potential marker to quantify central nervous system injury [14,15]. Unfortunately, the role of S100B in assessing the degree of multiple traumas and its underlying mechanism remain unclearly.
In this study, our aim was to investigate the changes of S100B concentrations in patients following multiple traumas. Moreover, the prognostic significance of S100B and underlying mechanism was also discussed.
Methods
Patient selection
The 212 patients with multiple trauma were enrolled into the study on the day of admission to the emergency department from June 2010 and January 2012 in the Shaanxi Provincial People’s Hospital. The study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital and accorded with the Helsinki Declaration. All patients met the criteria (age > 18 and < 70 years). Exclusion criteria were tumor, acute and chronic infectious diseases, urgent administration of major intravenous volume resuscitation before the first blood sample was obtained, any metabolic disorder, injuries secondary to acute heart attack or stroke. Crystalloids (contained in Ringer lactate solution) and hydroxyethyl starch solution (HAES 200/0.5) were used as resuscitation regimen. Finally, about 123 patients were chosen to perform the subsequent research. For control purposes, 26 normal volunteers were collected (average age 28.3 years). All of the patients gave written informed consent.
Trauma score
The abbreviated injury scale (AIS) was examined after a trauma scan of CT. The severity of injury was assessed by the Injury Severity Score (ISS) based on the final diagnosis of each patient. For ISS, it is the sum of squares of the highest AIS grades in each of the three most severely injured body regions. To clarify the correlation between S100B concentrations and the severity of multiple traumas, patients were divided into two groups: values less than 16, which mean moderate injury; values greater than 16, which indicate the serious injury.
Collection of serum specimens
All experimental procedures were undertaken with approval from Shaanxi Provincial People’s Hospital. EDTA-anticoagulated whole blood samples were taken within 2 h and 24 h of injury for each traumatized patient. Then, bloods were centrifuged at 1000 g for 10 min in a storage tube with a bullet cover and the resulting plasma was collected. All of the obtained samples were split into aliquots and stored at 70°C until analysis.
Enzyme-linked immunosorbent assay (ELISA) assay
The serum levels of S100B, sE-selectin, and von Willebrand factor (vWF) were measured by ELISA. Briefly, after coating with monoclonal antibody anti-S100B, 50 μl of sample plus 50 μl of barbital buffer (pH 8.6) was placed on microtiter plates, followed by blocking with 1% bovine serum albumin. Then, horseradish peroxidase-conjugated secondary antibody was added to incubation for 1 h. After washing, OPD (Sigma) was introduced as substrate and incubated for a further 30 min in the dark. The absorbance was measured at 492 nm on a microtiter plate reader.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA). Cells were cultured in M199 medium including 10% FBS, 5 mM L-glutamine, endothelial cell growth supplement, and 0.25 μg/ml fungizone. All cells were maintained at 37°C with 5% CO2. Cells from passages 4 to 6 were used for all experiments.
Construction of S100B recombinant expression vectors and stable transfection
To obtain S100B expression vectors, the obtained S100B cDNA was digested with BamHI and XhoI restriction enzymes, following the ligation into the corresponding enzyme sites of pcDNA3.1 (+) vector (Invitrogen) to construct the recombinant S100 expression vector (pcDNA3.1-S100B) (rS100B)). Then, the transfection was performed using 8 µl of Lipofectamine 2000 (Invitrogen) and 15 µg pcDNA3.1-syntenin or pcDNA3.1 empty vector in HUVECs. Forty-eight hours later, 400 µg/ml of G418 was used to select the stable transfectants.
Real-time PCR
Total RNA from HUVECs was extracted using the RNAiso plus kit (Roche Diagnostics, Mannheim, Germany), followed by the reverse-transcribe to synthesize the first strand cDNA with the cDNA Synthesis Kit (Fermentas). Then, the obtained cDNA was subjected to real-time PCR with specific primers for S100B (sense, 5’-TGGACAATGATGGAGACGG-3’; anti-sense, 5’-ATTAGCTACAACACGGCTGG-3’) in 20 μl reactive system. The reaction conditions were performed as the instructions of SYBR Premix Ex TaqTM II Kit (Takara Bio Inc., Otsu, Japan). β-actin was used as normalization. All samples were performed in triplicate.
Western blotting
Following lysis with TRIzol, the total protein concentrations in HUVECs were measured with the BCA assay (Pierce, Rockford, IL). Then, about 100 μl proteins were electrophoresed by SDS-PAGE, followed by the transferred onto a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% nonfat dry milk, the PVDF membrane was incubated prior with the antibody against S100B to the subsequent HRP-conjugated secondary antibodies for 1 h. Then, the LumiGLo reagent (Pierce) was introduced to display the bound antibodies.
Cell apoptosis analysis by annexin V-propidium iodide (AV-PI) staining
To investigate the effect of S100B on cell apoptosis, AV-PI staining was performed. Briefly, after transfected with pcDNA3.1-S100B, cells were harvested and washed three times with PBS. Following centrifuging, cells were resuspended in 500 μl of binding buffer supplement with 5 μl FITC-conjugated annexin V in the dark. Ten minutes later, 5 μl of PI was introduced. The specimens were then analyzed by flow cytometry using the CellQuest software (BDIS). All results were exhibited as a percentage of total cells counted.
Analysis of inflammatory cytokines levels by ELISA
ELISA assay was used to assess the function of S100B on inflammatory cytokine levels of interleukin 6 (IL-6) and interleukin 8 (IL-8). Briefly, HUVEC cells (2 × 104 per well) were were seeded into 24 well plates and incubated at 4°C overnight. After transfection with S100B recombinant vector, the concentrations of IL-6 and IL-8 in supernatants were measured using ELISA DuoSet Development systems according to the manufacturer’s instructions (R&D Systems).
Statistical analysis
All data were analyzed using SPSS 11.0, and results were shown as mean ± SEM. A typical image from at least three similar experiments was presented. Statistical analysis was performed by an independent Student t-test. P < 0.05 was considered statistically significant.
Results
Patients’ characteristics
All the patients’ characteristics were summarized in Table 1. Total 123 patients (87 male and 36 female) were aged 18 to 70 years (median, 27.8 years). Sixty-nine patients had severity injury (ISS ≥ 16), and head trauma was the common injury site (32.5%). Twenty-eight patients occured shock. About 109 patients survived the trauma, and 14 patients died of severe head injury (n = 9) or thoracic and abdominal injuries (n = 5) within 36 hours of the unintentional injury.
Table 1.
Characteristics of patients
| Characteristics | Number | % |
|---|---|---|
| total | 123 | 100 |
| Median age | 27.8 (18-70) | |
| Gender | ||
| male | 87 | 70.7 |
| female | 36 | 29.3 |
| Injury site | ||
| head | 50 | 40.6 |
| thorax | 41 | 33.3 |
| abdomen | 38 | 30.1 |
| spinal pelvis | 8 | 6.5 |
| other | 12 | 9.8 |
| Injury severity (ISS) | ||
| ≥ 16 | 69 | 56.1 |
| < 16 | 54 | 43.9 |
| Shock | 28 | 22.7 |
| Sepsis | 22 | 17.8 |
| Mortality | 14 | 11.4 |
High serum levels of S100B was detected in multi-trauma patients
To assess the circulating S100B serum levels in multi-trauma and healthy volunteers, we performed ELISA assay. As shown in Figure 1A, a mean serum S100B levels of 0.52 ± 0.16 μg/L was detected in control group (n = 26). After multiple traumas, the average serum S100B concentration was 1.77 ± 0.12 μg/L at 2 h post multi-trauma, indicating that a significantly higher serum concentration of S100B was observed in trauma patients than in health group. Additionally, we analyzed the serum levels of S100B in various trauma types, and the results indicated that a little higher concentration of S100B was observed in head injury patients than in others, but had no significant statistically difference (P > 0.05) (Figure 1B).
Figure 1.
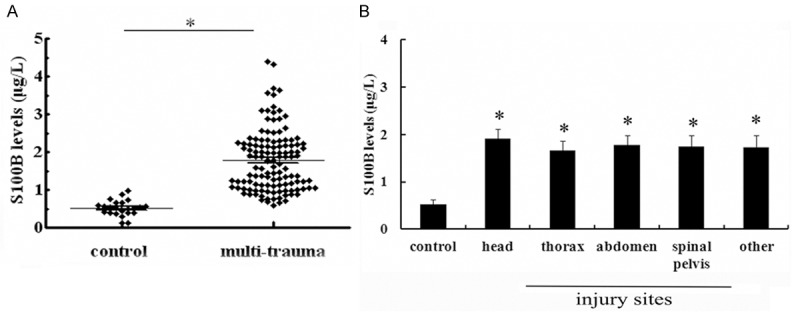
Individual serum concentrations of S100B in patients with multiple traumas and health volunteers. Total 123 patients (87 male and 36 female) were aged from 18 to 70 years (median, 27.8 years). For control purposes, 26 normal volunteers were collected. A. The serum levels of S100B were analyzed by ELISA assay within 2 h following multi-traumas. B. The serum levels of S100B in various trauma types. *P < 0.05 versus control group.
S100B expression was associated with injury severity in trauma patients
Based on the high expression levels of S100B in trauma patients, the correlation between S100B and injury severity was further discussed. Following unintentional injury, mean S100B concentrations in moderate trauma patients (ISS < 16; n = 54) was 1.26 ± 0.24 μg/L at 24 h post multi-trauma, compared to 0.52 ± 0.16 μg/L in control groups (Figure 2). Moreover, an obviously higher level of S100B was confirmed in severe trauma groups (ISS ≥ 16; n = 69), and the circulating S100B level was 2.18 μg/L. Taken together, these results suggested that S100B could be used as a marker to assess injury severity in multiple trauma patients.
Figure 2.
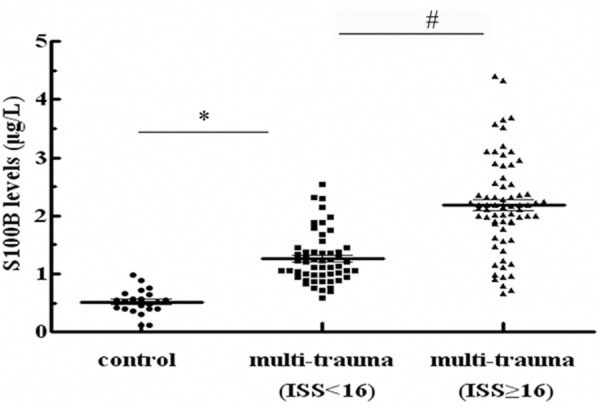
S100B concentrations were related to the severity degree of multiple traumas. ISS scoring system was used to evaluate the severity degree of injury. Patients with multi-traumas were divided into two groups: severe injury (ISS ≥ 16; n = 69) and moderate trauma (ISS < 16; n = 54). ELISA was performed to detect the levels of S100B. *P < 0.05 versus control group. #P < 0.05 versus severity injury groups (ISS ≥ 16).
Elevated expression levels of S100B was confirmed in fatal patients
To further assess the potential prognostic value of S100B in patients with multiple traumas, we discussed the expression levels of S100B in fatal and survival patients. About 14 patients died from severity injury, and the average concentration of S100B was 2.91 ± 0.33 μg/L (Figure 3). However, the expression levels of S100B in survivors (n = 109) were 2.21 ± 0.27 μg/L, suggesting that S100 levels were obviously higher in fatal patients than those in survivors.
Figure 3.
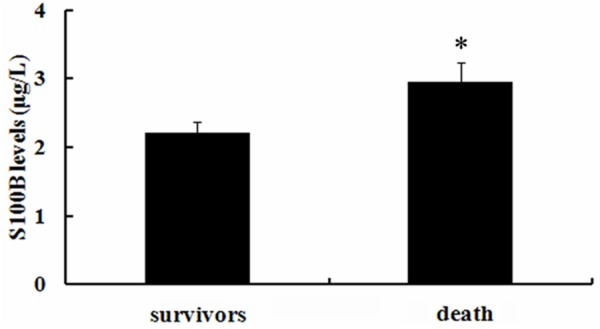
Higher serum levels of S100B were demonstrated in fatal patients compared to survivors. Following hospital admission, approximate 14 patients died of severe injury, and 109 patients survived the trauma. The serum levels of S100B between these two groups were assessed by ELISA assay. *P < 0.05.
Correlation between S100B levels and endothelial cell dysfunction
Endothelial cells are believed to be involved in various inflammatory diseases and their dysfunction can lead to organ failure and subsequent death in patients with severe sepsis [16]. To further analyze the underlying mechanism, we investigated the correlation between S100B levels and endothelial cell dysfunction. As shown in Table 2, the average mean of vWF, a marker for endothelial cell injury, was 205 ± 42% in moderate trauma patients (ISS < 16; n = 54) after unintentional injury compared with control health patients. With the increased degree of injury, higher levels of vWF were observed. Consistently, the similar increase in endothelial cell injury marker sE-selectin was also confirmed. Further correlation analysis indicated an obvious positive correlation between S100B and vWF, sE-selectin, suggesting that S100B might accelerated the pathological process of multi-trauma by regulating endothelial cell dysfunction.
Table 2.
Correlation between serum levels of vWF, TF and S100B
| Groups | S100B (μg/L) | vWF (%) | sE-selectin (ng/mL) | ||
|---|---|---|---|---|---|
|
|
|||||
| Content | R2 | Content | R2 | ||
| Multi-trauma (ISS < 16) | 1.26 ± 0.24 | 205 ± 42 | 0.61* | 57.5 ± 11 | 0.63* |
| Multi-trauma (ISS > 16) | 2.18 ± 0.32 | 265 ± 51 | 0.55* | 132.1 ± 36 | 0.57* |
| Control | 0.52 ± 0.16 | 115 ± 35 | 0.30 | 38.9 ± 5 | 0.28 |
P < 0.05.
Overexpression S100B enhanced HUVEC cell apoptosis
It is known that endothelial cell dysfunction is characterized with cell death. To clarify the potential mechanism of S100B involved in the development of multi-trauma, the recombinant pcDNA3.1-S100B was transfected into HUVEC and then a stable S100B overexpression clones were obtained. As shown in Figure 4A, an obvious up-regulation of S100B mRNA was detected after transfection with rS100B, concomitant with a similar increase in S100 protein levels (Figure 4B). Further analysis confirmed that S100B overexpression dramatically enhanced cell apoptosis. Quantified analysis indicated a 33.4% increase in cell apoptotic rate, compared with 8.6% in control groups (Figure 4C), implying a critical role of S100B in endothelial cell dysfunction.
Figure 4.
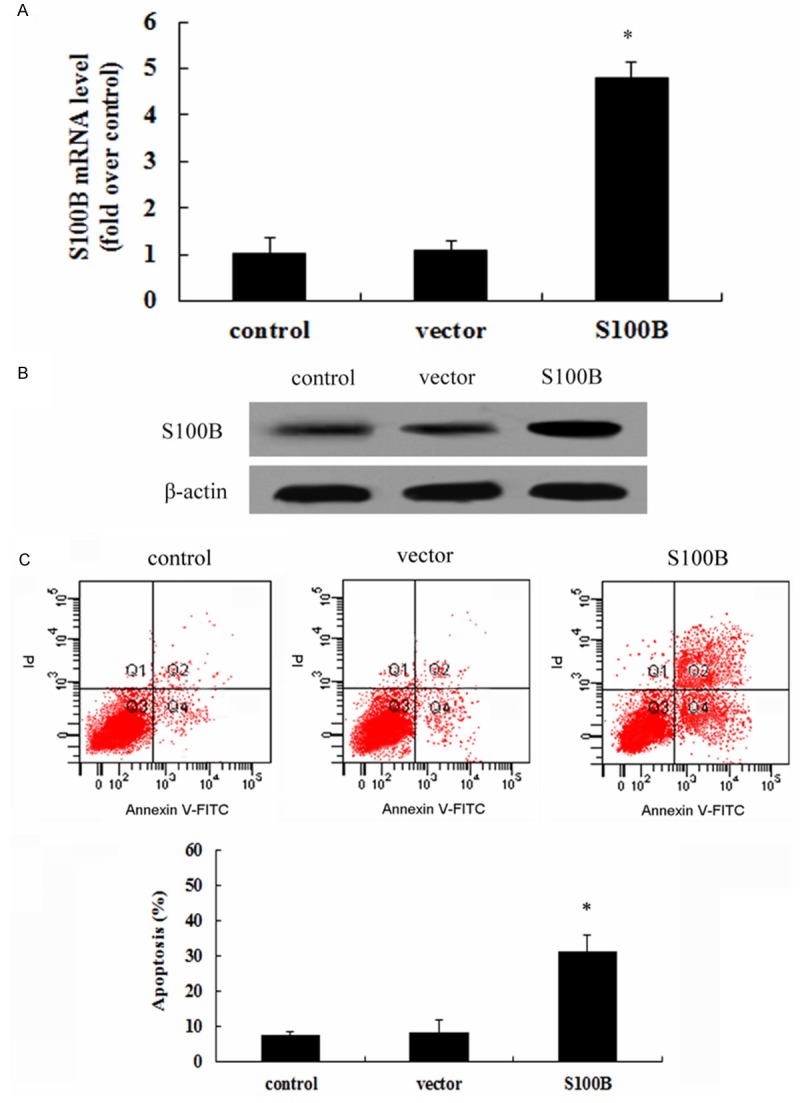
S100B overexpression enhanced HUVEC cell apoptosis. After transfection of recombinant pcDNA3.1-S100B and empty vector into HUVEC cells, the expression levels of S100B mRNA and protein were detected by RT-PCR (A) and western blotting (B), respectively. Furthermore, the effect of S100B overexpression on cell apoptosis was assessed by AV-PI staining (C). *P < 0.05.
Elevated S100B expression promoted inflammatory cytokines secretion
To further explore the effect of S100B on endothelial cell function, the pro-inflammatory cytokines of IL-6 and IL-8 was analyzed. Following transfection with rS100B, the concentration of IL-6 was 14.6 ng/ml and significant higher than 9.4 ng/ml in control groups (Figure 5). Consistently, S100B up-regulation also obviously induced the increase of IL-8 levels, compared with control and vector-treated groups. Together, these results might confirm an important function of S100B on endothelial-mediated pro-inflammatory response.
Figure 5.
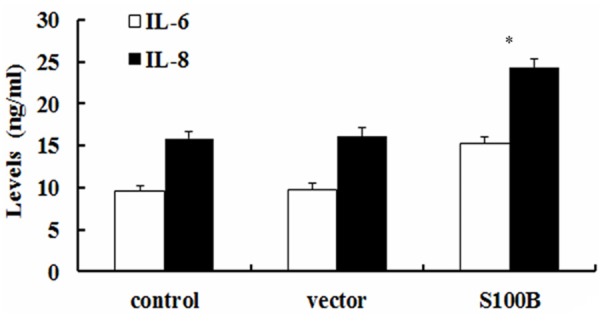
Elevated S100 expression promoted the pro-inflammatory cytokine levels of IL-6 and IL-8. Following transfection with pcDNA3.1-S100B, the inflammatory cytokines of IL-6 and IL-8 levels were evaluated by ELISA assay. *P < 0.05.
Discussion
Polytrauma is recognized to be life-threatening because of the high incidences and morbidity around the world [1]. As the fourth leading cause of death in the United States overall-followed heart disease, cancer and stroke, multi-trauma accounts for one in ten hospital discharges and one in six hospital days. Its serious complications commonly account for late fatalities after multiple injuries, such as organ dysfunction and systemic inflammatory response [17-19]. Therefore, to explore an effective method for assessing the outcome following multi-trauma has become clinically urgent.
Recently, several clinical studies have confirmed that some cytokines levels are associated with organ dysfunction, degree of injury, and fatality, which suggest a new approach to predict the outcome following multiple injuries [6,7,20]. S100 is a multi-genic family of small (~10 kDa) Ca2+-binding proteins, with a conserved calcium-binding motif termed the EF-hand. S100 proteins are markers for inflammatory diseases. As one of the major member, S100B is expressed in various cells and has multiple local regulatory effects, including cell division, proliferation, apoptosis, energy metabolism, and inflammation [21-23]. The important prognostic value of serum levels S100B are detected in patients with cutaneous melanoma and breast cancer [24,25]. Recent studies have been demonstrated a potential function of S100B as an outcome predictor in acute ischemic stroke and severe traumatic brain injury indicator [10,13,26]. However, the roles of S100B in polytrauma remain unclear.
In this study, we analyzed 123 multiple trauma patients with about one of third patients suffered from head injury. ISS scoring system exhibited that about 69 patients occurred severe injury. To explore the values of S100B as a predictor following multiple traumas, the serum levels of S100B were analyzed by ELISA. As expected, the expression levels of S100B in multi-trauma were significantly higher than those in healthy volunteers. Furthermore, ISS analysis confirmed that the serum levels of S100B were associated with the severity of multiple traumas because the higher serum levels of S100B were demonstrated in severity injury (ISS ≥ 16) groups than ISS < 16 groups, implying an important predictive value of S100B following traumas. Further analysis indicated that the expression levels of S100B in fatal patients were 2.91 μg/L, compared to 2.21 μg/L in survivors. Therefore, these results suggested a pregnant predictive function of S100B when evaluating the severity degree of multi-traumas. However, its underlying mechanism is still unclear.
Endothelial cells are believed to be involved in various inflammatory diseases and their injury exert the important role during the development of the trauma-triggered sepsis, MODS and SIRS [16,27,28]. During this process, endothelial cells may undergo apoptosis or necrosis, and ultimately led to the dysfunction of endothelial and accelerate the pathological progress [16]. In this study, we further confirmed an obvious correlation between S100B levels and vWF and sE-selectin, both of these are the marker of endothelial cell injury [29]. Therefore, these results indicated a correlation between S100B and endothelial cell dysfunction. To further clarify this mechanism, we constructed S100B-elevated HUVEC cell models and found that S100B overexpression enhanced HUVEC cell apoptosis. It is believed that endothelial cell apoptosis will accelerates the pro-inflammatory, which is the major contributor for the development of polytrauma and its serious complication [16,30]. Thus, the effect of S100B on endothelial cell inflammatory response was further explored. IL-6 and IL-8 are known to participate in the inflammatory cascade and rank as the reliable prognostic marker of inflammatory-related diseases. In this study, the elevated expression of S100B increased the levels of pro-inflammatory cytokines of IL-6, as well as IL-8 levels. Together, these results suggest that S100B may regulate the pathological process by mediating endothelial cell dysfunction through enhancing cell apoptosis and inflammatory response.
In conclusion, our research investigated for a potential predict role of S100B in polytrauma. Furthermore, S100B was further confirmed to be associated with endothelial cell dysfunction, which might relate with cell apoptosis and subsequent enhanced inflammatory response. Together, this study investigated the function of S100B on the pathological progression of multiple traumas and the underlying mechanisms. Accordingly, this research will support a potential aspect for the prediction and treatment of multiple traumas and its complications.
Disclosure of conflict of interest
None.
References
- 1.Søreide K. Epidemiology of major trauma. Bri J Surg. 2009;96:697–698. doi: 10.1002/bjs.6643. [DOI] [PubMed] [Google Scholar]
- 2.Leigh JP. Economic burden of occupational injury and illness in the United States. Milbank Quarterly. 2011;89:728–772. doi: 10.1111/j.1468-0009.2011.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peek-Asa C, Zwerling C, Stallones L. Acute traumatic injuries in rural populations. J Inform. 2004;94:1689–1693. doi: 10.2105/ajph.94.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouch DC, Thompson JP. Severity scoring systems in the critically ill. Continuing Education in Anaesthesia, Critical Care Pain. 2008;8:181–185. [Google Scholar]
- 5.Loh SA, Rockman CB, Chung C, Maldonado TS, Adelman MA, Cayne NS, Pachter HL, Mussa FF. Existing trauma and critical care scoring systems underestimate mortality among vascular trauma patients. J Vasc Surg. 2011;53:359–366. doi: 10.1016/j.jvs.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 6.Frink M, van Griensven M, Kobbe P, Brin T, Zeckey C, Vaske B, Krettek C, Hildebrand F. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med. 2009;17:49. doi: 10.1186/1757-7241-17-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Kanellakopoulou K, Pelekanou A, Tsaganos T, Kotzampassi K. Kinetics of angiopoietin-2 in serum of multi-trauma patients: correlation with patient severity. Cytokine. 2008;44:310–313. doi: 10.1016/j.cyto.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Piazza O, Leggiero E, De Benedictis G, Pastore L, Salvatore F, Tufano R, De Robertis E. S100B induces the release of pro-inflammatory cytokines in alveolar type I-like cells. Int J Immunopathol Pharmacol. 2012;26:383–391. doi: 10.1177/039463201302600211. [DOI] [PubMed] [Google Scholar]
- 9.Steiner J, Schroeter M, Schiltz K, Bernstein H, Müller U, Richter-Landsberg C, Müller W, Walter M, Gos T, Bogerts B. Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience. 2010;167:1025–1031. doi: 10.1016/j.neuroscience.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB. Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J Neuroinflam. 2010;7:71. doi: 10.1186/1742-2094-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, Antsaklis A, Sakas D. Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr Med Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- 12.Vos P, Jacobs B, Andriessen T, Lamers K, Borm G, Beems T, Edwards M, Rosmalen C, Vissers J. GFAP and S100B are biomarkers of traumatic brain injury An observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 13.Egea-Guerrero J, Revuelto-Rey J, Murillo-Cabezas F, Muñoz-Sánchez M, Vilches-Arenas A, Sánchez-Linares P, Dominguez-Roldan J, Leon-Carrion J. Accuracy of the S100 β protein as a marker of brain damage in traumatic brain injury. Brain Inj. 2012;26:76–82. doi: 10.3109/02699052.2011.635360. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Suh GI, Shin HE. Association between Cerebrospinal Fluid S100B Protein and Neuronal Damage in Patients with Central Nervous System Infections. Yonsei Med J. 2013;54:567–571. doi: 10.3349/ymj.2013.54.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6:121–138. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 16.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 17.Mommsen P, Andruszkow H, Frömke C, Zeckey C, Wagner U, Van Griensven M, Frink M, Krettek C, Hildebrand F. Effects of accidental hypothermia on posttraumatic complications and outcome in multiple trauma patients. Injury. 2013;44:86–90. doi: 10.1016/j.injury.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Giannoudis P, Harwood P, Pape H. Severe and multiple trauma in older patients; incidence and mortality. Injury. 2009;40:362–367. doi: 10.1016/j.injury.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Mica L, Vomela J, Keel M, Trentz O. The impact of body mass index on the development of systemic inflammatory response syndrome and sepsis in patients with polytrauma. Injury. 2012;45:253–258. doi: 10.1016/j.injury.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Mommsen P, Frink M, Pape H, van Griensven M, Probst C, Gaulke R, Krettek C, Hildebrand F. Elevated systemic IL-18 and neopterin levels are associated with posttraumatic complications among patients with multiple injuries: a prospective cohort study. Injury. 2009;40:528–534. doi: 10.1016/j.injury.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–3771. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 22.Riuzzi F, Sorci G, Donato R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J Cell Sci. 2011;124:2389–2400. doi: 10.1242/jcs.084491. [DOI] [PubMed] [Google Scholar]
- 23.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, J Weber D, L Geczy C. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 24.Mocellin S, Zavagno G, Nitti D. The prognostic value of serum S100B in patients with cutaneous melanoma: A meta - analysis. Int J Cancer. 2008;123:2370–2376. doi: 10.1002/ijc.23794. [DOI] [PubMed] [Google Scholar]
- 25.McIlroy M, McCartan D, Early S, Gaora PÓ, Pennington S, Hill AD, Young LS. Interaction of developmental transcription factor HOXC11 with steroid receptor coactivator SRC-1 mediates resistance to endocrine therapy in breast cancer. Cancer Res. 2010;70:1585–1594. doi: 10.1158/0008-5472.CAN-09-3713. [DOI] [PubMed] [Google Scholar]
- 26.Goyal A, Failla MD, Niyonkuru C, Amin K, Fabio A, Berger RP, Wagner AK. S100b as a Prognostic Biomarker in Outcome Prediction for Patients with Severe Traumatic Brain Injury. J Neurotrauma. 2013;30:946–957. doi: 10.1089/neu.2012.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M. Role of Endothelial Cell–Derived Angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscl Thromb Vasc Biol. 2014;34:790–800. doi: 10.1161/ATVBAHA.113.303116. [DOI] [PubMed] [Google Scholar]
- 28.Reinhart K, Bayer O, Brunkhorst F, Meisner M. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med. 2002;30:S302–S312. doi: 10.1097/00003246-200205001-00021. [DOI] [PubMed] [Google Scholar]
- 29.Schalkwijk C, Poland D, Van Dijk W, Kok A, Emeis J, Dräger A, Doni A, Van Hinsbergh V, Stehouwer C. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- 30.Probst C, Mirzayan M, Mommsen P, Zeckey C, Tegeder T, Geerken L, Maegele M, Samii A, van Griensven M. Systemic inflammatory effects of traumatic brain injury, femur fracture, and shock: an experimental murine polytrauma model. Mediat Inflamm. 2012;2012:136020. doi: 10.1155/2012/136020. [DOI] [PMC free article] [PubMed] [Google Scholar]


