Abstract
Breast cancer (BC) is the most common malignancy among women. We aimed to illuminate the molecular dysfunctional mechanisms of BC progression. The mRNA expression profile of BC GSE15852 was downloaded from Gene Expression Omnibus database, including 43 normal samples and 43 cancer samples. Differentially expressed genes (DEGs) in BC were screened using the t-test by Benjamin and Hochberg method. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of the selected DEGs were enriched using Hypergeomeric distribution model. In addition, functional similarity network among the enriched pathways was constructed to further analyze the collaboration of these pathways. We found 848 down-regulated DEGs were associated with 16 significant dysfunctional pathways, including PPAR signaling fatty acid metabolism, and 1584 up-regulated DEGs were related to 6 significant dysfunctional pathways, like cell cycle, protein export, and antigen processing and presentation in BC samples. Crosstalk network analysis of pathways indicated that pyruvate metabolism, propanoate metabolism, and glycolysis gluconeogenesis were the pathways with closest connections with other pathways in BC. In addition, other antigen processing and presentation, including 19 DEGs; PPAR signaling pathway, including 18 DEGs; and pyruvate metabolism pathway, including 13 DEGs were further analyzed. Our results suggested that dysfunctional of significant pathways can greatly affect the progression of BC. Several significant disorder pathways were enriched in our comprehensive study. They may provide guidelines to explore the dysfunctional mechanism of BC progression.
Keywords: Breast cancer, differentially expressed genes, pathway crosstalk, dysfunctional
Introduction
Breast cancer (BC) is one of the most common invasive malignancies in the world, and is the second leading cause of cancer death among women [1]. The incidence of BC is lower in Asia than in western countries, such as Europe and North America, and it varies four- to five fold across countries [2]. The percentage of female BC in all new female cancers is 27% in the developed countries [3], while the top one women malignant tumor both in urban areas and in cancer registration areas of China is BC [4]. Though the morbidity of BC is rising, the death rate of BC in America is declining in recent years, possibly in part, due to the reduced use of hormone replacement therapy [5]. Usually, the breast tumors generated on the mammary epithelial cells (MEC), and the incidence of BC is higher in pre-menopausal and post-menopausal women [6]. Hormone therapy, drug therapy, chemotherapy, and breast removed operation, are the mainly methods for BC treatment. Nevertheless, BC patients have an anomalously high rate of relapse after long periods of tumor remission [7]. In addition, current medical management of BC patients is not satisfactory, which has a great impact on the female’s life quality suffered from BC and places an enormous economic burden on the sick patients’ family [8-10].
Lots of reports have been published to investigate the mechanism of progression and relapse of BC. It is reported the existence of dangerous, tiny metastases such as regional lymph nodes, bone marrow, lung, proliferating cells and liver remain in the blood may cause the relapse of BC, which are under a condition of growth restricted and in a clinically undetectable size but can restart growth until transforming event occurs [11-13]. Studies demonstrated that physical activity is likely to decrease BC risk. Multiple interrelated biologic pathways may involve in BC including adiposity, sex hormones, adipokines, insulin resistance, and chronie inflammation [14,15]. Notch-family members have been linked to uPA signaling which were demonstrated to reduce cell proliferation and invasion of BC cells. Haffty said that the relapse endangers of HER-2 in BC is high, and has a poor prognosis [16]. In Kent’s study, the HER-2 receptor pathway could activate AIBI by phosphorylation signaling on the development and progression of BC [17]. HER-2/ErbB-2 receptor (HER-2) signaling pathways can indeed decrease recurrence rates for BC that are characterized by HER-2 overexpression [18]. A correlation between Notch signaling, Hedgehog (Hh) pathway, Wnt signaling and the BC recurrence has also been suggested.
With the search for BC susceptibility genes going at an enormous speed, BC has been proved to be a polygenic disease and associated with multifactor. Two major genes BRCA1 and BRCA2 are considered to lead to the pathology of tissue-specific somatic mutations in BC. One of Fanconi Anemia (FA) genes is identical to the well-known BC susceptibility gene, BRCA2 [19]. Nowadays, lots of researches supported that cancers are some kind metabolic diseases, which related to a wide range of metabolic disorders [14]. Although disordered intermediary metabolism in cancer cells has been known for the better part of these decades, little attention has been paid to fatty acid metabolism. Only Ellen reported the differences of fatty acid synthesis between cancer and normal cells in the vitro setting and in vivo of human BC [20]. In some studies, the positive association between obesity and BC was also found to be independent [21]. In spite of a number of molecules have been implicated in the metastasis of BC, the precise mechanisms of how these factors and pathways interact with each other are still remain to be established.
Microarray analysis is an effective approach to monitor the global alterations of gene expression and identify pathways that are important to BC-developing progress. In this present study, microarray analysis was used to monitor differential gene expression in BC samples compared to the control-group. Comprehensive bioinformatics analysis was used to enrich the pathways that were closely related to BC, as well as the collaboration of dysfunctional pathways to provide deeper insight into the biological mechanisms of female BC. Using this approach, we were able to predict the significant pathways that are most likely associated with BC and identify the molecular mechanisms that could serve as novel therapeutic methods.
Data and methods
Affymetrix microarray data and data preprocessing
The microarray and other forms of high flux data produced by scientific communities were archived and freely released in the Gene Expression Omnibus (GEO) database of NCBI [22], which is the biggest completely public storage. We extracted the mRNA expression profiles from the study of Pau et al [23], which were deposited in GEO database (ID: GSE15852) based on the Affymetrix Human Genome U133A Array. The study contains a total of 86 samples of BC, including 43 case-samples and 43 control-samples that paired with the case-samples.
The Robust Multichip Average (RMA) method in R software including background adjustment, quintile normalization and summarization, was used to preprocess the downloaded raw data. After the ID (Entrez gene ID) transformation between probes and genes, probe mean was used as the gene expression value in the case of different probes were mapped to the same gene. Finally, we constructed the gene expression spectrum matrix (12633*86), with rows delegated by genes and columns delegated by samples.
DEGs identification
Differentially expression analysis between the case-samples and control-samples were conducted using the double sampling T-test method [24], and the relevant P-value of each gene was collected. The FDR-value was adjusted by Benjamin and Hochberg (BH) method based on the multtest package, and 0.05 was used as the cut-off criterion.
Pathway enrichment analysis
The Kyoto Enrichment of Genes and Genomes (KEGG) pathway database contains information of how molecules or genes are networked, which is complementary to most of the existing molecular biology databases containing the information of individual genes [25]. The significant functions and pathways of the DEGs were analyzed on the hyper-geometric distribution KEGG database, which was downloaded from the molecular signatures database (MSigDB), containing 186 classical pathways and with a version number of c2.cp.kegg.v3.0.entrez.gmt [26], 0.05 was used as the cut-off criterion of FDR-value for the enrichment.
In addition, we supposed N is the total genes in KEGG pathways, n is the number of DEGs in these pathways, and m stands for the number of DEGs in one enriched pathway, and k represents the number of DEGs in each selected pathway (Equation 1).
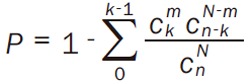 |
Pathway collaboration analysis
The functional similarity network of the enriched pathways was constructed to identify the collaboration of pathways. Jaccard Index [27] was used to calculate the crosstalk between the enriched pathways, which is calculated as follows:
Jaccard (pathA, pathB) = #intersection (pathA, pathB) / #union (pathA, pathB)
Whereas, #intersection (A, B) stands for the common gene sets between pathway A and pathway B, and #union (A, B) represents the union set of genes in pathway A and pathway B.
Results
Screening and functional analysis of DEGs
In order to get DEGs about BC, we obtained publicly available microarray dataset GSE15852 from GEO. A total of 2432 DEGs were selected with a FDR < 0.05, including 848 down-regulated DEGs and 1584 up-regulated DEGs.
To gain further insights into the biological functions and pathways of DEGs selected in this study, we used Hyper-geometric Enrichment Analytic method to identify the significant pathways in KEGG database. Six significant pathways of up-regulated DEGs were enriched, such as “cell cycle pathway”, protein synthesis and transport related pathway “ribosome”, “protein export”, and “antigen processing and presentation pathway” (Table 1). In addition, we enriched 16 significant pathways that the down-regulated DEGs involved in, mainly containing the classical cell signaling pathway in disorder cancers (Table 2).
Table 1.
Pathway enrichment analysis of up-regulated differential genes
| Pathway-name | #Gene | #DEGs | P-value | FDR |
|---|---|---|---|---|
| KEGG ribosome | 88 | 40 | 0 | 0 |
| KEGG spliceosome | 128 | 50 | 0 | 0 |
| KEGG cell cycle | 128 | 31 | 3.72E-06 | 0.000231 |
| KEGG protein export | 24 | 10 | 6.52E-05 | 0.003032 |
| KEGG Selenoamino acid metabolism | 26 | 9 | 0.000779 | 0.028986 |
| KEGG Antigen processing and presentation | 89 | 19 | 0.001508 | 0.046747 |
DEG: Differentially expressed gene; FDR: The False Discovery Rate.
Table 2.
Pathway enrichment analysis of down-regulated differential genes
| Pathway-name | #Gene | #DEGs | P-value | FDR |
|---|---|---|---|---|
| KEGG glycolysis gluconeogenesis | 62 | 12 | 0.000548291 | 0.007844774 |
| KEGG citrate cycle TCA cycle | 32 | 8 | 0.000801373 | 0.010646817 |
| KEGG fatty acid metabolism | 42 | 16 | 2.71E-09 | 5.03E-07 |
| KEGG PPAR signaling pathway | 69 | 18 | 2.40E-07 | 2.23E-05 |
| KEGG pyruvate metabolism | 40 | 13 | 7.53E-07 | 4.67E-05 |
| KEGG propanoate metabolism | 33 | 11 | 4.13E-06 | 0.000192151 |
| KEGG valine leucine and isoleucine degradation | 44 | 12 | 1.56E-05 | 0.000580369 |
| KEGG butanoate metabolism | 34 | 10 | 3.95E-05 | 0.001224683 |
| KEGG adipocytokine signaling pathway | 67 | 14 | 8.06E-05 | 0.002140577 |
| KEGG tryptophan metabolism | 40 | 10 | 0.00017942 | 0.004171524 |
| KEGG limonene and pinene degradation | 10 | 5 | 0.00022134 | 0.004574357 |
| KEGG beta alanine metabolism | 22 | 7 | 0.000345338 | 0.005839352 |
| KEGG glyoxylate and dicarboxylate metabolism | 16 | 6 | 0.000339575 | 0.005839352 |
| KEGG complement and coagulation cascades | 69 | 13 | 0.000427426 | 0.006625096 |
| KEGG lysine degradation | 44 | 9 | 0.001791461 | 0.02221412 |
| KEGG glycine serine and threonine metabolism | 31 | 7 | 0.003196366 | 0.037157757 |
DEG: differentially expressed gene; FDR: false discovery rate.
Pathway collaboration analysis
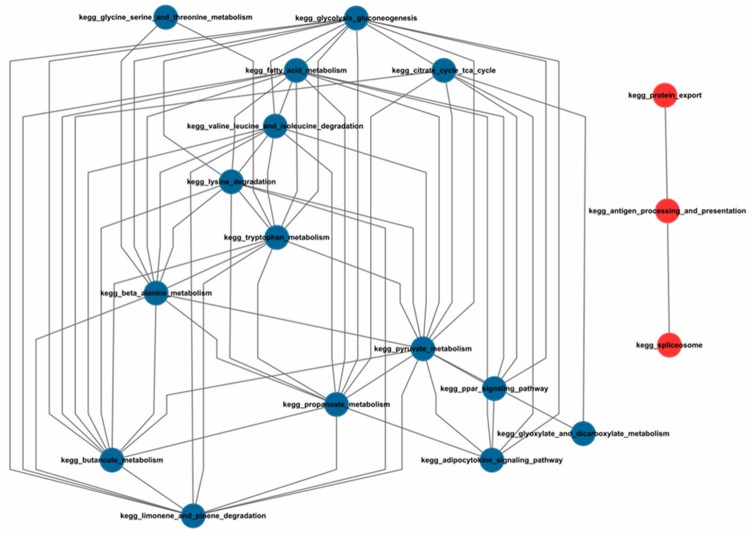
In order to identify the collaboration of the enriched pathways, a functional similarity network was constructed: nodes represent pathways, while edges represent the Jaccard value. The Jaccard Index was used to calculate the crosstalk between pathways, which was ruled with two conditions: (1) the Jaccard value of genes in the two pathways was over zero, (2) at least one DEG was overlapped in the two pathways. We got 65 pairs of pathways based on the 22 enriched pathways, of which 18 pathways had the crosstalk (Figure 1). According to the node numbers in the crosstalk network, we collected 3 pathways with most close connections with others, which were pyruvate metabolism (13 nodes), glycolysis gluconeogenesis (12 nodes) and propanoate metabolism (11 nodes).
Figure 1.
Collaboration network of pathways. *The up-regulated pathways are marked in red; the down-regulated pathways are marked in blue.
Dysfunctional analysis of crucial pathways
To obtain a deep investigation of the dysfunctional mechanism of BC, we further studied the function crosstalk network, and found that many dysfunctional pathways were involved in BC, such as the immuno-inflammatory pathway (antigen processing and presentation), the classical down-regulated cell signaling pathway (PPAR signaling pathway), and the most closely interacted metabolic pathway (pyruvate metabolism).
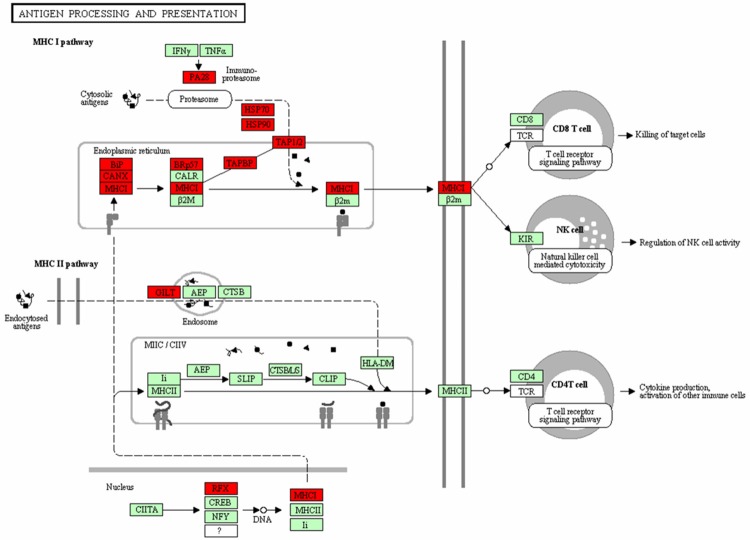
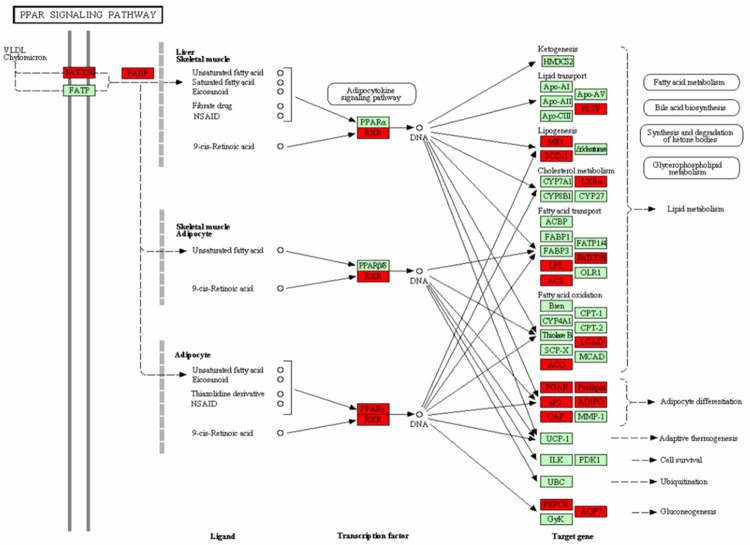
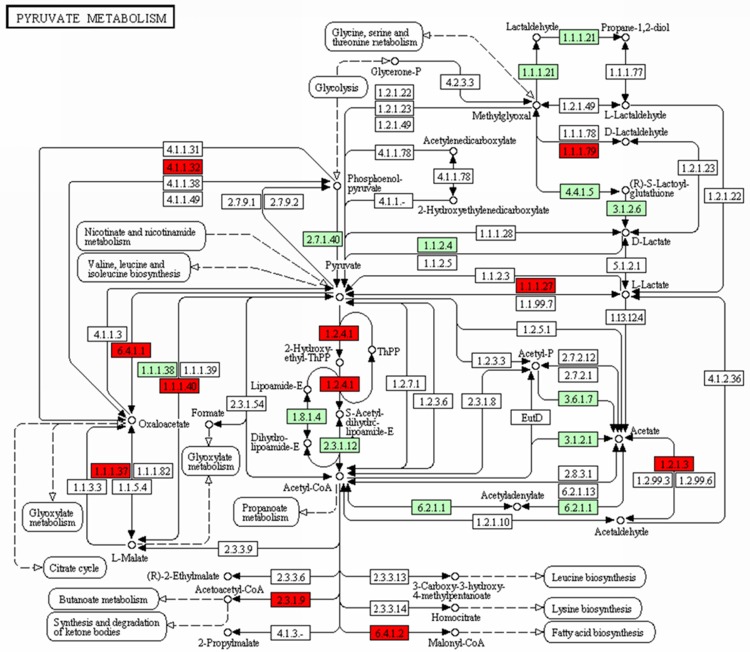
Antigen processing and presentation pathway was up-regulated, which contained 19 DEGs, such as HSP70, HSP90, TAPBP, and MHC (Figure 2, MHC I pathway), while the signaling pathway and pyruvate metabolism pathway were down-regulated, which containing 18 DEGs and 13 DEGs respectively. Targets genes such as LPL, LEP, PLTP, and PPAR, and transcription factors like PPARγ and RXR were the DEGs enriched in signaling pathway (Figure 3), Moreover, ACAT1, ALDH2, ACACB, MDH1, and PDHA1, were the selected DEGs involved in the pyruvate pathway (Figure 4). Additionally, LEP was the gene in adipocytokine signaling pathway and LPL in lipid metabolism pathway.
Figure 2.
The antigen processing and presentation pathway. *Genes with red are differentially expressed genes while genes with green are non-differentially expressed genes.
Figure 3.
The PPAR signaling pathway. *Genes with red are differentially expressed genes while genes with green are non-differentially expressed genes.
Figure 4.
The pyruvate metabolism pathway. *Genes with red are differentially expressed genes while genes with green are non-differentially expressed genes.
Discussion
Breast cancer is one of the most common malignancies among women, which is second only to the metrocarcinoma of all the female tumors and being a main threaten to female’s health [1,2]. Most BC cases arising at a young age, and to a great extent, it is due to the inheritance of dominant susceptibility genes and easy to relapse even after surgery removed of the primary tumor [7]. However, investigations in histological sections of BC patients, as well as clinical statistics, have demonstrated a pathway dysfunctional process of BC that appears to contribute to the process of BC [28,29]. We screened 848 down-regulated DEGs and 1584 up-regulated DEGs in the 43 samples of BC, and they were found mainly function in the metabolism, immuno-inflammatory response, protein synthesis and transport, and signaling related pathways, of which “cell cycle, antigen processing and presentation, and ribosome” were the up-regulated pathways, while “fatty acid metabolism, PPAR signaling pathway, and adipocytokine signaling pathway” were the down-regulated pathways. Besides, the 19 up-regulated DEGs in antigen processing and presentation pathway included HSP70, HSP90, TAPBP, and MCH. PPAR signaling pathway included 18 down-regulated DEGs, such as PPARγ, RXR, LPL, LEP, and ACS, while 13 down-regulated DEGs, such as ACAT1, ALDH2, ACACB, MDH1, and PDHA1 were in the pyruvate metabolism pathway.
The disorder mechanism of pathways in BC remains unknown. Glycolysis metabolism is very activate in malignant cells, which is called the Warburg effect [30], and some glycolysis enzymes, like HK-II, LDH, and GAPDH have over-expression levels in tumor cells [31]. Studies demonstrated that a high rate of glycolytic flux is a central metabolic hallmark of neoplastic tumors because glycolysis was utilized by cancer cells preferentially to satisfy their increased energetic and biosynthetic requirements [32]. In Gonzalez’s study, the propanoate metabolism pathway, which commutated with 11 pathways, was most consistently up-regulated in basal-like residual cancers [33]. In this work, we can see from the crosstalk network that the progression of BC were involved in multiple function disorders, such as propanoate metabolism and glycolysis gluconeogenesis pathway, which were not isolated but interacted with each other orderly and synergistically.
Heat shock protein (HSP) gene, which encodes HSP proteins, participating in several biological processed, such as the subunit composition, protein folding and degradation, and activity and function regulation of targeted protein. Many reports indicated that HSP was related to the proliferation, apoptosis, differentiation, immunity, and drug resistance of tumor cells [34]. In Jolly’s study, the expression level of HSP90 in tumor cells was twice- to ten-fold as in normal cells, which indicated HSP90 can mediate the invasion and metastasis of tumor cells [35]. Besides, HSP70 has the ability to enhance the heat resistance and proliferation of BC cells [36]. Transporter that associated with antigen processing (TAP) gene, can encode “the transporter involve in antigen processing” proteins [37]. The high expression level of TAP mRNA could contribute to the processing and presenting to the cell surface of tumor antigens, so as to be recognized and killed by the T lymphocytes [38]. In recent years, some researchers have pointed out that the decreased expression of cell histocompatibility complex (MHC) antigen is an important mechanism for tumors escaping the body immune surveillance [39]. In Zuo’s study, the down-regulated expression of MCH I antigen was found on the surface of many human malignancies, which indicated its relationship with tumor progression and exacerbation [40]. Janice said that T cells could recognize the antigen-presenting cells depending on their expression of a spectrum of peptides bound to major MHC I molecules [41]. Also, Harumichi and his colleagues proved the inflammatory cytokines TGF-βplayed an important role in the generation of effector and memory CD8+ T cells [42]. On the basis of our research, both HSP70 and HSP90 were genes in the MHC I pathway, and the up-regulated expression of HSP70 and HSP90 may indicate that HSP70 plays a role in preventing the BC progress, while HSP90 contributes to the BC developing via MHC I pathway.
1-Aminocyclopropane-1-carboxylate synthase (ACS) gene is involved in fatty acids synthesis. A biologically aggressive of human BC and other malignancies is characterized by elevated fatty-acid synthase enzyme expression and elevated fatty acid synthesis [43]. Leptin (LEP) is a gene associated with obesity, which is significantly down-regulated in BC tumors. The association between obesity and BC has been reported in recent years. Compared to obese female before menopause, obese females after menopause have a higher risk of developing BC. LEP act as cell growth regulator in breast tumor-genesis pathways [44]. Another fatty acid and lipid associated gene, lipoprotein lipase (LPL), can encode the regulator protein LPL. Reports have demonstrated that LPL deficiency could prevent the breakdown of lipid and result in its accumulation in the blood. In addition, LPL gene was observed in BC, prostate cancer, and B-cell chronic lymphocytic leukemia [45-47]. Peroxisome proliferators-activated receptor-gamma (PPARγ), which is a ligand dependent type nuclear transcription factor, is a member of the nuclear hormone receptor superfamily. Only combined with retinoid X receptor (RXR), which was another nuclear hormone receptor superfamily member, could transcription factor PPARγ recognize the target genes by the combination with product heterogeneous dimer PPARγ/RXR [48]. Researches showed that PPARγ expressed in many kinds of tumor tissues, and had the ability to inhibit the proliferation of malignant cells after being activated by the ligand, such as human BC, prostatic cancer, and lung cancer [49]. Brockmann proved that the growth inhibition of PPARγ ligand for tumor cells is mediated by the PPARγ pathway [50]. In this work, from the PPAR signaling pathway, we can see that the expression level of LEP in adipocytokine signaling pathway and LPL in lipid metabolism pathway were both down-regulated, which suggested the two DEGs may be crucial in suppressing the tumor cell proliferation. Down regulation of LPL and LEP may contribute the propagation of tumor cells in BC.
ACACB (Acetyl Coenzyme A carboxylase β), a down-regulated gene in pyruvate metabolism pathway, is the rate-limiting enzyme and key regulator in fatty acid oxidation pathway [51]. Acyl-CoA cholesterol acyl transferases-1 (ACAT1) is a key protein that regulates the cholesterol metabolism balance, playing a crucial role in the absorption, transportation and storage of cholesterol in body [52], also is the main existence form in mononuclear macrophages [53]. ACTC1 and ACTC2 were the two types of ACTC. Mononuclear macrophage is a cell that has the ability to hematopoiesis, and can stimulate the proliferation, differentiation and functional activity of neutrophils, monocytes and macrophages, mainly being used in the granulocytopenia caused by the tumor chemotherapy [54]. A high mRNA expression level of ACTC1 will lead to the increase of cholesteryl-ester synthesis in cells [55]. In Chinetti’s study, PPARγ with activated ligand can descend the mRNA and protein expression level of ACAT1 in human macrophages to inhibit the progression of BC [56]. The down-regulated expression of ACAT1 gene and ACACB was observed in our analysis results, this phenomenon may illustrate both the two genes play a key role in inhibiting the BC progress. Besides, pyruvate metabolism pathway may have a close relationship with PPAR signaling pathway in BC regulating.
To sum up, pyruvate metabolism pathway and PPAR signaling pathway with down-regulated DEGs, and the antigen processing and presentation pathway with up-regulated DEGs were the dysfunctional pathways that involved in BC. Although their role is different, they function on the BC progression together somehow. Our study provides a basis for future experiments of human BC that may enhance our understanding of the pathogenesis of BC. However, further experiments are still needed to confirm our study.
Disclosure of conflict of interest
None.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Huang ZZ, Chen WQ, Wu CX. Incidence and mortality of female breast cancer in China—a report from 32 Chinese cancer registries, 2003-2007. Tumor. 2012;32:435–439. [Google Scholar]
- 5.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 6.Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 7.Willis L, Graham TA, Alarcón T, Alison MR, Tomlinson IP, Page KM. What can be learnt about disease progression in breast cancer dormancy from relapse data? PLoS One. 2013;8:e62320. doi: 10.1371/journal.pone.0062320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persson I. Estrogens in the causation of breast, endometrial and ovarian cancers—evidence and hypotheses from epidemiological findings. J Steroid Biochem Mol Biol. 2000;74:357–364. doi: 10.1016/s0960-0760(00)00113-8. [DOI] [PubMed] [Google Scholar]
- 9.Mols F, Vingerhoets AJ, Coebergh JW, Van De Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41:2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39:261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- 11.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 12.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, Mcclanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86:823S–835S. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 15.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up-and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 16.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 17.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 18.Janz M, Harbeck N, Dettmar P, Berger U, Schmidt A, Jürchott K, Schmitt M, Royer HD. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int J Cancer. 2002;97:278–282. doi: 10.1002/ijc.1610. [DOI] [PubMed] [Google Scholar]
- 19.Alan D, D’andrea M. The Fanconi Anemia and Breast Cancer Susceptibility Pathways. N Engl J Med. 2010;362:1909. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745–2747. [PubMed] [Google Scholar]
- 21.Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131:794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 22.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar R. NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res. 2007;35:D760–D765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pau Ni IB, Zakaria Z, Muhammad R, Abdullah N, Ibrahim N, Aina Emran N, Hisham Abdullah N, Syed Hussain SN. Gene expression patterns distinguish breast carcinomas from normal breast tissues: the Malaysian context. Pathol Res Pract. 2010;206:223–228. doi: 10.1016/j.prp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Fox RJ, Dimmic MW. A two-sample Bayesian t-test for microarray data. BMC Bioinformatics. 2006;7:126. doi: 10.1186/1471-2105-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shmelkov E, Tang Z, Aifantis I, Statnikov A. Assessing quality and completeness of human transcriptional regulatory pathways on a genome-wide scale. Biol Direct. 2011;6:15. doi: 10.1186/1745-6150-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 29.Deberardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Pelicano H, Martin D, Xu R, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 32.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Angulo AM, Iwamoto T, Liu S, Chen H, Do KA, Hortobagyi GN, Mills GB, Meric-Bernstam F, Symmans WF, Pusztai L. Gene expression, molecular class changes, and pathway analysis after neoadjuvant systemic therapy for breast cancer. Clin Cancer Res. 2012;18:1109–1119. doi: 10.1158/1078-0432.CCR-11-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 36.Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol Immunother. 2003;52:80–88. doi: 10.1007/s00262-002-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozbaş-Gerçeker F, Ozcelik U, Kiper N, Anadol D, Ayhan G, Yilmaz E, Erdem-Yurter H, Ozgüç M. Analysis of the modifying effects of TAP 1/2 genes on cystic fibrosis phenotype. Turk J Pediatr. 2002;44:91–97. [PubMed] [Google Scholar]
- 38.Tam KW, Ho CT, Lee WJ, Tu SH, Huang CS, Chen CS, Lee CH, Wu CH, Ho YS. Alteration of α-tocopherol-associated protein (TAP) expression in human breast epithelial cells during breast cancer development. Food Chem. 2013;138:1015–1021. doi: 10.1016/j.foodchem.2012.09.147. [DOI] [PubMed] [Google Scholar]
- 39.Aptsiauri N, Cabrera T, Garcia-Lora A, Ruiz-Cabello F, Garrido F. MHC Class I Antigens and the Tumor Microenvironment, in The Tumor Immunoenvironment. Springer; 2013. pp. 253–286. [Google Scholar]
- 40.Zuo J, Hislop AD, Leung CS, Sabbah S, Rowe M. Kaposi’s sarcoma-associated herpesvirus-encoded viral IRF3 modulates major histocompatibility complex class II (MHC-II) antigen presentation through MHC-II transactivator-dependent and -independent mechanisms: implications for oncogenesis. J Virol. 2013;87:5340–5350. doi: 10.1128/JVI.00250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishigame H, Mosaheb MM, Sanjabi S, Flavell RA. Truncated form of transforming growth factor-β receptor II, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. Cytokine. 2013;63:273. doi: 10.4049/jimmunol.1300397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 44.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 45.Kim JW, Cheng Y, Liu W, Li T, Yegnasubramanian S, Zheng SL, Xu J, Isaacs WB, Chang BL. Genetic and epigenetic inactivation of LPL gene in human prostate cancer. Int J Cancer. 2009;124:734–738. doi: 10.1002/ijc.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomassen M, Tan Q, Kruse TA. Gene expression meta-analysis identifies chromosomal regions and candidate genes involved in breast cancer metastasis. Breast Cancer Res Treat. 2009;113:239–249. doi: 10.1007/s10549-008-9927-2. [DOI] [PubMed] [Google Scholar]
- 47.Heintel D, Kienle D, Shehata M, Kröber A, Kroemer E, Schwarzinger I, Mitteregger D, Le T, Gleiss A, Mannhalter C. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1216–1223. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 48.Segawa Y, Yoshimura R, Hase T, Nakatani T, Wada S, Kawahito Y, Kishimoto T, Sano H. Expression of peroxisome proliferator-activated receptor (PPAR) in human prostate cancer. Prostate. 2002;51:108–116. doi: 10.1002/pros.10058. [DOI] [PubMed] [Google Scholar]
- 49.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 50.Brockman JA, Gupta RA, Dubois RN. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 51.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 52.Chang T, Chang A, Catherine Cy, Cheng D. Acyl-coenzyme A: cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 53.Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR. ACAT-2, a second mammalian acyl-CoA: cholesterol acyltransferase its cloning, expression, and characterization. J Biol Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Zhang Z, Barletta KE, Burdick MD, Mehrad B. Heterogeneity of lung mononuclear phagocytes during pneumonia: contribution of chemokine receptors. Am J Physiol Lung Cell Mol Physiol. 2013;305:L702–11. doi: 10.1152/ajplung.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo T, Oelkers PM, Giattina MR, Worgall TS, Sturley SL, Deckelbaum RJ. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry. 2001;40:4756–4762. doi: 10.1021/bi0022947. [DOI] [PubMed] [Google Scholar]
- 56.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]