Abstract
Purpose: A great deal of studies have been performed on the prognostic value of monocyte chemotactic protein-1 (MCP-1) in solid tumors in recent years. However, no consistent outcomes are reported. Therefore, the prognostic value of MCP-1 still remains controversial in patients with solid tumors. Here we aimed to evaluate the prognostic value of MCP-1 expression for patients with solid tumors. Methods: Comprehensive literature was selected from PUBMED and EMBASE and clinical studies which reported analysis of survival data about MCP-1 in solid tumors were included. Stata 11.0 was used for performing a meta-analysis on evaluating the relation between MCP-1 and clinical staging, overall survival (OS) and disease free survival (DFS). Results: Eleven studies with a total of 1324 patients with solid tumors were included into our meta-analysis. The result showed that high concentration of MCP-1 was related to a worse OS (HR = 1.95, 95% CI 1.32-2.88). The subgroup analysis on different location of tumors showed that high concentration of MCP-1 meant bad prognosis in patients with digestive cancer (HR = 2.66, 95% CI 1.44-4.91) and urogenital cancer (HR = 2.23, 95% CI 1.61-3.10), even head and neck cancer (HR = 1.99, 95% CI 0.95-4.18) other than respiratory cancer (HR = 1.10, 95% CI 0.39-3.11). Another subgroup analysed on different sites of cancer and indicated a poor prognosis on adenocarcinoma (HR = 2.10, 95% CI 1.63-2.69). Conclusions: Our findings suggest that MCP-1 can be regarded as a poor prognostic maker for solid tumors and may represent important new therapeutic targets.
Keywords: Monocyte chemotactic protein-1, meta-analysis, cancer, prognosis, overall survival
Introduction
Chemokines have attracted broad interests and discussions in recent years. This has occurred, to some extent, because of an enlarging view on what chemokines do on tumor progression.Chemokines are signaling proteins and act as a chemoattractant to guide the migration of cells by a signal of increasing chemokine concentration, which also raise the possibility of controling the migration of tumor cells in humman bodies.
Monocyte chemotactic protein-1 (MCP-1), also called Chemokine (C-C motif) ligand 2 (CCL2), is a 76-amino acid protein and a member of the C-C subfamily of chemokines [1], was first purified by Matsushima. from serum-free culture supernatant of human myelomonocytic cells in 1989 [2]. It is produced by many activating cells (fibroblasts, endothelial cells, lymphocytes, smooth muscle cells, B cells and macrophages) under the stimulation of lipopolysaccharide (LPS), Poly I-C, IL-1, IFN-γ, polyhydroxyalkanoates (PHA), platelet-derived growth factor (PDGF), epidermal growth factor (EGF) or some viruses [3-7]. Many recent studies have reported that MCP-1 can also be expressed by several malignant tumor cells, including liver cancer, prostate cancer, pancreatic cancer, breast cancer, lung cancer, colorectal cancer and ovarian cancer [8-12].
Huang S. said MCP-1 is associated with reducing the metastatic potential, probably by enhancing monocyte infiltration in a murine model of colon cancer in 1994 [13], and Zia A.D. also told that high serum MCP-1 expression is correlated with favorable prognostic variables via increased preexisting HER-2/neu immunity in patients with breast cancer [14]. However, more studies have indicated that MCP-1 expression is related to tumor progression, matrix formation and dissolution, clinical aggressiveness and promoting metastasis to local and distant sites [15-19]. Therefore, the prognostic value of MCP-1 still remains unclear in patients with solid tumors.
In this study, we attempted to conduct a meta-analysis to estimate the relationship between MCP-1 expression and clinical staging, overall survival (OS) and disease free survival (DFS) among patients with solid tumors, and we sought to find out whether MCP-1 could provide helpful guidance in the treatment and prognosis of cancer.
Materials and methods
Identification and eligibility of relevant studies
Literature selected from PubMed (MEDLINE) and EMBASE was conducted by combing search terms “cancer”, “cancers”, “tumor”, “tumors”, “neoplasm”, “neoplasms”, “carcinoma”, “carcinomas” with “monocyte chemoattractant protein-1”, “monocyte chemotactic protein 1” , “monocyte chemotactic protein-1”, “MCP-1”, “CCL2”, “Chemokine ligand 2”, “C-C motif ligand 2” and “monocyte chemoattractant protein 1”. The deadline of the literature search was July 19th, 2013. To prevent the omission of any research via electronic search strategy, reference list from primary identified studies were also searched.
Study inclusion or exclusion criteria
Inclusion criteria for the study were as follows: (1) all cases were confirmed diagnosis of solid tumor in humans; (2) reviews, letters to the editors, articles published in a book and only summaries of the literature or in languages other than English were excluded; (3) clinical research association of MCP-1 with overall survival (OS), and/or disease free survival (DFS), not basic research and animal experiments; (4) no duplicate data. The same sample in multiple reports was included once; (5) having survival data about MCP-1. Literature must provide prognostic hazard ratio (HR) or provide sufficient information that can calculate HR value, incomplete information were excluded.
Data extraction
We extracted the useful data from included studies by using a standard information collection form with the following items: (1) article data including first author’s name, publication date and country of origin; (2) demographic data regarding inclusion number, mean or median age, sex of patients and percentage of MCP-1 positive; (3) tumor data including tumor location, histological type, percentage of distant metastasis, tumor-node-metastasis (TNM) staging; (4) survival data including OS, DFS; (5) technology of MCP-1 measurement, cut-off used for assessing MCP-1 positivity.
Statistical analyses
The effect of MCP-1 expression on OS and DFS were assessed by calculating the value of hazard ratios (HR) with its corresponding 95% confidence interval (95% CI). For those whose HRs were not available in the literature, the published data including the number of patients at risk in the negative and positive group, the survival rate or figures from original studies were used to estimate the HR via the methods described by Parmar [20]. If the only exploitable survival data were survival curves, we read them by Engauge Digitizer version 4.1 (free software downloaded from http://sourceforge.net) and extracted survival rate from them to estimate the HR, 95% CI and its standard error (SE). All the data was analyzed by Stata version 11.0 (Stata Corporation, College Station, TX, USA). Q-tests and P-values were assessed to estimate the effect of between-study heterogeneity in our meta-analyses. When there was no significant heterogeneity across the included studies (P-values >0.05), the fixed effects model was used to calculate the HR and its 95% CI according to the method of Mantel and Haenszel [21]. Otherwise, a random-effects model (the DerSimonian-Laird method) was used. Visual assessment of Begg’s funnel plots and Egger’s test was used to assess the possibility of publication bias on the outcomes.
Results
Study selection and characteristics
By searching in PubMed and EMBASE databases, a total of 1631 references were primary retrieved and we evaluated 73 candidate studies in full text. Upon full articles review, 62 studies were excluded including 51 studies for no prognostic analysis, 8 for no usable data and 3 for irrelevant studies. The processes of search strategy for article were summarized in Figure 1.
Figure 1.
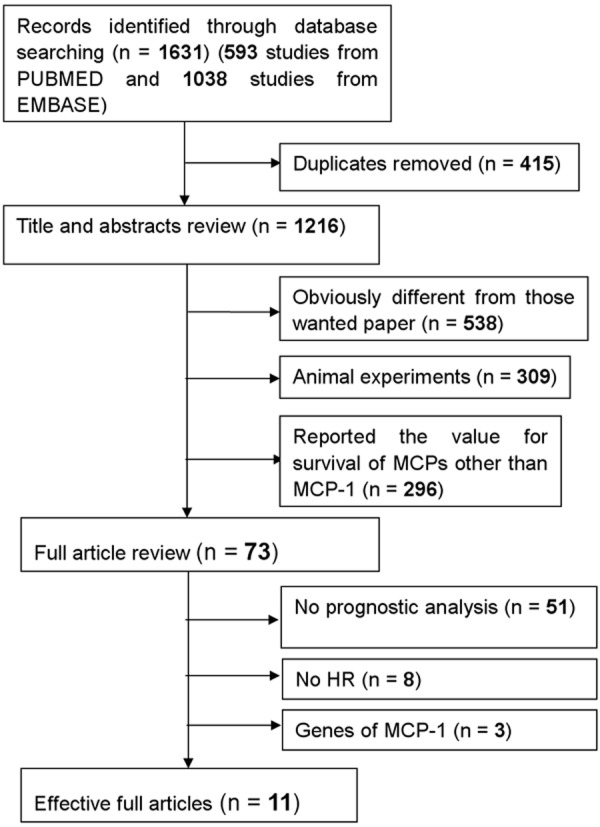
Methodological flow chart of the literature search and selection of included studies.
Study results
Eleven studies with a total of 1324 patients of solid tumors (ranging from 56 to 297 per study) were included into our meta-analysis [22-32], there were 725 patients with high MCP-1 expression and the remaining 605 patients with low or undetectable MCP-1 expression. All of the eleven studies were published in English.
In total, four studies with 677 patients provide data on disease-free survival (DFS) [24,25,27,29] and ten studies with 1214 patients provide data on overall survival (OS) [22-24,26-32]. Three reports originated from China, five from Japan, and three from Europe. The range of the eligible studies’ mean age was 46 to 66. But the percentage of the number of male was from 0% to 100% because of the patients with breast or prostate cancer and men just accounted for 50% of the enrolled patients in the 9 studies with information of gender (Table 1).
Table 1.
Main characteristics of the studies relating MCP-1 to Patients’ Prognosis
| First author (years) | Country | Mean age (years) | N (Male %) | Technology | Distant metastasis (%) | MCP-1 + (%) | Tumor location | Analysis | Cut-off for MCP-1 + | TNM (+/-) |
|---|---|---|---|---|---|---|---|---|---|---|
| Qian et al (2012) | China | 65.5 | 48 (60.76) | ELISA | 28.57 | 55.7 | Lung | OS, STAGE | >3301 pg/mL | 79 (55/24) |
| Zhang et al (2012) | China | 60.4 | 79 (58.96) | HIER | 3.73 | 79.85 | Lung | OS, STAGE | >10% | 134 (107/27) |
| Suguru et al (2011) | Japan | 66 | 110 (100) | ELISA | NR | 19.09 | Prostate | DFS | score 3 to 7 | NR |
| Lu et al (2010) | China | 46 | 227 (76) | ELISA | 24 | 54 | Nasopharynx | OS, DFS, STAGE | >311 pg/ml | 297 (205/92) |
| Yoshidome et al (2009) | Japan | 60.3 | 55 (63.22) | MART | 43.68 | 56.32 | Liver | OS | >10% | NR |
| JOHN et al (2008) | Norway | 62.2 | NR | ELISA | NR | 78.31 | Head and neck | OS, STAGE, DFS | >7207pg/ml | 65 (34/31) |
| Hideki et al (2008) | Japan | 65.2 | 66 (65.35) | ELISA | 41.58 | 50 | Ccolorectal | OS, STAGE | Ca/N Ratio >1.8 | 101 (41/60) |
| Annechien et al (2007) | Netherlands | 59 | 0 (0) | CBA | NR | 50 | Ovarian cancer | OS, PFS, STAGE | >253.28 pg/mL | 186 (119/67) |
| Naohiko et al (2004) | Japan | NR | 42 (75) | ABC | 28.57 | 55.36 | Esophagus | OS | >10% | NR |
| Paolo et al (2003) | Italy | 66 | 35 (56.14) | ELISA | 30.4 | 61.29 | Pancreatic | OS | >91 pg/ml | NR |
| Takayuki et al (2000) | Japan | 55 | NR | ELISA | 39.7 | 48.15 | Breast | OS | >10% | NR |
Abbreviations: NR, not reported; IHC, immunohistochemistry; MART, Microwave Antigen Retrieval Technique; CBA, Cytokine Bead Array; ABC, Avidin-Biotin Complex method; MCP-1 Ca/N ratio, the cancer tissue MCP-1 concentration divided by normal mucosa MCP-1 concentration.
The positive rate of MCP-1 ranged from 50.77% to 81.08% in all studies. The percentage of patient with distant metastasis of tumor was reported in 8 articles [22,23,25,26,28,30-32] ranging from 3.73% to 43.68%. The percentage of solid tumors with diameters more than 5 centimeters was told in 4 studies [23,26,28,32] and ranged from 19.54% to 59.70%.Patients with stage information were reported in six studies (ranging from 65 to 297) and the ratio in stage III and IV was from 40.60% to 79.85%.
Enzyme-linked immunosorbent assay (ELISA) was the mostly common technique used to detect MCP-1 expression. In the other four trails, microwave antigen retrieval technique (MART), cytokine bead array (CBA), immunohistochemical method (IHC) and avidin-biotin complex method (ABC) were also used to assess MCP-1 separately. Because of the differences between the detecting technology, the cut-off value for definition of MCP-1 positive was also different. The most common cut-off value was more than 10% of cancer cells stained in each section [23,26,30,32]. The second principle to define MCP-1 positive was in line with the concentration of MCP-1 (from 91 to 3301 pg/ml) in 5 included studies [22,25,27,29,31].
Meta-analysis
In the meta-analysis of the effect of MCP-1 expression on overall survival, there was significant heterogeneity among those 10 studies [22-24,26-32] (I squared = 69.3%), so the random-effect model was used to calculate the ORs and 95% CIs. We found that compared with cancer patients with low or negative MCP-1 expression, high concentration of MCP-1 was associated with a bad prognosis (HR = 1.95, 95% CI 1.32-2.88) (Figure 2). For DFS in overall population, the random-effect model also used because of the obvious heterogeneity (I squared = 66.6%) and a weak worse prognosis (HR = 1.29, 95% CI 0.75-2.22) was observed among patients considered MCP-1 positive.
Figure 2.
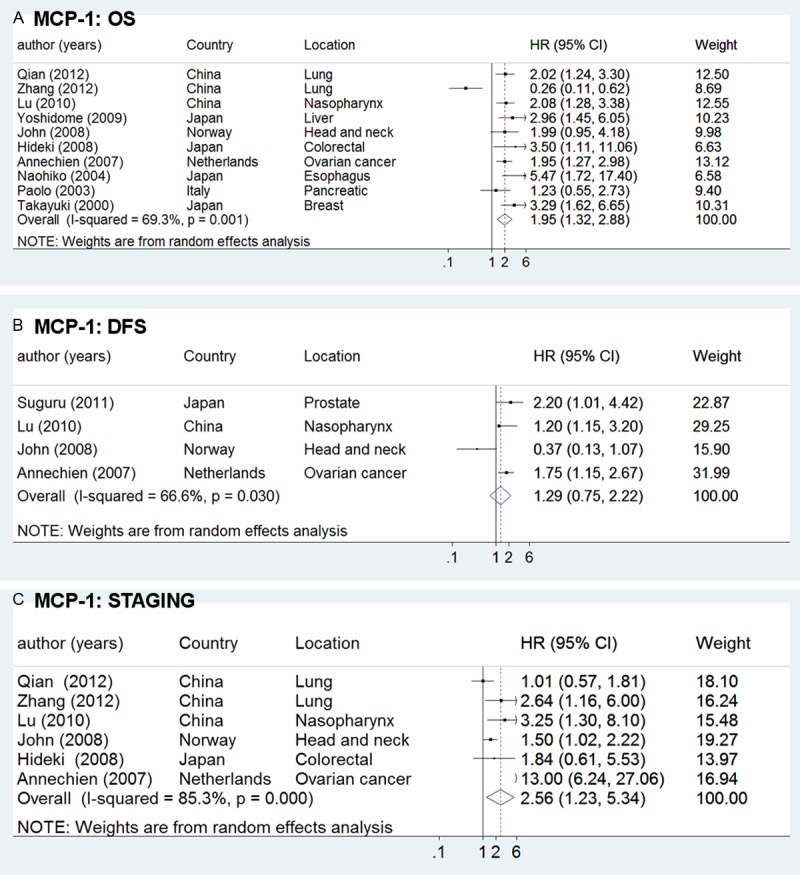
Forrest plots in studies of MCP-1 expression in patients with solid tumor by HR estimation. Clinical staging and survival data are reported as (A) Overall survival (OS), (B) disease free survival (DFS) and (C) staging.
The first subgroup analysis was assessed according to the location of tumors (Figure 3A).High concentration of MCP-1 was significantly related to poor OS in patients with digestive cancer (HR = 2.66, 95% CI 1.44-4.91) and urogenital cancer (HR = 2.23, 95% CI 1.61-3.10). However, three studies reported that there was no significant correlation between high density of MCP-1 and OS in patients with respiratory cancer (HR = 1.10, 95% CI 0.39-3.11) [22,23,25]. Only one study reported that the MCP-1 density was negatively correlated with extended survival in patients with head and neck cancer (HR = 1.99, 95% CI 0.95-4.18) [27].
Figure 3.
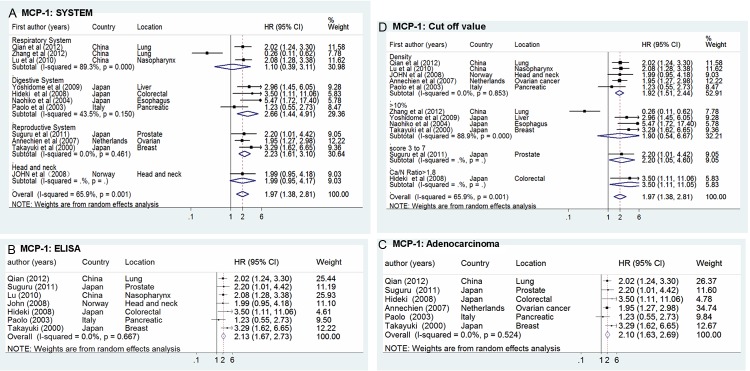
Forrest plots in studies of MCP-1 expression in patients with solid tumor by HR estimation for OS in subgroups. Survival data are reported as (A) System, (B) ELISA, (C) Adenocarcinoma and (D) Cut off value.
The impact of MCP-1 concentration on clinic-pathological in patients with different solid cancer was further analyzed and described, six studies told about adenocarcinoma and indicated a bad prognosis (HR = 2.18, 95% CI 1.72-2.76) with high concentration of MCP-1 (Figure 3C). To analyze different technology on evaluating MCP-1, Figure 3B showed an HR of 2.23 (95% CI 1.67-2.73) by the method of ELISA. There was also significant difference in the summary estimate of MCP-1 on overall survival when cut-off value was in line with the concentration of MCP-1 (HR = 1.92, 95% CI 1.51-2.44), especially when it is above 10% (HR = 1.90, 95% CI 0.54-6.67) (Figure 3D).
TNM staging is connectted with the prognosis of tumors closely [33,34]. Therefore, it is necessary to analyze the value of MCP-1 sub-grouped by TNM staging. In Figure 2C showed a statistically significant HR of 2.56 (95% CI 1.23-5.34). At last, subgroup analysis was performed according to countries. In Asian countries, with 8 studies evaluable, showed a significant HR of 2.09 (95% CI 1.25-3.49), and European countries showed an HR of 1.81 (95% CI 1.29-2.53).
Evaluation of publication bias
Visual assessment of Begg’s funnel plots and Egger’s test was used to assess the possibility of publication bias on the outcomes in all studies evaluating OS, DFS staging separately, and evaluation was also performed in sub-group analysis. Begg’s funnel plot did not find any evidence of asymmetry in overall meta-analysis of OS (P = 0.815), DFS (P = 0.174) and staging (P = 0.573) (Figure 4). In addition, no indication of publication was shown in Egger’s test of OS (p = 0.981), DFS (P = 0.387), staging (P = 0.365). For those sub-groups, there were also no significant evaluation of publication bias shown from Egger’s or Begg’s test.
Figure 4.
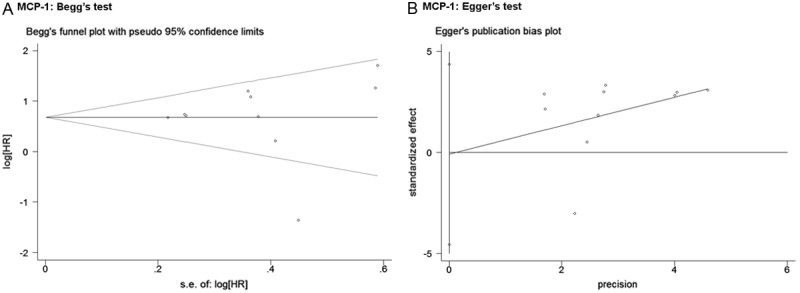
Funnel graph of Begg’s test (A) and Egger’s test (B) in studies of MCP-1 expression in patients with solid tumor by HR estimation for the assessment of potential publication bias in Figure 1 (A). No indication of publication was shown in Begg’s test (P = 0.815) and Egger’s test (P = 0.981) of OS.
Discussion
So far, plenty of original articles and reviews have studied the prognostic value of MCP-1 in solid tumors [8,11,32,35] and addressed the importance of MCP-1 on survival, which made it necessary to perform a quantitative aggregation of the survival results. According to the literature we found by searching the PubMed (MEDLINE) and EMBASE, this is the first study conducted by meta-analysis to clarify the prognostic value of MCP-1 for OS and DFS in patients with solid cancer. The results of our meta-analysis show that compared with cancer patients with low or negative MCP-1 expression, the high MCP-1 expression in solid tumors is a worse prognostic factor with statistical significance for OS (HR = 1.95, 95% CI 1.32-2.88), which suggests a 1.95-fold higher OS for cancer patients with the positive detection of MCP-1. This final result about OS is consistent with 9 of 10 included studies with a HR above 1. No significant effect on poor DFS (HR = 1.29, 95% CI 0.75-2.22) was observed among patients considered MCP-1 positive. Using Begg’s, Egger’s test and funnel plot, we regard an absent publication bias in our analysis. Therefore, the findings from our meta-analysis suggest that MCP-1 expression is an effective biomarker of prognosis in patients with solid tumors. These results may provide further basis for the development of new marker for cancer prognosis and for the development of anti-angiogenic drugs for cancer therapy.
With the deepening study of cancer pathophysiology and the characteristic of MCP-1, the value of MCP-1 in cancer has drawed our attention. The expression of MCP-1 at least contains four following aspects of biological effects on solid tumors growth: first, MCP-1 can promote the infiltrating of Tumor-associated macrophages (TAM) in the tumor tissues. Most authors think that the differentiation and maturation of TAM can activate the transcriptional program by hypoxia-inducible factor 1 (HIF-1) and Nuclear factor κB (NF-κB), then secrete IL-1, IL-4, IL-10, TNF-α, VEGF and MMP, which promote the growth, invasion and metastasis of tumor cells [36-38]. Second, recent studies have reported that MCP-1 has a biological effect on promoting angiogenesis [39-43] in two ways. On the one hand, MCP-1 recruits TAM into the tumor tissues and secrete VEGF, TGF-β, other angiogenesis factors and ELR + CXC chemokine, which stimulates angiogenesis indirectly. In the test of bird chorio allantoic membrane (CAM) and rat aorta germination test, MCP-1 still promote the generation of new blood vessels on the base of exclusing inflammatory cell infiltration [37,38,43]; On the other hand, MCP-1 acts with CCR2 receptors directly on vascular endothelial cell membrane and guides the directional movement of vascular smooth muscle cells [44-46]. Third, MCP-1 may play a similar role as growth factor and has a direct effect on tumor cells to promote growth and survival of tumor cells. It was reported by Song G that CXCL12/CXCR4 can inhibit the apoptosis of tumor cell and promote the cell growth by activatinc the Akt/PKB signaling pathway [47]. And MCP-1 is able to activate the IP3-dependent Akt/PKB signaling pathway. Fourth, MCP-1 not only induces TAM into tumor tissues to secrete VEGF, TGF-β, TNF-α and other cytokines that promote the growth of tumor cells and angiogenesis [47], but also secrete matrix metalloproteinases 2 (MMP2) and matrix metalloproteinases 9 (MMP9), involved in the destruction and reconstruction of extracellular matrix, to promote tumor cell invasion and metastasis.
As we all know, tumor marker works better with specific. Carcinoembryonic antigen (CEA) measurement is mainly used as a tumor marker of monitor colorectal carcinoma treatment to identify recurrences after surgical resection [48]; Alpha-fetoprotein (AFP) is the specific markers of the diagnosis of primary liver cancer [49]. To further investigate the prognostic value of MCP-1 in different site of cancer and examine the specificity of MCP-1, we analyzed the relation between the density of MCP-1 and tumor-location factors that was also associated with outcome of cancer patients. In most cancers including digestive and urogenital cancers, even head and neck cancers, high concentration of MCP-1 was significantly related to poor OS in patients. However, three studies reported that there was no significant correlation between high density of MCP-1 and OS in patients with respiratory cancer (HR = 1.10, 95% CI 0.39-3.11). These results indicate that MCP-1 can be a helpful tumor marker of prognosis in patients with digestive and urogenital cancers, even head and neck cancers, other than respiratory cancer.
To further investigate the prognostic value of MCP-1 in different site of cancer, we analyzed the relation between the density of MCP-1 and clinic-pathological factors that was also associated with outcomes of cancer patients. In the 11 eligible studies, seven of these studies told about adenocarcinoma and indicated a bad prognosis (HR = 2.10, 95% CI 1.63-2.69). In general, we consider HR >2 that means strongly predictive [50]. These results indicated that high density of MCP-1 has a negative effect on survival in patients with solid tumor, especially for patients with adenocarcinoma.
The results of meta-analysis are considered as gold standards by authors worldwide [51-53], however, there are several limitations of the meta-analysis and that might present a potential source of variability of meta-analysis. The first main limitation in our meta-analysis was the item of primary outcome. Different specimen from tissue or plasma, different survival rate, different methods (IHC, ELISA, ABC or CBA) identify MCP-1 expression and no standard of cut-off value brings variability for MCP-1 positive and negative. These differences may cause the obvious between-study heterogeneity among those eleven studies in the meta-analysis of the effect of MCP-1 expression. Therefore, to provide further evidence for the prognostic role of MCP-1 expression in patients with solid tumors, more studies that are well designed and having the same items of primary outcome are needed. Second, a phenomenon of “file-drawer problem” might appear because the included articles are all published in English. It means that positive studies would be easier to be accepted by editors of English magazine while negative studies are often published in native languages or even not received by the journal [54-56], which could arise publication bias in our meta-analysis and overestimate the prognostic significance of MCP-1 in cancer [57].
In conclusion, MCP-1 can be regarded as a prognostic marker for solid tumors, especially for OS, and may represent as important new therapeutic targets, which was supported by our meta-analysis. To achieve a more definitive conclusion enabling the clinical use of MCP-1 in cancer, we need more high-quality interventional original studies following agreed research approach or standard.
Acknowledgements
Thank Dr Yan Xin, West China School of Medicine, Sichuan University, 37 Guo Xue Xiang, Chengdu, China for his statistical guidance and all authors whose articles have been included in this meta-analysis.
Disclosure of conflict of interest
None.
References
- 1.Frade J, Mellado M, del Real G, Gutierrez-Ramos J, Lind P, Martinez-A C. Characterization of the CCR2 chemokine receptor: functional CCR2 receptor expression in B cells. J Immunol. 1997;159:5576–5584. [PubMed] [Google Scholar]
- 2.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101:676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 5.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–62. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allavena P, Bianchi G, Zhou D, Van Damme J, Jílek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 7.Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J Leukoc Biol. 1996;59:67–74. doi: 10.1002/jlb.59.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Dwyer R, Potter-Beirne S, Harrington K, Lowery A, Hennessy E, Murphy J, Barry F, O’Brien T, Kerin M. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 10.Negus R, Stamp G, Relf M, Burke F, Malik S, Bernasconi S, Allavena P, Sozzani S, Mantovani A, Balkwill FR. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95:2391. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 12.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085–1091. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Singh RK, Xie K, Gutman M, Berry KK, Bucana CD, Fidler IJ, Bar-Eli M. Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunol Immunother. 1994;39:231–238. doi: 10.1007/BF01525986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehqanzada ZA, Storrer CE, Hueman MT, Foley RJ, Harris KA, Jama YH, Kao TC, Shriver CD, Ponniah S, Peoples GE. Correlations between serum monocyte chemotactic protein-1 levels, clinical prognostic factors, and HER-2/neu vaccine-related immunity in breast cancer patients. Clin Cancer Res. 2006;12:478–486. doi: 10.1158/1078-0432.CCR-05-1425. [DOI] [PubMed] [Google Scholar]
- 15.Mestdagt M, Polette M, Buttice G, Noël A, Ueda A, Foidart JM, Gilles C. Transactivation of MCP-1/CCL2 by β-catenin/TCF-4 in human breast cancer cells. Int J Cancer. 2006;118:35–42. doi: 10.1002/ijc.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Khayat A, Cheng H, Graves DT. The pattern of monocyte recruitment in tumors is modulated by MCP-1 expression and influences the rate of tumor growth. Lab Invest. 1997;76:579–590. [PubMed] [Google Scholar]
- 18.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein-and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem. 2012;287:36593–36608. doi: 10.1074/jbc.M112.365999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainz C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81:855. doi: 10.1038/sj.bjc.6690776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freels S. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints. Stat Med. 2004;23:1817. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. The Challenge of Epidemiology: Issues and Selected Readings. 2004;1:533–553. [PubMed] [Google Scholar]
- 22.Qian Q, Sun WK, Zhan P, Zhang Y, Song Y, Yu LK. Role of monocyte chemoattractant protein-1, tumor necrosis factor-alpha and interleukin-6 in the control of malignant pleural effusion and survival in patients with primary lung adenocarcinoma. Int J Biol Markers. 2012;27:e118–124. doi: 10.5301/JBM.2012.9197. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XW, Qin X, Qin CY, Yin YL, Chen Y, Zhu HL. Expression of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in non-small cell lung cancer and its significance. Cancer Immunol Immunother. 2013;62:563–570. doi: 10.1007/s00262-012-1361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirotake S, Miyajima A, Kosaka T, Tanaka N, Kikuchi E, Mikami S, Okada Y, Oya M. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol. 2012;180:1008–1016. doi: 10.1016/j.ajpath.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Qian CN, Mu YG, Li NW, Li S, Zhang HB, Li SW, Wang FL, Guo X, Xiang YQ. Serum CCL2 and serum TNF-α–Two new biomarkers predict bone invasion, post-treatment distant metastasis and poor overall survival in nasopharyngeal carcinoma. Eur J Cancer. 2011;47:339–346. doi: 10.1016/j.ejca.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Nakatani Y, Miyazaki M. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009;34:923–930. doi: 10.3892/ijo_00000218. [DOI] [PubMed] [Google Scholar]
- 27.Heimdal JH, Kross K, Klementsen B, Olofsson J, Aarstad HJ. Stimulated monocyte IL-6 secretion predicts survival of patients with head and neck squamous cell carcinoma. BMC Cancer. 2008;8:34. doi: 10.1186/1471-2407-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe H, Miki C, Okugawa Y, Toiyama Y, Inoue Y, Kusunoki M. Decreased expression of monocyte chemoattractant protein-1 predicts poor prognosis following curative resection of colorectal cancer. Dis Colon Rectum. 2008;51:1800–1805. doi: 10.1007/s10350-008-9380-7. [DOI] [PubMed] [Google Scholar]
- 29.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, Klaske A, Braid M, van der Zee AG, Daemen T, Nijman HW, Kast WM. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13:2385–2391. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 30.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagusa. Am J Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 31.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, Allavena P, Piemonti L. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- 32.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 33.Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–1095. doi: 10.1016/s0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 34.Javidan J, Stricker HJ, Tamboli P, Amin MB, Peabody JO, Deshpande A, Menon M, Amin MB. Prognostic significance of the 1997 TNM classification of renal cell carcinoma. J Urol. 1999;162:1277–1281. [PubMed] [Google Scholar]
- 35.Valković T, Lučin K, Krstulja M, Dobi-Babić R, Jonjić N. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract. 1998;194:335–340. doi: 10.1016/S0344-0338(98)80057-5. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 37.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, Iannettoni MD, Strieter RM. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 40.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 41.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 42.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:220–224. doi: 10.1002/ijc.10705. [DOI] [PubMed] [Google Scholar]
- 44.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Wang Q, Fei T, Han JD, Chen YG. MCP-1 mediates TGF-β–induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109:987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- 47.Vitiello PF, Shainheit MG, Allison EM, Adler EP, Kurt RA. Impact of tumor-derived CCL2 on T cell effector function. Immunol Lett. 2004;91:239–245. doi: 10.1016/j.imlet.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 49.Abelev G. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:278. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- 50.Ferlay J, Parkin D, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 52.Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–392. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]
- 53.Tong J, Sun X, Cheng H, Zhao D, Ma J, Zhen Q, Cao Y, Zhu H, Bai J. Expression of p16 in non-small cell lung cancer and its prognostic significance: a meta-analysis of published literatures. Lung Cancer. 2011;74:155–163. doi: 10.1016/j.lungcan.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Rosenthal R. The file drawer problem and tolerance for null results. Psychological bulletin. 1979;86:638. [Google Scholar]
- 55.Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]
- 56.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scargle JD. Publication bias: the “file-drawer” problem in scientific inference. Journal of Scientific Exploration. 2000;14:91–106. [Google Scholar]


