Abstract
HMGB1 is a necessary and critical mediator of acute lung injury and can act as a chemoattractant and anti-apoptosis factor in injury or repair in diseases. In this study we sought to determine whether HMGB1 is involved in the remodeling of pulmonary artery and investigate the mechanism. A rat model of pulmonary artery remodeling was successful induced with LPS infusion and the increasing of pulmonary arteries media was obviously inhibited in rats treated with thrice inject of HMGB1 neutralizing antibody. The percent of areas of tunica media to total artery wall was (0.53±0.15), (0.81±0.10) and (0.59±0.11) in control, LPS and antibody group respectively (p<0.05). Meanwhile, treatment with HMGB1 neutralizing antibody not only decreased the level of HMGB1 mRNA and protein significantly, but inhibited the expression of PCAN and Bcl-2 as well. On the contrary, Bax, a gen which represented the apoptosis, revealed an absolutely reversed trend to Bcl-2 in pulmonary arteries. Experiments in vitro showed that HMGB1 could stimulate the proliferation of hPASMC in MTT test and increase the number of migrated cells in a concentration-dependent manner in chemotaxis assay using modified Boyden chambers. In conclusion, data from this study support the concept that HMGB1 is involved in the remodeling of pulmonary artery by enhancing proliferation and migration of smooth muscle cell. Inhibiting HMGB1 may be a new target to deal with the remodeling of pulmonary artery.
Keywords: HMGB1, pulmonary artery remodeling, proliferation, migration, apoptosis
Introduction
High mobility group box 1 (HMGB1) which present in nuclei of all mammalians cells has originally been discovered as a chromatin-binding protein that participates in maintaining nucleosome structure and regulating gene transcription, it is currently thought to be a cytokine-like molecule when it is released from necrotic cells, or actively secreted from activated macrophages, dendritic cells, and natural killer cells [1]. Accumulated evidences indicate that HMGB1 is a necessary and critical mediator of various acute lung injury (ALI) [2-4] and chronic pulmonary diseases (CPD) [5,6] in mice and in patients.
Pulmonary artery remodeling (PAR) is one of the base pathogenesis of various forms pulmonary hypertension resulting from CPD. The present and progress of PAR are the main cause leading PH to irreversible and resulting in poor survival [7]. It has long been known that most CPD result from ALI and PAR begin even in the early stage of ALI [8]. Although HMGB1 is well known to be an important molecule in the pathogenesis of ALI and CPD, its role in PAR is still to be explored especially in the early stage as ALI.
The remodeling of pulmonary artery is through to be the result of increased proliferation and resistance to apoptosis of endothelial cells and smooth muscle cells (SMCs) [7]. Studies showed that HMGB1 could act as a chemoattractant factor not only for immunity cells [9], but for SMCs [10] and stem cells [11], and give raise to injury or repair in many diseases. In addition, HMGB1 could protect yeast [12] and mammalian cells [13] against Bax-induced apoptosis. So we hypothesized that HMGB1 may contribute PAR by its chemoattractant and anti-apoptosis function to pulmonary vascular SMC.
Thus, the present study sought to determine whether HMGB1 neutralizing antibody treatment could reduce the thickening of pulmonary arteries tunica media induced with lipopolysaccharide (LPS), and explore that weather HMGB1 could induce the proliferation, migration, and anti-apoptosis of human pulmonary SMC (hPASMC).
Materials and methods
Animal
Adult male Wistar rats weighing 220~250 g were obtained from the Experimental Animals Centre of Shandong University. All experimental protocols were approved by the Experiments Animal Ethics Committee of Shandong University.
The rats were housed five per cage in the regular animal room and given standard laboratory food and tap water ad libitum. All rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital 50 mg/kg and kept under anesthesia during the entire surgical procedures. A set of 30 rats were randomly assigned into three groups: control group (C, n=10), LPS group (L, n=10), and anti-HMGB1 treated group (A, n=10). The PAR rat model was induced with LPS according to [8]. In briefly, LPS (0111: B4, Sigma-Aldrich, St. Louis, MO, USA) 1 mg/kg was dissolved in 2 ml normal saline (NS) and was infused using a micro pump in 30 min after 3 ml of NS administrated in 1 h. The successful model preparation was observed for signs of ALI including: gray and violet lips, followed by listlessness and erect hair after reviving from anesthesia. In control group, 5 ml of NS was pump in 1.5 h. In anti-body group, neutralizing antibody of HMGB1 (2 mg/kg dissolved in NS 2.0 ml) was injected at 12, 24, 48 h separately following the LPS infusion.
Sample
Lungs were taken from anesthetized animal 72 h after treated with NS or LPS. The arteries from right lungs were frozen and stored under -80 for PCR and western blotting experiments. While, the left one were fixed in 30×4% paraformaldehyde for 48 h and used for histopathology and immunohistochemistry test.
Histopathology
Fixed lungs were washed with running water at least for 2 h, dehydrated by a descending ethanol series, cleared in 2 changes of xylene, and mounted with neutral gum, then stained with hematoxylin and feosin. 4 μm sections were examined and photographed by an Olympus BX51 microscope (Olympus Philippines inc., Greenhills, Philippines).
Immunohistochemistry
Paraffin sections were dried for 2 h, de-waxed in 2 changes of xylene, and antigen retrieval in citric acid buffer. After treated in 3% hydrogen peroxide and sealed with 10% goat serum, sections were incubated with polyclonal rabbit anti-rat PCNA, bax or bcl-2 (1:300, 1:50, 1:200; Abcam, Cambridge, UK) 4°C overnight. Washed sections then incubated with secondary antibodies (Rabbit two stage method kit, BIO-LAB, Beijing, China) at 37°C for 60 min. Sections were made visible by adding 3,3’-diaminobenzidine, counterstained with Harris-hematoxylin and mounted with neutral gum. The sections were examined and photographed with light microscope at 400×. An image analysis system (Imagre-Pro Plus Versoin 5.1, Media cybernetics, Inc., Bethesda, MD, USA) was used to measure the thickness of the media in the pulmonary arterioles accompanying respiratory bronchi with outside diameters between 50 to 100 µm in each section according to [8].
Real-time polymerase chain reaction analysis (PCR)
Total RNA was extracted from pulmonary arteries (ID from 50 to 100 μm) by the TRIzon Reagent kit (invitrogen Life Technologies, Grand Island, NY, USA). RNA was reverse-transcribed using the PrimeScript RT reagent Kit (TOYOBO Bio Inc, Osaka, Japan). Relative expression levels of mRNA were determined using a Roche Diagnostics Light Cycler 2.0 Real-Time PCR System (Roche Diagnostics, Shanghai, China) with a SYBR® Premix Ex TaqTM (TaKaRa Bio Inc, Shiga, Japan) and gene-specific primers (β-actin for, GTCGTACCACTGGCATTGTG, β-actin rev CTCTCAGCTGTGGTGGTGAA; Bcl-2 for CTGGTGGACAACATCGCTCTG, Bcl-2 rev GGTCTGCTGACCTCACTTGTG; Bax for CTGCAGAGGATGATTGCTGA, Bax rev GATCAGCTCGGGCACTTTAG. HMGB1 for, GCTCAGAGAGGTGGAAGACCA; HMGB1 rev, GGGGCATTGGGGTCCTTGAA.) A total volume of 20 μl reaction system liquid was subjected to the following PCR program: 30 s at 95°C (initial denaturation) and then a 20°C/s temperature transition rate up to 95°C for 5 s, 60°C for 20 s (HMGB1: 57°C for 20s), 72°C for 15 s and repeated 40 times. All protocols were performed according to the instructions from manufacturer.
Western blotting
Cell lysates from pulmonary arteries were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (PVDF). Subsequently, the membranes were probed with antibodies to mouse Bax, Bcl-2, HMGB1 and β-actin (Abcam, Cambridge, UK). Signals were visualized by the ECL detection system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Cell and culture
hPASMC purchased from Sciencell (Carlsbad, CA, US) were cultivated in DMEM medium containing 100 U/ml of penicillin, 100 ug/ml of streptomycin, and 10% FBS at 37°C and 5% CO2. Medium was changed every other day, and cells passaged to five to eight generations were used for the following experiments.
Proliferation assay
Cells were seeded in 96-well plates (2×105 cells/well) and grown in DMEM supplemented with 10% FBS for 16 h, and then the medium was replaced with serum-free DMEM for 24 h. Subsequently, the cells were grown with medium alone, or medium with the addition of 10% FBS or HMGB1 at the concentration of 1, 10, 100, and 1000 ng/ml for 24 h or 48 h. 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazlium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) 20 μl were added into the medium and incubated for 4 h and then dimethyl sulfoxide 150 μl for 15 min before cells were tested. The absorbance value of colored solution was quantified by measuring at 490 nm wavelength with a multimode microplate readers (TECAN, infinite M200, Männedorf, Switzerland). All experiments were performed in five times.
Chemotaxis assay
Cell migration was assayed using transwell Boyden chambers (corning, Tewksbury, MA, USA). In brief, PVP-free polycarbonate filters with 8 μm pores (Costar) were coated with 0.1% poly-L-lysine (wt/vol). Serum-free DMEM (negative control), DMEM containing 1, 10, 100 or 1000 ng/ml HMGB1, and RPMI with 20% FBS (positive control) were placed in the lower chambers.
hPASMC were grown in DMEM plus 10% FBS, starved overnight, washed twice with PBS to eliminate any floating cells, and harvested with trypsin. hPASMC 5×105 resuspended in 100 μl DMEM were placed in the upper chambers and incubated at 37°C in 5% CO2 for 20 h. Cells remaining on the upper surface of the filters were mechanically removed, and those which had migrated to the lower surface were fixed with methanol, stained with crystal violet stain (Sigma-Aldrich, St. Louis, MO, USA), and counted at 400× in 5 random fields per filter with OLYMPUS CKX41 (Olympus Philippines inc., Greenhills, Philippines).
TUNEL assay
A terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) assay was performed to determine the degree of apoptosis. Starved hPASMC (2×106 cells in 1ml /well) were plated into 6-well plates with slides for 24 h, and then the medium was replaced with DMEM containing 8% FBS only or 1, 10, 100 or 1000 ng/ml HMGB1 and treated for 48 h. Cells fixed with 4% polyformaldehyde were permeabilized in 0.1% triton X-100 solution and marked with biotin. 3’-diaminobenzidine tetrahydrochloride (DAB) mix in an equilibration buffer (0.5 ml) was added to cover the slides. Then the sections were counterstained with hematoxylin, dehydrated by a descending ethanol series, made visible by adding 3,3’-diaminobenzidine, and mounted with neutral gum. Sections were examined using light microscope and the cells colored brown were identified the apoptosis cells. The percentage of apoptosis was defined as apoptosis/all cells in view of one high magnification ×100%. The assay was repeated in five independent experiments.
Statistical analysis
The statistical analysis was employed by SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare mean values across multiple treatment groups, least significant difference (LSD) test to pairwise comparison, and Chi-Square test to assess rates form groups. A value of p<0.05 was considered significant.
Results
HMGB1 neutralizing antibody treatment reduced LPS-induced pulmonary arteries media thickening
The increased thickness of pulmonary arterioles media are the mark of PAR. A PAR rat model was successful induced with LPS infusion as shown in Figure 1A and signs of ALI. Compared to the control group, the media of pulmonary arteries (ID from 50 to 100 μm) increased obviously in LPS group (Figure 1A). However, they were thinner in rats of anti-HMGB1 group than those in LPS group (Figure 1A). The areas percent of tunica media to total artery was (0.53±0.15), (0.81±0.10), (0.59±0.11) in control, LPS and antibody group respectively, and there was a significantly different in statistics (Figure 1B).
Figure 1.
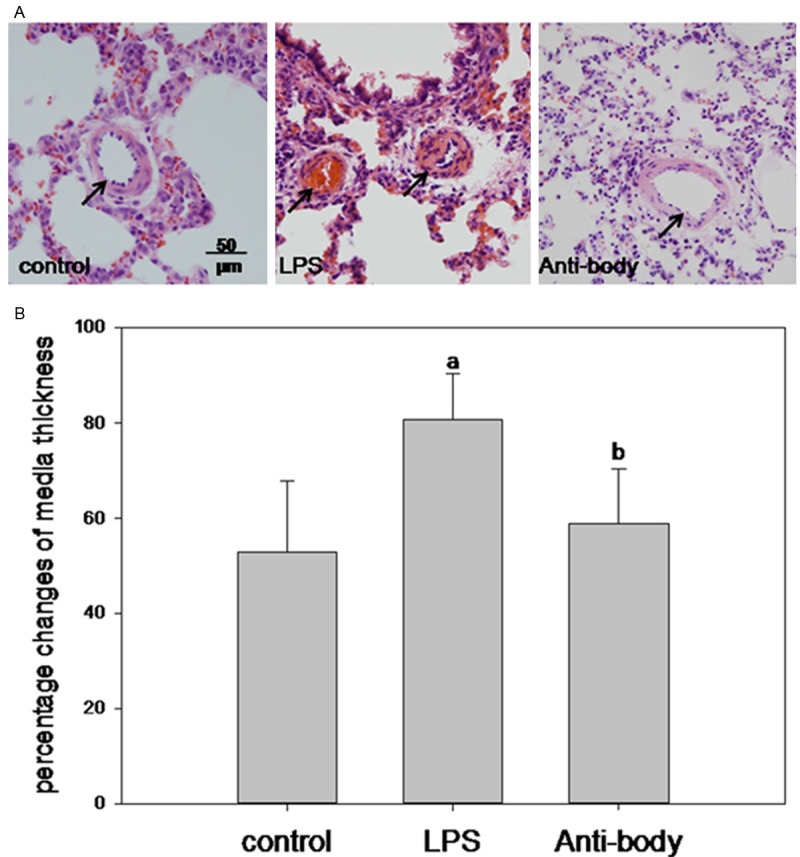
Changes of pulmonary arteries media thickness. (A) Representative pulmonary arterioles sections of HE staining (media thickness marked with arrow), and (B) percent of media areas to total artery in different groups. ap<0.05 vs control; bp<0.05 vs LPS.
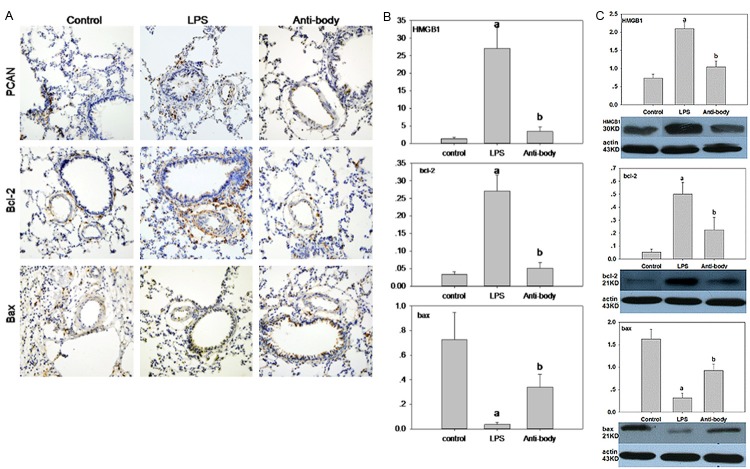
HMGB1 neutralizing antibody treatment inhibited LPS-induced expression of PCNA and Bcl-2 of pulmonary arteries
To gain insight into the mechanism of HMGB1 involving in the progress of PAR, we tested the effect of HMGB1 on the expression of PCNA, Bcl-2 and Bax which are thought to be responsible for the proliferation and apoptosis in many kind of cell. HMGB1, PCNA and Bcl-2 had a high expression in LPS group detected with immunohistochemistry (Figure 2A), real-time PCR (Figure 2B), or western blotting (Figure 2C). Treatment with HMGB1 neutralizing antibody decreased not only the level of HMGB1 mRNA and protein significantly, but the level of PCAN and Bcl-2 as well. On the contrary, Bax, a gen which represented the apoptosis, revealed an absolutely reversed trend to Bcl-2 (Figure 2).
Figure 2.
Expression of PCNA, Bcl-2, Bax and HMGB1 in different groups. A. Representative immunohistochemistry sections of PCNA, Bcl-2 and Bax. B. Relative expression of HMGB1 mRNA, Bcl-2 mRNA, Bax mRNA. C. Relative protein levels of HMGB1, Bcl-2 and Bax detected with western blotting. ap<0.05 vs control; bp<0.05 vs LPS.
HMGB1 stimulated the proliferation of hPASMC
To gain further insight into the mechanism of HMGB1 in the remodeling of pulmonary artery, we next tested the effect of HMGB1 on the proliferation, chemotaxis and apoptosis of hPASMC in vitro. Figure 3 showed that there was a concentration-dependent increase in the absorbance value of hPASMC after stimulation with grade HMGB1 and a time-dependent increase with up to 48 h, whereas only slight proliferation occurred between 100 ng/ml and 1000 ng/ml tested at 24 and 48 h. It suggested that HMGB1 could promote the proliferation of hPASMC at indicated concentration at least lasting up to 48 h.
Figure 3.
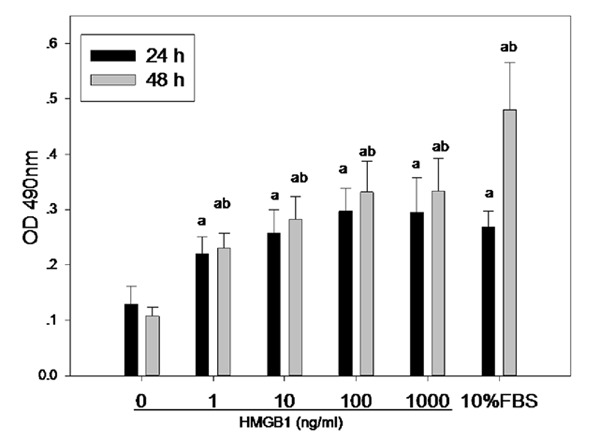
Effect of HMGB1 on the proliferation of hPASMC. Optical density (OD) value under 490 nm was detected with MTT assay. ap<0.05 vs control (0 ng/ml); bp<0.05 vs 24 h.
HMGB1 induced hPAMSC migration
The chemotactic effect of HMGB1 was determined with a chemotaxis assay using modified Boyden chambers. Compared to the control group, there was a concentration-dependent increase in the number of hPASMC migrated to the lower surface of filters with HMGB1 concentration up to 100 ng/ml, whereas the increase ceased when the concentration of HMGB1 in media reached 1000 ug/ml (Figure 4).
Figure 4.
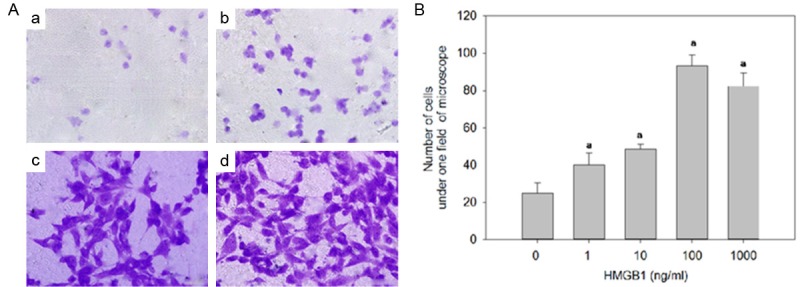
Effect of HMGB1 on the migration of hPASMC. A. Representative photos of hPASMC migrated to the lower surface stained with crystal violet stain. B. Number of hPASMC counted in one field of light microscope (400×). ap<0.05 vs. control (0 ng/ml).
HMGB1 didn’t impacted the apoptosis of hPASMC
To detect the apoptotic cells, hPASMC were incubated with graded concentration of HMGB1 (0, 1, 10, 100, 1000 ng/ml) for 48 h. TUNEL staining was performed and TUNEL-positive cells were counted with light microscope. A prospective increasing tread of apoptosis was not shown in Figure 5. On the contrary, a clearly decreasing was revealed even though a significantly different was not detected in statistics (p>0.05).
Figure 5.
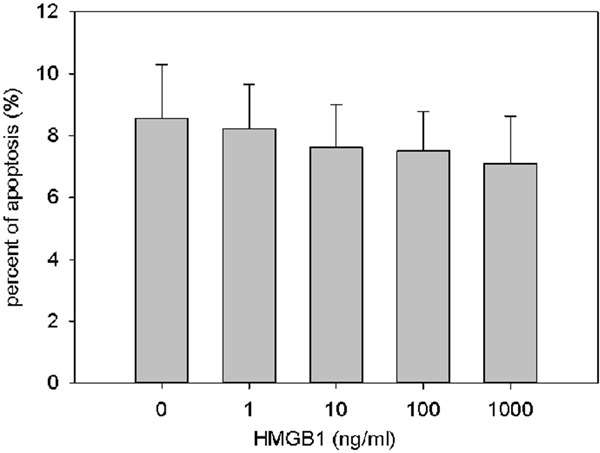
Effect of HMGB1 on apoptosis of hPASMC. Percent of the apoptotic cell.
Discussion
HMGB1 can be released into intravascular spaces by cells of the innate immune system, damaged tissues and necrotic cells in response to bacterial endotoxin and/or hypoxia [14-16], and its plasma levels correlate well with poor prognosis and high mortality in patients with ALI [3,4]. Previous studies have documented that administration of anti-HMGB1 antibodies or inhibitors can protect mice from various ALI significantly [2-4]. In the present study, we showed that HMGB1 neutralizing antibody treatment could reduce the thickening of pulmonary arteries tunica media and inhibit the expression of PCNA and Bcl-2 induced with LPS infusion in vivo. We also showed that the nuclear protein HMGB1, when presented in the medium, could promote hPASMC proliferation and chemotaxis in vitro. In other word, HMGB1 is involved in the development of PAR by enhancing proliferation and migration of SMC.
Endotoxin, an intrinsic component of the outer membrane of gram-negative bacteria, is composed of LPS and is known to cause acute lung injury (ALI) [3,8]. The study of Wang et al [8] has documented that there is remodeling of the pulmonary arteries in ALI induced with a small dose LPS constant infusion. In our present study, rats in model group revealed appreciable signs of ALI accompanied with thickening of pulmonary arteries tunica media at 72 h after LPS infusion just like those in Wang’s experiment. This indicated that the PAR model of acute lung injury was induced successfully. As a late cytokine mediator of lethal systemic inflammation, HMGB1 is time-dependently released from endotoxin-stimulated ALI, with serum concentrations of HMGB1 rising within 8 to 32 hours after the administration of LPS [3,14]. Based on the kinetics of HMGB1 accumulation in serum, and the relatively short biological half-life of antibodies of HMGB1 [17,18], we reasoned that repeated dose of antibodies might be required to neutralize HMGB1 completely. Results indicated that administration of anti-HMGB1 in three doses at 12, 24, 48 h after LPS infusion could decrease the media thickness in the pulmonary arterioles significantly.
Studies have demonstrated HMGB1 can act as a chemoattractant factor and promote the proliferation for many kinds of cell including SMC from thoracic aorta [10]. The results in vivo of us in the present study from PCNA detected with immunohistochemistry suggest that HMGB1 promoting the progress of PAR may be result from its effect of accelerating proliferation and facilitating migration of SMC, and this was further demonstrated with our experiments in vitro.
Previous studies have also demonstrated that HMGB1 can inhibit the expression of pro-apoptosis protein Bax and promote the expression of anti-apoptosis protein Bcl-2 in yeast [12], cancer cell [19] and PMN [20] against apoptosis. Our experiments in vivo revealed that the Bax protein, a proapoptotic gene product, was strongly expressed in medial media of pulmonary arteries in group C and A, whereas it was weakly expressed in group L. In contrast, the expression of bcl-2 protein, an antiapoptotic gene product, was rarely observed in medial media of pulmonary arteries in group C and A, whereas it was strongly expressed in group L. Thus, HMGB1 promoting the progress of PAR may be results from its regulating apoptosis gene expressions. But, TUNEL test of hHPASMC can’t provide further evidence supporting our results in vivo. Literatures have indicated that HMGB1 can also trigger apoptosis of T lymphocytes [21], cardiomyocytes [22], and hepatocytes [23] after a certain stimulus or injury in vitro or in vivo. So if the apoptosis of hPASMC involving in the pathology of PAR are still to be explored in the future.
There are other limitations in this study. First, PAR model was successfully induced with LPS and treatment with HMGB1 neutralizing antibody certainly did reverse the PAR partly in the present study, but it will be more well-grounded and reliable if a PAR model induced with HMGB1 used. Second, even though the previous findings have demonstrated that the receptor of advanced glycation end products and the mitogen-activated protein kinase contributed to the HMG1-induced cell migration [10] and proliferation [24], the precise mechanism of HMGB1 promoting the PAR are need to be elicited.
In conclusion, data from this study may give us the impression that HMGB1 is involved in the progress of pulmonary artery remodeling by enhancing proliferation and migration of SMC. Inhibiting HMGB1 may be a new target to deal with the remodeling of pulmonary artery.
Acknowledgements
This work was partly supported by Shandong Provincial Natural Science Foundation, P.R.China (Y2007C115, ZR2011HM028, H.W., 2009ZRB14031, W.L.ZR2010HM120, C.W.) and Shandong Province Science and Technology Plan Project (2010GWZ20246, B.S.).
Disclosure of conflict of interest
None.
References
- 1.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005 Jul;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000 Sep;165:2950–54. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 3.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004 Dec 15;170:1310–6. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 4.Luan ZG, Zhang XJ, Yin XH, Ma XC, Zhang H, Zhang C, Guo RX. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology. 2013 Oct;218:1261–70. doi: 10.1016/j.imbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, Dombret MC, Sims GP, Kolbeck R, Coyle AJ, Aubier M, Pretolani M. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010 May 1;181:917–27. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 6.Ko HK, Hsu WH, Hsieh CC, Lien TC, Lee TS, Kou YR. High expression of high-mobility group box 1 in the blood and lungs is associated with the development of chronic obstructive pulmonary disease in smokers. Respirology. 2013 Dec 24; doi: 10.1111/resp.12209. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Grant JS, White K, MacLean MR, Baker AH. MicroRNAs in pulmonary arterial remodeling. Cell Mol Life Sci. 2013 Dec;70:4479–94. doi: 10.1007/s00018-013-1382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Gong J, Pei L, Shan S, Tan W. The effect of rhBMP-2 on pulmonary arterioles remodeling in endotoxin-induced acute lung injury in rats. Clin Exp Med. 2013 Aug;13:187–92. doi: 10.1007/s10238-012-0197-2. [DOI] [PubMed] [Google Scholar]
- 9.Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol. 2009 Sep;86:609–15. doi: 10.1189/jlb.0908576. [DOI] [PubMed] [Google Scholar]
- 10.Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001 Mar 19;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004 Feb 2;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brezniceanu ML, Völp K, Bösser S, Solbach C, Lichter P, Joos S, Zörnig M. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003 Jul;17:1295–307. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Zhu H, Wang T, Sun Y, Ni P, Liu Y, Tian S, Amoah Barnie P, Shen H, Xu W, Xu H, Su Z. Exogenous high mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/TLR4-dependent signal pathways. Scand J Immunol. 2014;79:386–94. doi: 10.1111/sji.12174. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMGB-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 15.Sama AE, D’Amore J, Ward MF, Chen G, Wang H. Bench to bedside: HMGB1-a novel proinflammatory cytokine and potential therapeutic target for septic patients in the emergency department. Acad Emerg Med. 2004;11:867–73. doi: 10.1197/j.aem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 16.De Marco RA, Fink MP, Lotze MT. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Mol Immunol. 2005;42:433–44. doi: 10.1016/j.molimm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5:338–43. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- 18.Walker RE, Spooner KM, Kelly G, McCloskey RV, Woody JN, Falloon J, Baseler M, Piscitelli SC, Davey RT Jr, Polis MA, Kovacs JA, Masur H, Lane HC. Inhibition of immunoreactive tumor necrosis factor-alpha by a chimeric antibody in patients infected with human immunodeficiency virus type 1. J Infect Dis. 1996 Jul;174:63–8. doi: 10.1093/infdis/174.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Yang Q, Xu J, Qian G, Liu Y. Effects of HMGB1 on PMN apoptosis during LPS-induced acute lung injury. Exp Mol Pathol. 2008 Dec;85:214–22. doi: 10.1016/j.yexmp.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Z, Li Q, Wu P, Ye Y, Tseng HY, Zhang L, Zhang XD. Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1. Autophagy. 2012 Jan;8:109–21. doi: 10.4161/auto.8.1.18319. [DOI] [PubMed] [Google Scholar]
- 21.Wu ZS, Yao YM, Hong GL, Xu XP, Liu Y, Dong N, Zheng JY, Lu ZQ, Zhao GJ, Zhu XM, Zhang QH, Sheng ZY. Role of mitofusin-2 in high mobility group box-1 protein-mediated apoptosis of T cells in vitro. Cell Physiol Biochem. 2014;33:769–83. doi: 10.1159/000358651. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda N, Inoue K, Ikeda T, Hara Y, Wake K, Sato T. Apoptotic response through a high mobility box 1 protein-dependent mechanism in LPS/GalN-induced mouse liver failure and glycyrrhizin-mediated inhibition. PLoS One. 2014 Apr 1;9:e92884. doi: 10.1371/journal.pone.0092884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Zhou X, Hu X, Jiang H. H2O2 evokes injury of cardiomyocytes through upregulating HMGB1. Hellenic J Cardiol. 2014 Mar-Apr;55:101–6. [PubMed] [Google Scholar]
- 24.Xu X, Zhu H, Wang T, Sun Y, Ni P, Liu Y, Tian S, Amoah Barnie P, Shen H, Xu W, Xu H, Su Z. Exogenous high mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/TLR4-dependent signal pathways. Scand J Immunol. 2014;79:386–94. doi: 10.1111/sji.12174. [DOI] [PubMed] [Google Scholar]