Abstract
Paraneoplastic Ma1 (PNMA1) is a member of an expanding family of ‘brain/testis’ proteins involved in an autoimmune disorder defined as paraneoplastic neurological syndrome (PNS). Although it is widely studied in PNS, little is known about the underlying clinical significance and biological function of PNMA1 in tumors. Here, we find that elevated PNMA1 expression is more commonly observed in pancreatic ductal adenocarcinoma (PDAC) cell lines, compared with normal pancreatic cell and tissues from pancreatic ductal adenocarcinoma patient. Besides, higher PNMA1 expression is closely correlated with large tumor size. Suppression of endogenous PNMA1 expression decreases cell viability and promotes cell apoptosis. Subsequent studies reveal that the PI3K/AKT, MAPK/ERK pathway and members of the anti-apoptotic Bcl-2 family may be involved in the pro-survival and anti-apoptotic effect of PNMA1 on PDAC. Taken together, this study provides evidence that PNMA1 is involved in tumor growth of pancreatic carcinoma and PNMA1-related pathways might represent a new treatment strategy.
Keywords: PNMA1, pancreatic ductal adenocarcinoma, growth, cell survival, apoptosis
Introduction
Pancreatic ductal adenocarcinoma remains one of the most malignancies characterized by an insidious onset, late diagnosis and poor prognosis with annual mortality being equivalent to annual morbidity [1,2]. Many risk factors including smoking, obesity, alcohol consumption, and chronic pancreatitis have been recognized as potential risk factors for the development of PDAC [3]. Due to its aggressive growth, most cases are diagnosed in advanced stages, which makes the vast majority of patients suffered from pancreatic cancer are not amenable to surgical resection by the time of diagnosis and leads to a poor prognosis. Although much progress has been made in developing new diagnostic methods and novel targeted therapies, the overall survival rate has changed very little in the past 40 years [4]. Thus, it is urgent to find more effective therapeutic approaches to treat this deadly disease.
PNMA1 is a member of a putative family of six proteins that known as PNMA1, PNMA2, PNMA3, PNMA4 (also termed as MAP-1), PNMA5, PNMA6A [5,6]. These proteins are predominantly expressed in the brain. PNMA1 and PNMA3 are also with expression in adult testis and PNMA4 with expression in other organs and tissues [5-7]. PNMA1 and PNMA2 proteins were first discovered as one of the marker genes in tumors of patients suffered from paraneoplastic neurological syndromes, an autoimmune disorder triggered by onconeuronal proteins “ectopically” expressed in tumors [8-10]. PNMA5 gene is preferentially expressed in the association areas during primate evolution [11]. The functions of the proteins encoded by PNMA family of genes are little known. However, PNMA4, the best studied protein, has been widely reported as a modulator of cell death. It regulates cell apoptosis by connecting RASSF1A, a tumor suppressor that plays a key role in the development of human cancer, to Bax via the BH3 domain, which results in Bax conformational change, mitochondrial membrane insertion of Bax, and ultimately, cytochrome c release and apoptosis [12-14]. PNMA1 has been reported induction of mouse neuronal cell death because of behaving a motif resembling the BH3 consensus sequence, while without similar researches in human beings, especially in tumors [15]. Consideration the potential malignancies of PNMA1, we evaluated its clinical significances and biological functions in human pancreatic cancer.
In the present study, we report that elevated PNMA1 expression in human pancreatic cancer cell lines and tissues compared to normal controls or chronic pancreatitis controls. Suppressed PNMA1 expression results in decreased cell viability and pronounced cell apoptosis. The down-regulation of PNMA1 expression also correlated with inactivation of the PI3K/AKT, MAPK/ERK1 pathway and decreased expression of the anti-apoptotic molecules of Bcl-2 family members.
Materials and methods
Cell culture
Human PDAC cell lines AsPC-1, BxPC-3, Capan-2, CFPAC-1, HPAC, PANC-1, SW1990 were all purchased from Cell Bank of the Chinese Academy of Sciences and normal human pancreatic duct cell line hTERT-HPNE was purchased from American Type Culture Collection. All of these cells were cultured in specific medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% antibiotics at 37°C in a humidified incubator under 5% CO2 condition.
Clinical tissue samples and immunohistochemical staining
Human pancreas tissue microarrays (OD-CT-DgPan01-006) contained 81 cases of pancreatic ductal adenocarcinoma, 44 cases of normal pancreas tissues and 32 cases of chronic pancreatitis tissues were purchased from Shanghai Outdo Biotech Inc. Tissue sections were deparaffinized and rehydrated. The sections were then incubated with 0.3% hydrogen peroxide for 30 minutes and blocked with 10% BSA (Sangon, Shanghai, China). Slides were first incubated using the antibody for PNMA1 (Proteintech, US) at 4°C overnight with optimal dilution, labeled by HRP (rabbit) second antibody (Thermo Scientific, US) at room temperature for 1 hour, incubated with DAB substrate liquid (Gene Tech, Shanghai), and counterstained by hematoxylin. All the sections were observed and photographed with a microscope (Carl Zeiss, Germany). Scoring was conducted according to the ratio and intensity of positive-staining cells: 0-5% scored 0; 6-35% scored 1; 36-70% scored 2; more than 70% scored 3. The final score was designated as low or high expression group as follows: score 0-1, low expression, score 2-3, high expression. These scores were determined independently by two senior pathologists. The scoring by the pathologists was done in a blinded manner.
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Takara, Japan), and reversely transcribed through PrimeScript RT-PCR kit (Takara, Japan) according to the protocol. Quantitative real-time PCR was performed with SYBR Premix Ex Taq (Takara, Japan) on a 7500 Real-time PCR system (Applied Biosystems, Inc. USA). Primer sequences used for PNMA1 detection were as follows, forward: 5’-AGCTCTGTTAGTCTGGGGCA-3’; reverse: 5’-CTGCTTTCGCATTTTCTTCC-3’. The relative expression of PNMA1 was normalized to β-actin RNA (forward: 5’-ACTCGTCATACTCCTGCT-3’, reverse: 5’-GAAACTACCTTCAACTCC-3’). Primers for cyclin genes are available if needed.
Western blotting
Western blotting was performed as previously described [16]. The following antibodies were used in this assay: Erk1/2, Phospho-Erk1/2, Akt, Phospho-Akt (Cell Signaling, Beverly, MA), PNMA1, Bcl-2, Bcl-xL, Bax, Bak1, β-actin (Proteintech, US) and species-specific secondary antibodies. Bound secondary antibodies were detected by Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Establishment of stable PNMA1 knockdown cell lines
Lenti-virus was packaging in 293T cells using Lipofectamine2000 (Invitrogen, Carlsbad, CA) and virus titers were determined. Target cells, including AsPC-1, BxPC-3 cells, were infected with 1×106 recombinant lentivirus-transducing units in the presence of 6 μg/ml polybrene (Sigma). Short hairpin RNA (shRNA) sequences targeting PNMA1 are as follows. Sh-1: 5’-CCGGGAGAATGTTCTGGAGGGAAGACTCGAGTCTTCCCTCCAGAACATTCTCTTTTTG-3’; Sh-2: 5’-CCGGGGGTCTGGAAAGTGTTATTTACTCGAGTAAATAACACTTTCCAGACCCTTTTTG-3’. The efficiency of knock-down was tested by western blot.
Cell viability assay
Cells were seeded into a 96-well plate at 3×103 cells per well with 100 μl culture medium supplemented in the presence or absence of 10% FBS and cultured at 37°C. The cell viability was quantified by addition 10 µl of Cell Counting Kit-8 (CCK8, Dojindo, Japan). After 1 hour of incubation, WST-8 was metabolized to produce a colorimetric dye, which can be monitored by measuring absorbance at 450 nm using a Power Wave XS microplate reader (BIO-TEK). Viable cells were distinguished with the fluorescent dyes Hoechest 33342 (Dojindo, Japan). Staining was performed according to the manufacturer’s instructions.
Apoptosis assay
20×105 cells per well were cultured in 6-well plates in the absence of 10% FBS for 48 hours. Adherent cells were detached with 0.25% trypsin without EDTA in 1×PBS. Cells were harvested in complete RPMI 1640 medium and centrifuged at 1000 rpm for 5 minutes. Each of the cells were washed with 1×PBS and stained with 50 ug/ml propidium iodide and Annexin V-FITC (BD Pharmingen) following the manufacturer’s instructions.
Statistical analysis
Data were presented as the means ± SD. Correlation of PNMA1 expression with clinicopathologic parameters was evaluated by chi-square test. The student’s t-test was used for comparison between groups. Values of P<0.05 were considered statistically significant.
Results
Elevated PNMA1 expression in human pancreatic ductal adenocarcinoma
To observe the expression change happened in PDAC, we first measured the PNMA1 level in PDAC cell lines and tissues. The expression of PNMA1 mRNA was significantly up-regulated (from 1.5-fold to 3.5-fold) in six of seven pancreatic cancer cell lines when compared with the nonmalignant hTERT-HPNE cells (Figure 1A). This trend was amplified by PNMA1 protein expression, as shown by western blotting and immunohistochemistry (Figure 1B, 1C). Notably, PNMA1 was localized predominantly in the cytoplasm of PDAC cells (Figure 1C) not as well as in the subnuclear elements (including the nucleoli) of neurons and testicular germ cells [5]. In addition, immunohistochemical analysis of a tissue microarray (TMA) containing 81 cases of PDAC samples, 44 cases of normal control samples and 32 cases of chronic pancreatitis tissues indicated that there is also increased PNMA1 immunoreactivity in pancreatic carcinoma tissues compared to normal pancreas or chronic pancreatitis tissues. In addition, high expression of PNMA1 was significantly associated with larger tumor size, no significant difference was found in age, gender, TNM stages, tumor location and neuronal invasion (Table 1). Together, these results indicate that elevated expression of PNMA1 may contribute to tumor growth in pancreatic ductal adenocarcinoma.
Figure 1.
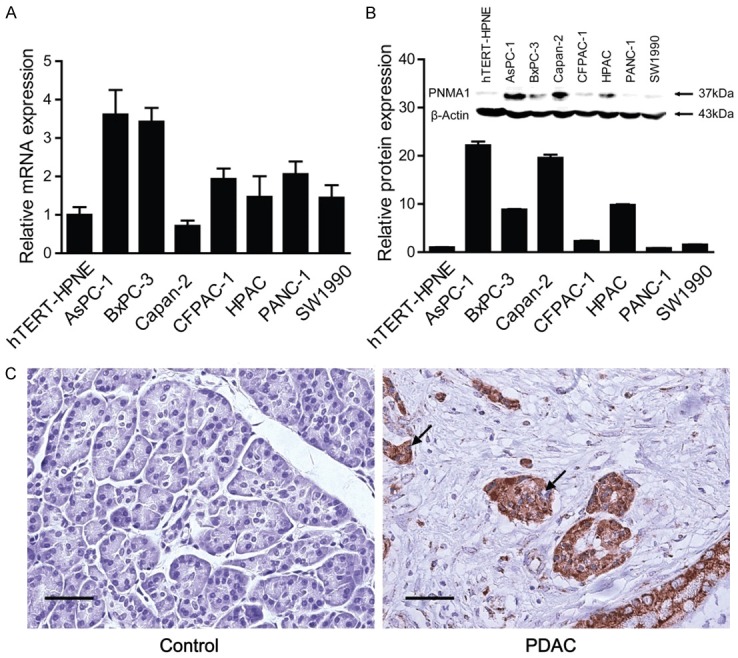
PNMA1 expression is increased in pancreatic ductal adenocarcinoma. PNMA1 levels were assessed in seven pancreatic cancer cell lines, as well as a nonmalignant cell line hTERT-HPNE, by quantitative real-time PCR (A) and immunobloting (B). PNMA1 levels were also detected in surgical samples from 81 pancreatic carcinoma patients and healthy controls by immunohistochenistry. (C) Representative photomicrographs of the PNMA1 immunoreactivity in pancreatic carcinoma and normal control. The arrows represent PNMA1 positive staining without reaction in the nuclei (scale bar: 50 um).
Table 1.
Patient’s clinical and pathological parameters and their association with PNMA1 expression
| Variable | PNMA1 (n) | |||
|---|---|---|---|---|
|
| ||||
| High | Low | P | ||
| Samples | Carcinoma | 56 (69.14) | 25 (30.86) | |
| normal | 12 (27.27) | 32 (72.73) | <0.001 (versus carcinoma) | |
| Chronic pancreatitis | 14 (43.75) | 18 (56.25) | <0.001 (versus carcinoma) | |
| Age | ≤60 years | 23 (60.53) | 15 (39.47) | 0.115 |
| >60 years | 33 (76.74) | 10 (23.26) | ||
| Gender | Female | 34 (70.83) | 14 (29.17) | 0.690 |
| Male | 22 (66.67) | 11 (33.33) | ||
| Tumor size | ≤4 cm | 31 (62.00) | 20 (38.00) | 0.034 |
| >4 cm | 25 (83.33) | 5 (16.67) | ||
| Tumor location | Head | 21 (84.00) | 4 (16.00) | 0.053 |
| Body + Tail | 35 (62.50) | 21 (37.50) | ||
| TNM stage | I | 30 (75.00) | 10 (25.00) | 0.259 |
| II-III | 26 (63.41) | 15 (36.59) | ||
| Neuronal invasion | Yes | 30 (73.17) | 11 (26.83) | 0.426 |
| No | 26 (65.00) | 14 (35.00) | ||
Values in parentheses indicate percentage values. The bold number represents the P-values with significant differences.
Silencing of PNMA1 inhibits cell growth in vitro
To test whether PNMA1 has implications on cell proliferation we measured CCK8 assay in two human pancreatic cancer cell lines, AsPC-1 and BxPC-3. Stable expression of two PNMA1 short hairpin RNA (sh-1, sh-2) in AsPC-1 and BxPC-3 cells resulted in >70% decrease in PNMA1 expression (Figure 2A). In the presence of 10% FBS, silencing of PNMA1 caused significantly decrease in cell viability in AsPC-1 cells, but not in BxPC-3 cells (Figure 2B). To further investigate the effect of PNMA1 on PDAC cell growth, we detected the cyclin gene expression. Compared to scrambled control cells, silencing of PNMA1 contributed to uniformly reduced expression of cyclin genes in BxPC-3, while showing faint influence on AsPC-1 cells (Figure 2C). To best simulate the undernourishment character of the microenvironment of PDAC, we examined cell viability when cells were cultured in serum-free medium. Surprisingly, knockdown of PNMA1 markedly decreased cell viability after serum deprivation for 48 hours (Figure 3A). Meanwhile, we tested whether PNMA1 knockdown increased cell death under starvation conditions. After 48 hours of serum deprivation cells underwent death as demonstrated by Hoechest 33342 staining. As shown in Figure 3B, cell death could be pronounced promoted in AsPC-1 and BxPC-3 cells after silencing of PNMA1. Collectively, these data suggest that PNMA1 promotes cell survival under conditions of nutrient deficiency and this prerequisite facilitates cell growth.
Figure 2.
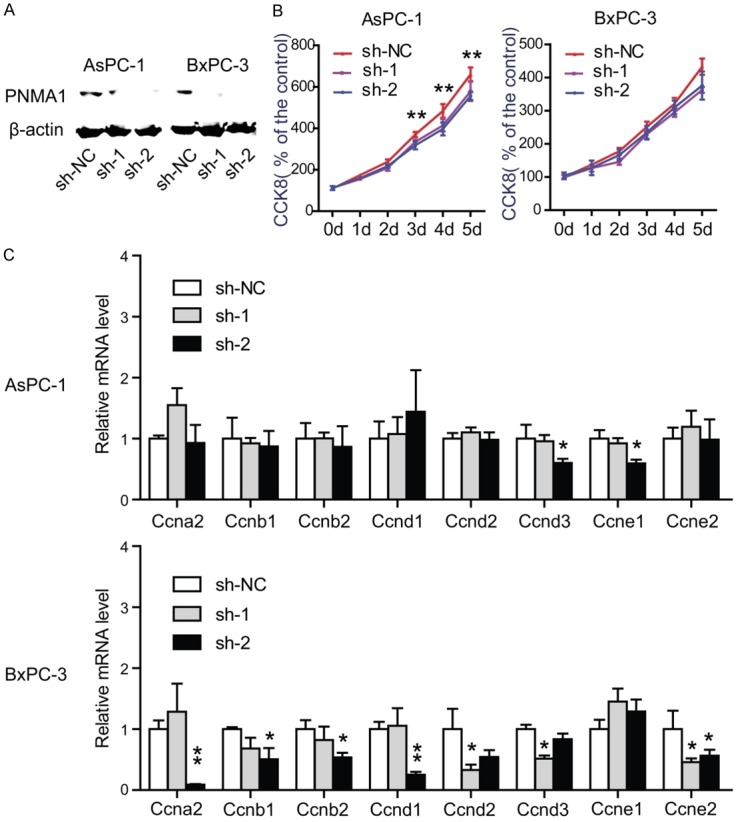
Knockdown of PNMA1 inhibits cell proliferation in AsPC-1 and BxPC-3 cells. A: The expression level of PNMA1 was detected by western-bolting in AsPC-1, BxPC-3 cells after PNMA1 knockdown. B, C: Effect of PNMA1 knockdown on cell growth of AsPC-1 and BxPC-3 cells in the presence of 10% FBS was analyzed by measuring cell viability (sh-NC versus sh2, **, P<0.01) and detecting the expression of cyclins (sh-NC versus sh1 or sh2, *, P<0.05, **, P<0.01). Data are representative of three independent experiments.
Figure 3.
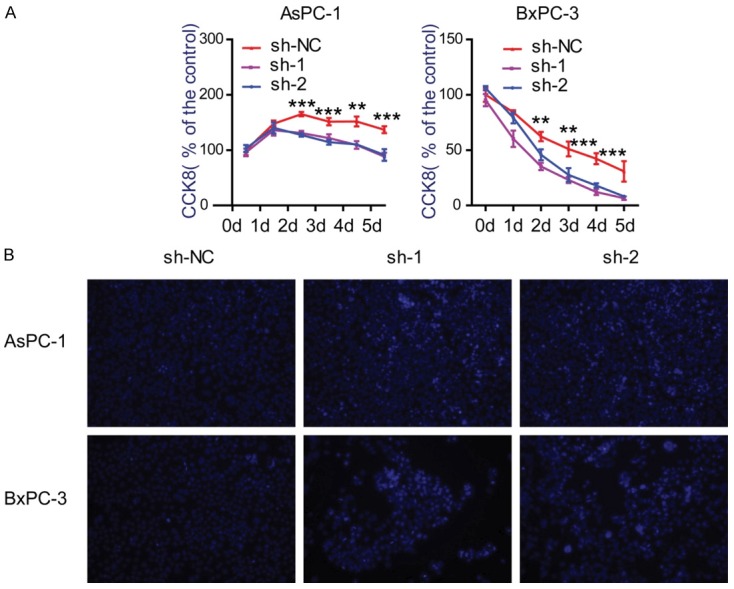
Silencing of PNMA1 decreases cell viabilities and promotes cell death under serum deprivation. A: Effect of PNMA1 knockdown on cell survival of AsPC-1 and BxPC-3 cells with serum deprivation were analyzed by CCK8 assay (sh-NC versus sh-1 or sh-2, **, P<0.01, ***, P<0.001). B: Hoechest 33342 staining with living cells (light blue) and dead cells (dark blue) under fluorescent microscopy.
Silencing of PNMA1 promotes cell apoptosis in vitro
Previous work performed in neurons has shown that ectopic expression of PNMA1 promotes apoptosis even in a condition that normally ensures survival of mouse neurons [15]. Given the cell viabilities were greatly decreased after PNMA1 knockdown, we hypothesized whether there is a different relationship between increased PNMA1 expression and cell apoptosis in human PDAC cells. Annexin V/PI staining was performed in AsPC-1 and BxPC-3 cells after cultured in serum-free medium for 48 hours. As shown in Figure 4, expression of sh-1 or sh-2 in AsPC-1 and BxPC-3 both displayed lower early apoptosis ratio. Therefore, above data suggest that PNMA1 may functions as a critical inhibitor of cell apoptosis.
Figure 4.
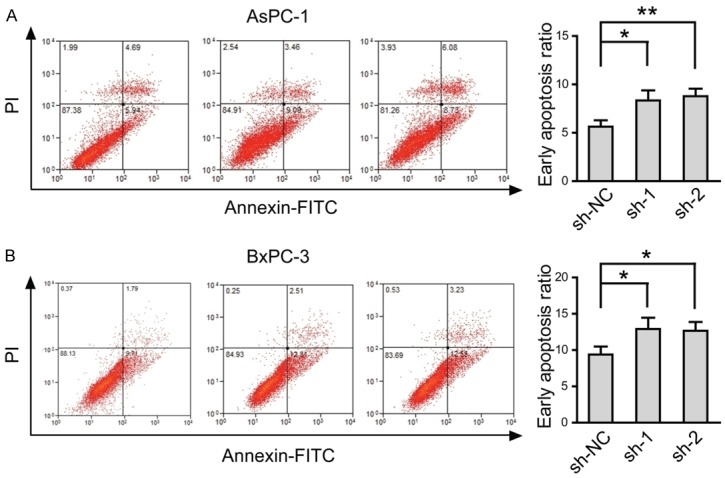
Silencing of PNMA1 promotes cell apoptosis induced by serum deprivation. Apoptosis ratio of AsPC-1 and BxPC-3 cells were analyzed by Annexin V and PI staining and flow cytomeric analysis (sh-NC versus sh-1 or sh-2, *, P<0.05; **, P<0.01). Statistic data shown right are means ± SD of early apoptotic cell rates from triplicate samples. Data are representative of three independent experiments.
Silencing of PNMA1 results in inactivated PI3K/AKT, MAPK/ERK signaling pathway and altered Bcl-2 family proteins
Consideration the decreased cell viabilities and increased cell apoptosis after PNMA1 knockdown, we examined whether PI3K/AKT and MAPK/ERK pathways, which play an important role in tumor growth [17], and Bcl-2 family proteins, which function as critical modulator in cell apoptosis [18], are involved in the function of PNMA1 in pancreatic cancer cells. As shown in Figure 5A, knockdown of PNMA1 led to a decrease in p-Akt and p-Erk1/2 levels in AsPC-1, BxPC-3 cells, respectively. Meanwhile, the anti-apoptotic protein of Bcl-2 family, both Bcl-2 and Bcl-xL significantly decreased after PNMA1 knockdown, while Bax or Bak1, the pro-apoptotic protein, remained unchanged (Figure 5B). These results indicate that PNMA1 promotes PDAC cell growth may via activating PI3K/AKT, MAPK/ERK pathways and altering the expression level of Bcl-2 family proteins.
Figure 5.
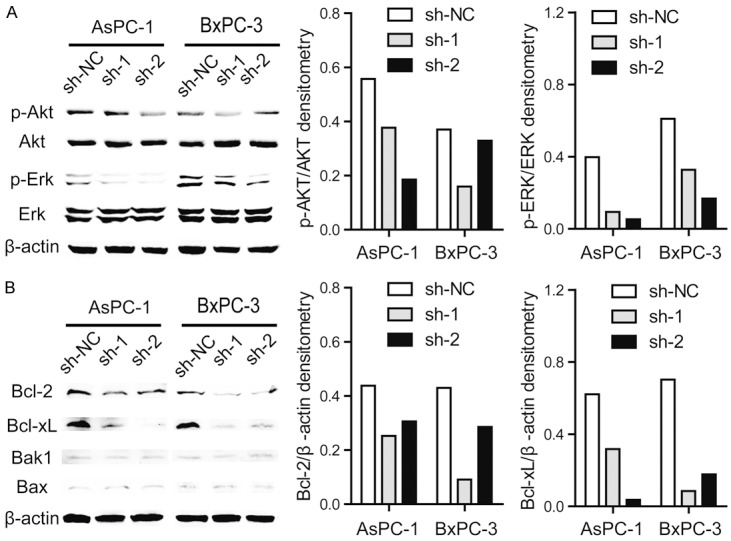
Silencing of PNMA1 reduces activation of p-Akt, p-Erk1/2 and alters the expression of Bcl-2 family proteins. A, B: Western blot analysis of activation of p-Akt, p-Erk1/2 and expression level of Bcl-2, Bcl-xL, Bax and Bak1 in PNAM1 knockdown AsPC-1 and BxPC-3 cells. The photographs are representative immunoblots.
Discussion
The current study focuses on PNMA1, one poorly studied cellular protein whose clinical significances and biological functions in tumors remains unknown. We find that PNMA1 is more commonly observed in PDAC compared with normal pancreas and chronic pancreatitis tissues. In contrast to the previous view of PNMA1 as a pro-apoptotic factor in neuron studies, our present findings indicate that PNMA1 play a pro-survival and anti-apoptotic role in human PDAC cells, thus promoting cell growth.
In normal human and rat tissues, PNMA1 is exclusively distributed in the nuclei and nucleoli of neurons with no or low expression in other organs and tissues except the testis. Previous researches mainly focus on PNS study, while the underlying malignancy remains unknown. By examining multiple pancreatic cancer cell lines we revealed that PNMA1 expression is merely detected in human normal pancreatic duct cells hTERT-HPNE and is amplified during its malignant transformation (Figure 1A, 1B). By analyzing on a TMA of PDAC, we show that the reaction of anti-PNMA1 antibodies was mainly with the cytoplasm (Figure 1C) and elevated PNMA1 expression is closely correlated with larger tumor size (Table 1).
PNMA1 is a member of a family of six proteins that includes MAP-1, PNMA5. Previous studies have demonstrated that PNMA1 and MAP-1 have a pro-apoptotic effect in mouse neurons and several human derived cell lines, respectively [15,19]. MAP-1 was found to connect RASSF1A to Bax physically through a BH3-like (BH: Bcl-2 homology) motif leading to the activation of Bax and cytochrome c release, while apoptosis induced by PNMA1 is likely to involve association of proteins with PNMA1 through its BH3-like sequence. Up-regulation of PNMA5 expression in neurons primed to die raises the possibility that under certain circumstances PNMA5 might participate in inducing neuronal death [11]. All these results support a pro-apoptotic function of PNMA proteins. Since PNMA1 is also abnormally expressed in PDAC cells and tissues, we investigated the effect of PNMA1 on PDAC cells after knockdown. Surprisingly and expectedly, we find suppressed PNMA1 expression decreases the viabilities of cancer cells and promotes cell apoptosis (Figures 3, 4), which are both indispensable for PDAC growth. Compared to the previous studies in PNMA proteins, our results do not stay alone. Firstly, PNMA1 is mainly localized in the nuclei of neurons and concentrated in the cytoplasm of tumor cells, respectively. The abnormal expression of PNMA1 in cytoplasm remains unknown. Secondly, although PNMA1 shares 58% identical amino acid sequence similarity with human MAP-1 protein, an extensive search of available databases did not reveal homology to any known proteins or motifs [5]. Thirdly, overexpression of PNMA1 does not affect viability of the human embryonic kidney HEK293T cells. Taken together, these discrepancies suggest that the pro-survival and anti-apoptotic effect of PNMA1 may depend on a specific mechanism in PDAC. The most possibility is that PNMA1 interacts with another cytoplasmic protein to promote cell growth. In this study, we find PNMA1 knockdown resulted in decreased activation of the PI3K/AKT, MAPK/ERK pathway and deceased expression of the anti-apoptotic proteins of Bcl-2 family (Figure 5B). However, whether the functions of PNMA1 on cell survival and cell apoptosis are mediated by the PI3K/AKT, MAPK/ERK pathways or Bcl-2 proteins remains unconfirmed.
In summary, this study show for the first time that PNMA1 functions to enhance cell viabilities and inhibit cell apoptosis, which ultimately favors cell growth in human PDAC. Although the detailed mechanism remains to be delineated, this preliminary study provides a further consideration the neglected biological functions of PNMA1 in tumors and suggests PNMA1 could be set as a potential therapeutic target of PDAC.
Acknowledgements
We appreciate Drs Jun Li, Ya-Hui Wang, Xiao-Mei Yang and Yan-Li Zhang for assistance in experiments and comments on the manuscript. This work was supported by grants from National High Technology Research and Development Program of China (no.2014AA020609) and additional grants from State Key Laboratory for Oncogenes and Related Genes (YSJJ-2014-01).
Disclosure of conflict of interest
None.
References
- 1.Kaltsas S, Syrigos KN, Saif MW. Pancreatic cancer in 2014. JOP. 2014;15:84–86. doi: 10.6092/1590-8577/2403. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.01.038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.The Lancet Oncology. Pancreatic cancer in the spotlight. Lancet Oncol. 2014;15:241. doi: 10.1016/S1470-2045(14)70097-X. [DOI] [PubMed] [Google Scholar]
- 5.Dalmau J, Gultekin SH, Voltz R, Hoard R, DesChamps T, Balmaceda C, Batchelor T, Gerstner E, Eichen J, Frennier J, Posner JB, Rosenfeld MR. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain. 1999;122:27–39. doi: 10.1093/brain/122.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Schuller M, Jenne D, Voltz R. The human PNMA family: novel neuronal proteins implicated in paraneoplastic neurological disease. J Neuroimmunol. 2005;169:172–176. doi: 10.1016/j.jneuroim.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Tan KO, Tan KM, Chan SL, Yee KS, Bevort M, Ang KC, Yu VC. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J Biol Chem. 2001;276:2802–2807. doi: 10.1074/jbc.M008955200. [DOI] [PubMed] [Google Scholar]
- 8.Voltz R, Gultekin SH, Rosenfeld MR, Gerstner E, Eichen J, Posner JB, Dalmau J. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med. 1999;340:1788–1795. doi: 10.1056/NEJM199906103402303. [DOI] [PubMed] [Google Scholar]
- 9.Albert ML, Darnell RB. Paraneoplastic neurological degenerations: keys to tumour immunity. Nat Rev Cancer. 2004;4:36–44. doi: 10.1038/nrc1255. [DOI] [PubMed] [Google Scholar]
- 10.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 11.Takaji M, Komatsu Y, Watakabe A, Hashikawa T, Yamamori T. Paraneoplastic antigen-like 5 gene (PNMA5) is preferentially expressed in the association areas in a primate specific manner. Cereb Cortex. 2009;19:2865–2879. doi: 10.1093/cercor/bhp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley CJ, Freedman H, Choo SL, Onyskiw C, Fu NY, Yu VC, Tuszynski J, Pratt JC, Baksh S. Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol. 2008;28:4520–4535. doi: 10.1128/MCB.02011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos MD, Dallol A, Eckfeld K, Allen NP, Donninger H, Hesson LB, Calvisi D, Latif F, Clark GJ. The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem. 2006;281:4557–4563. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- 14.Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18:637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Chen HL, D’Mello SR. Induction of neuronal cell death by paraneoplastic Ma1 antigen. J Neurosci Res. 2010;88:3508–3519. doi: 10.1002/jnr.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Yang XM, Wang YH, Feng MX, Liu XJ, Zhang YL, Huang S, Wu Z, Xue F, Qin WX, Gu JR, Xia Q, Zhang ZG. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014;60:1225–34. doi: 10.1016/j.jhep.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15:245–246. doi: 10.4161/cbt.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu ZH, Wang MH, Ren HJ, Qu W, Sun LM, Zhang QF, Qiu XS, Wang EH. Interleukin 7 signaling prevents apoptosis by regulating bcl-2 and bax via the p53 pathway in human non-small cell lung cancer cells. Int J Clin Exp Pathol. 2014;7:870–881. [PMC free article] [PubMed] [Google Scholar]
- 19.Fu NY, Sukumaran SK, Yu VC. Inhibition of ubiquitin-mediated degradation of MOAP-1 by apoptotic stimuli promotes Bax function in mitochondria. Proc Natl Acad Sci U S A. 2007;104:10051–10056. doi: 10.1073/pnas.0700007104. [DOI] [PMC free article] [PubMed] [Google Scholar]


