Abstract
The expression of hypoxia-induced factor (HIF)-1α is up-regulated in tumor microenvironments under hypoxia condition. However, the prognostic significance of HIF-1α in esophageal squamous cell carcinoma (ESCC) is still elusive. We measured the HIF-1α expression by immunochemistry in tumor specimens from 136 resected ESCC; in the current study, the HIF-1α expression in tumor cells was significantly associated with tumor stage (P = 0.003) and lymph node metastasis (P = 0.006); whereas the HIF-1α expression in tumor-infiltrating lymphocytes (TILs) had no relationship with patients’ clinicopathological parameters. Patients with high HIF-1α expression in tumor cells or in TILs showed worse survival related to those with low HIF-1α expression. Multivariate analysis demonstrated that expression of HIF-1α in TILs was an independent factor for DFS (P = 0.007) and OS (P = 0.013). Additionally, the expression of HIF-1α in tumor cells was an independent factor for DFS (P = 0.037) and OS (P = 0.033) in locoregional ESCC patients, whereas the expression of HIF-1α in TILs was an independent factor for DFS (P = 0.048) and OS (P = 0.039) in metastatic ESCC patients. Correlation analysis revealed that expressions of HIF-1α in tumor cells and in TILs were positively correlated, and patients with combined high HIF-1α in both tumor cells and TILs had the worst survivals (P < 0.05). These findings suggest that the HIF-1α expressions in different cell populations of ESCC microenvironments have different clinical relevance and prognostic impact on patients.
Keywords: Esophageal squamous cell carcinoma, HIF-1α, tumor microenvironments, clinical prognosis, tumor-infiltrating lymphocytes
Introduction
Esophageal squamous cell carcinoma (ESCC), one of the major histopathological subtypes of esophageal cancer, is the fourth most prevalent malignancy in China and a leading cause of cancer-related death. The prognosis of ESCC is generally unfavorable, with a five-year survival rate of less than 30% [1,2]. Despite general advances in diagnosis and treatment, the prognosis of ESCC patients remains poor because of high rates of recurrence/metastasis and resistance to adjuvant therapy [3,4]. Therefore, there is an urgent clinical need to explore novel prognostic markers for ESCC patients. In addition to the traditional prognostic factors determined at diagnosis, such as TNM stage and cell differentiation, the molecular markers related to tumor cell apoptosis, epithelial-mesenchymal transition (EMT), the function of infiltrated immune cells and angiogenesis in tumor microenvironments have been evaluated for their contribution to the prognoses of ESCC patients in recent studies [5-10]. However, reliable markers are still lacking in ESCC. Furthermore, to the best of our knowledge, there are no reports on the correlation between the expression level of a given molecular marker in different cell populations within the tumor microenvironment and the clinical prognosis of patients with ESCC, although it has been noted that the biological function of a gene product, as well as its effect on the clinical progression of cancer, varies depending on the cell population in which it is located.
Hypoxia is one of the defining features and poor prognostic markers of several solid cancers. Under low oxygen conditions (hypoxia), transcription of the a-subunit of hypoxia-induced factor (HIF) is rapidly induced. Hypoxic HIF activity is controlled primarily through post-translational modification and stabilization of the HIF-1α and HIF-2α subunits, leading to the transcription of a diverse set of target genes that are broadly involved in the adaptation to low oxygen and contribute to metabolic changes, angiogenesis and survival [11,12]. Recent studies demonstrated that HIF-1α was overexpressed in a number of cancers, including gastric cancer, breast cancer, prostate cancer and colon cancer, furthermore, overexpression of HIF-1α had an important effect on the biological action of tumor cells, the recruitment of infiltrated lymphocytes and angiogenesis in tumor microenvironments [13-16]. To date, however, the of the expression status of HIF-1α in ESCCs, particularly in its tumor microenvironment, and their clinico-prognostic significances remain to be elucidated [17-19].
In the present study, we measured the expression pattern of the hypoxia-induced protein HIF-1α in different cell populations, including tumor cells and tumor-infiltrating lymphocytes (TILs) in tumor microenvironment of ESCC and investigated their associations with patients’ clinical outcomes.
Methods
Patients and tissue samples
Paraffin-embedded tumor tissue samples were obtained from 136 ESCC patients who underwent surgery at Sun Yat-Sen University Cancer Center in Guangzhou City of China from November of 2000 to December of 2002. None of the patients had received anticancer treatment prior to surgery, and all of the patients had histologically confirmed primary ESCC in this retrospective study. The follow-up data from the 136 patients with ESCC in this study were available and complete. The diagnostic examinations consisted of esophagography, CT, chest X-ray, abdominal ultrasonography and bone scan when necessary to detect recurrence and/or metastasis. The OS was defined as the time interval from the date of surgery to the date of cancer-related death or the end of follow-up (December 2011), and the DFS was defined as the time interval from the date of surgery to the date of tumor recurrence or tumor metastasis. This study was approved by the Research Ethics Committee of the Sun Yat-Sen University Cancer Center.
Immunohistochemistry
The paraffin-embedded tissues were sectioned continuously into 4-μm-thick sections. The tissue sections were dewaxed in xylene, rehydrated and rinsed in graded ethanol solutions. The antigens were retrieved by heating the tissue sections at 100°C for 30 min in citrate (10 mmol/L, pH 6.0) or EDTA (1 mmol/L, pH 9.0) solution when necessary. The sections were then immersed in a 0.3% hydrogen peroxide solution for 30 min to block endogenous peroxidase activity, rinsed in phosphate-buffered saline (PBS) for 5 min, and incubated with the primary antibody mouse anti-human HIF-1α (Clone H1alpha67; Novus Biologicals, Inc., Littleton, CO) at 4°C overnight. A negative control was performed by replacing the primary antibody with a normal murine IgG antibody. The sections were then incubated with a horseradish peroxidase-labeled goat antibody against a mouse/rabbit secondary antibody (Envision; Dako, Glostrup, Denmark) at room temperature for 30 min. Finally, the signal was developed for visualization with 3, 3’-diaminobenzidine tetrahydrochloride (DAB), and all of the slides were counterstained with hematoxylin.
Evaluation of immunohistochemical staining
Two independent observers (Lin Zhang and Xiao-Feng Tang) blinded to the clinicopathological information scored the HIF-1α expression level in tumor cells by assessing (a) the proportion of positively stained cells (0, < 5%; 1, 6 to 25%; 2, 26 to 50%; 3, 51 to 75%; 4, > 75%) and (b) the intensity of staining (0, negative staining; 1, mild staining; 2, moderate staining; 3, strong staining). The score was the product of a × b. The levels of HIF-1α expression in lymphocytes were obtained by counting the positively and negatively stained lymphocytes in five to ten separate 400 × high-power microscopic fields and calculating the mean percentage of positively stained lymphocytes among the total lymphocytes per field. The patients were divided into subgroups: a high-level group (an X b ≥ 7 score in tumor cells; or ≥ 44% of HIF-1α-positive cells in lymphocytes) and a low-level group (an X b < 7 score in tumor cells; or < 44% of HIF-1α-positive cells in lymphocytes) based on the medians of immunohistochemical variable values in diverse cell subsets in our data.
Statistical analysis
All analyses were conducted with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Pearson’s chi-square test and Fisher’s chi-square test were used to analyze the correlation between HIF-1α expression in different cell subsets and the patients’ clinicopathological parameters. HIF-1α expression level was examined in tumor cells and in TILs in relation to the patients’ clinical prognosis using the Kaplan-Meier method and the log-rank survival analysis. Prognostic factors were assessed by univariate and multivariate analyses using the Cox proportional hazards model. The correlations among the expression levels of HIF-1α in tumor cells and in TILs were determined using Pearson’s correlation coefficient and linear regression analyses. A two-tailed P-value < 0.05 was considered statistically significant in this study.
Results
Patient characteristics
A total of 136 eligible patients were enrolled in the current study. The patients had a median age of 62 years (range, 35 to 90 years); 111 (81.6%) were males and 25 (18.4%) were females. There were 74 (54.4%) cases of Stage I and II tumors and 62 (45.6%) cases of Stage III and IV tumors based on the International Union against Cancer 2002 TNM staging system and WHO classification criteria [20]. Of the 136 patients, 103 (75.7%) had died. Of the136 patients one-hundred and seven cases (78.7%) didn’t get any anti-tumor therapy until tumor progression, whereas 10 cases (7.4%) received adjuvant radiotherapy, twelve cases received adjuvant chemotherapy (8.8%), and seven cases (5.1%) received both of radiotherapy and chemotherapy. The patients’ clinical parameters are detailed in Table 1.
Table 1.
Clinical characteristic of 136 patients with ESCC
| Characteristics | No. (%) |
|---|---|
| Total case | 136 |
| Age (Years) | |
| Median | 62 |
| Range | 35-90 |
| Gender | |
| Male | 111 (81.6%) |
| Female | 25 (18.4%) |
| WHO degree | |
| G1 | 40 (29.4%) |
| G2 | 59 (43.4%) |
| G3 | 37 (27.2%) |
| Tumor (T) status | |
| T1 | 8 (5.9%) |
| T2 | 36 (26.5%) |
| T3 | 88 (64.7%) |
| T4 | 4 (2.9%) |
| Lymphoid Nodal (N) status | |
| N0 | 69 (50.7%) |
| N1 | 67 (49.3%) |
| Distant metastasis (M) status | |
| M0 | 130 (95.6%) |
| M1 | 6 (4.4%) |
| TNM stage | |
| I | 6 (4.4%) |
| IIa-IIb | 68 (50.0%) |
| III | 56 (41.2%) |
| IV | 6 (4.4%) |
| Death | |
| No | 33 (24.3%) |
| Yes | 103 (75.7%) |
| Therapy after surgery | |
| Radiation therapy alone | 10 (7.4%) |
| Chemotherapy alone | 12 (8.8%) |
| Radiation therapy + chemotherapy | 7 (5.1%) |
| No | 107 (78.7%) |
Expression patterns of HIF-1α in ESCC and their correlations with clinicopathological parameters
In the present study, the protein expression level of HIF-1α was examined in tumor specimens from 136 patients with ESCC. HIF-1α was expressed mainly in the nucleus and cytoplasm of tumor cells and TILs (Figure 1). Based on the criteria described in the Methods section, high expression level of HIF-1α in tumor cells was noted in samples from 71 (52.2%) of the 136 patients. The mean percentage and the range of the percentage of patients with TILs positive for HIF-1α expression per high-power light microscopic field were 43% (range, 0 to 95%) among the 136 patients assessed.
Figure 1.
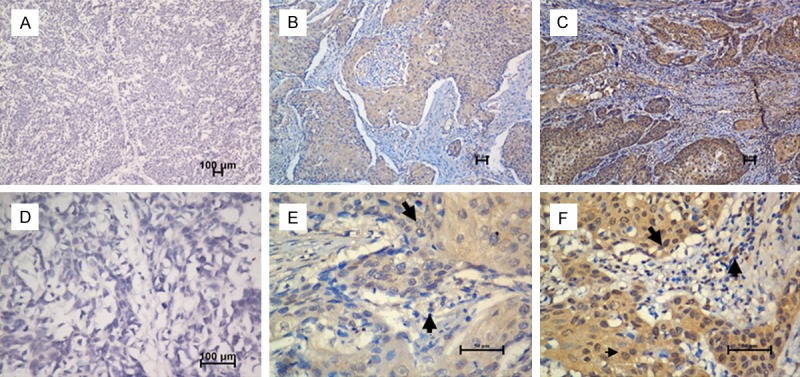
Immunohistochemical staining for HIF-1α in human esophageal carcinoma. Our data showed negative expression level (A, X 100; D, X 400), low expression (B, X 100; E, X 400) and high expression level of HIF-1α (C, X 100; F, X 400) in tumor tissues from patients with ESCC. The arrows point to the positive staining of tumor cells or TILs.
The associations between clinicopathological features and HIF-1α in different cell subsets within the tumor microenvironment in samples from 136 ESCC patients are summarized in Table 2. In the present study, high expression level of HIF-1α in tumor cells were closely associated with advanced clinicopathological characteristics, including tumor (T) status (P = 0.006), node (N) status (P = 0.006) and clinical stage (P = 0.019). Furthermore, the expression level of HIF-1α in lymphocyte was not related to any of the clinicopathological parameters, including age, gender, WHO grade, T status, N status and clinical stage.
Table 2.
Clinicopathological associations of HIF-1α expression levels and the density of tumor-infiltrating lymphocytes in 136 patients with ESCC
| Clinicopathologic parameter | Total case (n = 136) | High level of HIF-1α in tumor (%) | P | High level of HIF-1α in TIL (%) | P |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 62 y | 71 | 34 (47.9) | 0.292 | 34 (47.9) | 0.574 |
| > 62 y | 65 | 37 (56.9) | 28 (43.1) | ||
| Gender | |||||
| Female | 25 | 10 (40.0) | 0.176 | 12 (48.0) | 0.789 |
| Male | 111 | 61 (55.0) | 50 (45.0) | ||
| WHO grade | |||||
| G1 | 40 | 24 (60.0) | 0.213 | 20 (50.0) | 0.221 |
| G2 | 59 | 32 (54.2) | 22 (37.3) | ||
| G3 | 37 | 15 (40.5) | 20 (54.1) | ||
| Tumor size | |||||
| < 12 cm3 | 63 | 30 (47.6) | 0.320 | 26 (41.3) | 0.348 |
| ≥ 12 cm3 | 73 | 41 (56.2) | 36 (49.3) | ||
| T status | |||||
| T1-2 | 44 | 15 (34.1) | 0.003* | 21 (47.7) | 0.729 |
| T3-4 | 92 | 56 (60.9) | 41 (44.6) | ||
| N status | |||||
| N0 | 69 | 28 (40.6) | 0.006* | 29 (42.0) | 0.398 |
| N1 | 67 | 43 (64.2) | 33 (49.3) | ||
| Clinical stage | |||||
| I-II | 74 | 34 (45.9) | 0.110 | 34 (45.9) | 0.927 |
| III-IV | 62 | 37 (33.3) | 28 (45.2) |
P < 0.05, as determined by Pearson’s X2 test.
Expression level of HIF-1α in diverse cell subsets and ESCC patient survival
Among the 136 patients with ESCC, the median survival time was 25 months (range: 0 to 33 months). The cumulative five-year OS rate and DFS rate of the patients in this study were 29 and 31%, respectively. The statistical analysis showed a significant negative correlation between DFS, OS and the HIF-1α expression in tumor cells and TILs (P < 0.05, Figure 2).
Figure 2.
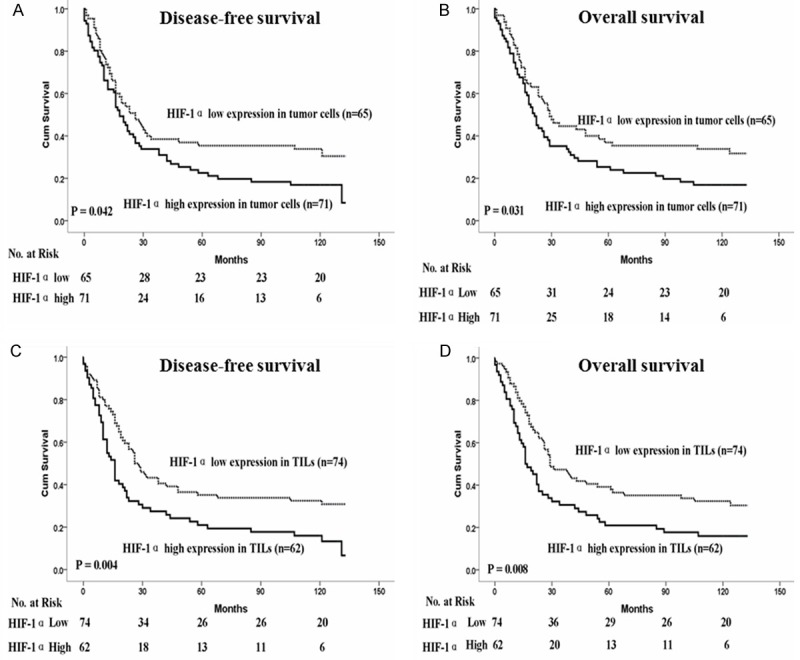
Kaplan-Meier survival analysis in patients with ESCC. A, B. Disease-free survival and overall survival curves for patients according to the low and high expression level of HIF-1α in tumor cells. C, D. Disease-free survival and overall survival curves for patients according to the low and high expression level of HIF-1α in TILs.
The multivariate analysis demonstrated that except some conventional clinicopathological parameters such as gender, WHO grade, nodal status and TNM stage the HIF-1 in TILs but not in tumor cells was an independent unfavorable factor for DFS (P = 0.007) and OS (P = 0.013) (Table 3).
Table 3.
Multivariate Cox regression analysis for DFS and OS of 136 patients with ESCC
| Variablesa | DFS | OS | ||
|---|---|---|---|---|
|
|
||||
| HR (95% CI) | p | HR (95% CI) | p | |
| HIF-1α expression in tumor cells | ||||
| Gender (Male/Female) | 0.462 (0.255-0.838) | 0.011* | 0.422 (0.235-0.760) | 0.004* |
| WHO Grade (1/2/3) | 1.321 (1.009-1.730) | 0.043* | 1.310 (1.000-1.716) | 0.050* |
| Tumor (T) status (1-2/3-4) | 0.944 (0.510-1.746) | 0.853 | / | |
| Nodal (N) status (0/1) | 2.205 (1.015-4.789) | 0.046* | 2.158 (1.029-4.527) | 0.042* |
| TNM stage (I-II/III-IV) | 0.875 (0.567-1.350) | 0.547 | 0.855 (0.594-1.231) | 0.400 |
| HIF-1α in tumor cells (low/high) | 1.234 (0.794-1.920) | 0.350 | 1.297 (0.856-1.964) | 0.220 |
| HIF-1α expression in TILs | ||||
| Gender (Male/Female) | 0.461 (0.254-0.837) | 0.011* | 0.412 (0.229-0.741) | 0.003* |
| WHO Grade (1/2/3) | 1.289 (0.990-1.677) | 0.059 | 1.263 (0.971-1.644) | 0.082 |
| Tumor (T) status (1-2/3-4) | 0.977 (0.544-1.752) | 0.937 | / | |
| Nodal (N) status (0/1) | 2.215 (1.070-4.585) | 0.032* | 2.156 (1.064-4.367) | 0.033* |
| TNM stage (I-II/III-IV) | 0.896 (0.590-1.361) | 0.606 | 0.885 (0.623-1.258) | 0.497 |
| HIF-1α in TILs (low/high) | 1.697 (1.152-2.500) | 0.007* | 1.635 (1.108-2.412) | 0.013* |
| HIF-1α expression in locoregional ESCC | ||||
| Gender (Male/Female) | 0.406 (0.168-0.981) | 0.045* | 0.341 (0.132-0.886) | 0.027* |
| WHO grade (1/2/3) | 1.897 (1.204-2.989) | 0.006* | 1.881 (1.181-2.994) | 0.008* |
| HIF-1α in tumors cells (low/high) | 1.938 (1.040-3.611) | 0.037* | 1.991 (1.058-3.748) | 0.033* |
| HIF-1α expression in metastatic ESCC | ||||
| HIF-1α in TILs (low/high) | 1.669 (1.005-2.772) | 0.048* | 1.711 (1.028-2.847) | 0.039* |
Variables with P values greater than 0.05 in the univariate models were not included in the multivariate analysis.
Significant.
DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Among the 136 patients with ESCC, there were 67 (49.3%) patients with locoregional ESCC and 69 cases (50.7%) with metastastic ESCC. The multivariate analysis demonstrated that the high expression of HIF-1α in tumor cells was noticeably associated with reduced DFS (P = 0.051) and OS (P = 0.039) in patients with locoregional ESCC (Figure 3A), whereas high expression level of HIF-1 in TILs was only significantly correlated with poor DFS (P = 0.042) and OS (P = 0.035) in patients with metastatic ESCC (Figure 3B). Furthermore, our multivariate analysis revealed that the expression of HIF-1α in tumor cells was an independent prognostic marker for patients with locoregional ESCC; and the expression of HIF-1α in TILs was an independent prognostic marker for patients with metastatic ESCC (Table 3).
Figure 3.
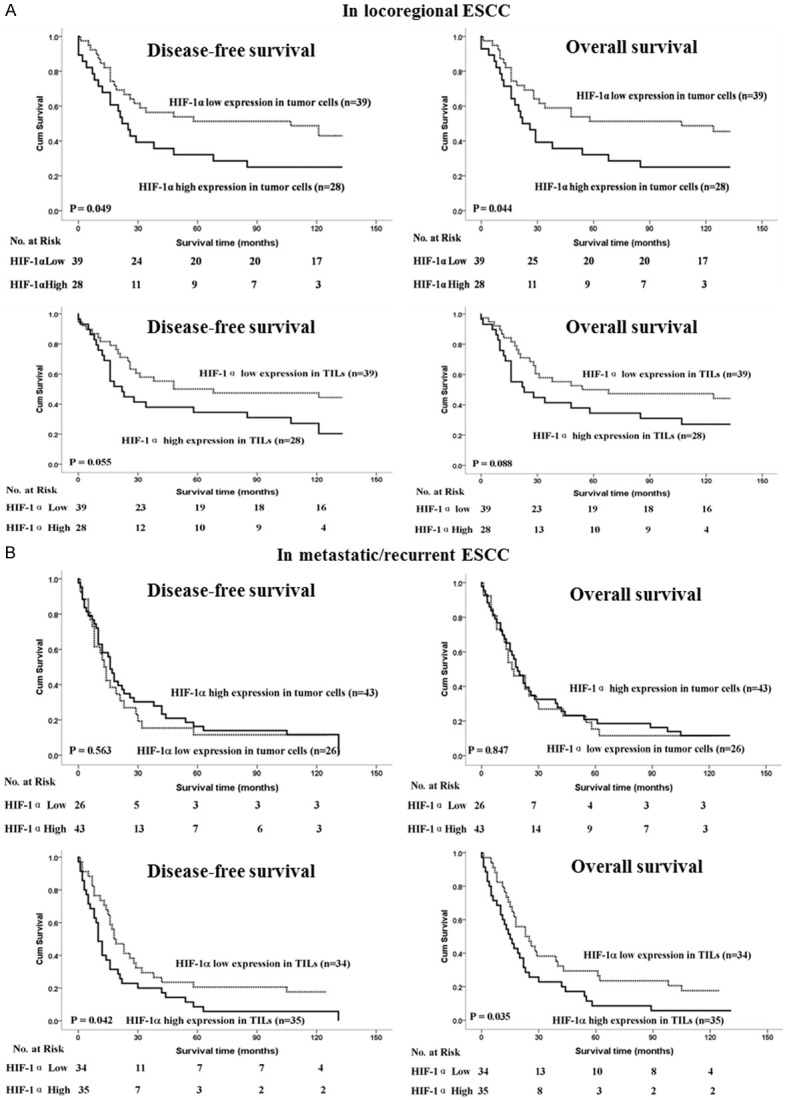
Kaplan-Meier survival analysis in patients with locoregional ESCC and metastatic ESCC. A. Disease-free survival and overall survival curves for patients with low and high expression levels of HIF-1α in tumor cells of locoregional ESCC. B. Disease-free survival and overall survival curves for patients with low and high expression levels of HIF-1α in TIL of metastatic ESCC.
The combined expression levels of HIF-1 in both tumor cells and TILs and the survival of ESCC patients
In the current study, Pearson’s correlation coefficient and a linear regression analysis were applied to evaluate the correlation between the expression levels of HIF-1α in tumor cells and in TILs. The HIF-1α expression level in tumor cells were significantly positively associated with the HIF-1α expression level in TILs (P < 0.001, R = 0.376). Therefore, the patients with a combined low expression level of HIF-1α both in tumor cells and TILs had the longest DFS (Median 32 months versus 18, 16 and 12 months) and OS (median 48 months versus 21, 16 and 16 months) related to those with a single high expression level only in tumor cells or in TILs or with a combined high expression level both in tumor cells and in TILs (Figure 4 and Table 4).
Figure 4.
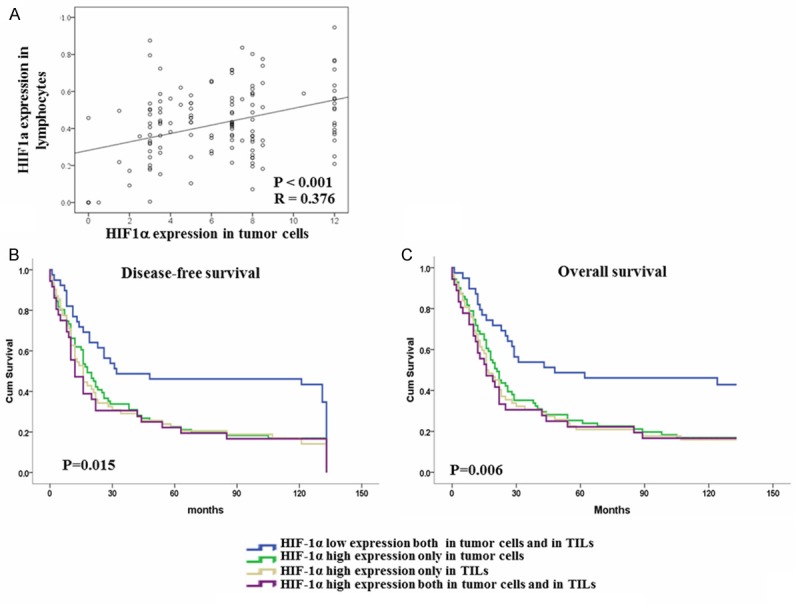
Correlation between the expression levels of HIF-1α in diverse cell populations and survival curves for ESCC patients according to the HIF-1α expression in diverse cell populations. A. The expression level of HIF-1α in tumor cells and TILs were significantly positively correlated (P < 0.001, R = 0.376). B, C. Disease-free survival and overall survival curves for patients according to the combined low expression level and combined high expression level of HIF-1α in tumor cells and TIL, single high expression level in tumor cells or in TILs.
Table 4.
Kaplan-Meier Survival Analysis (Log-Rank Test) analyses of factors associated with OS and DFS
| Variables | DFS (n = 136) | OS (n = 136) | ||
|---|---|---|---|---|
|
| ||||
| Median (Months) | p | Median (Months) | p | |
| Low expression of HIF-1α in tumor cells and in lymphocytes | 32 | 0.015 | 48 | 0.06 |
| High expression of HIF-1α only in tumor cells | 18 | 21 | ||
| High expression of HIF-1α only in lymphocytes | 16 | 16 | ||
| High expression of HIF-1α in tumor cells and in lymphocytes | 12 | 16 | ||
Discussion
It has been suggested that the tumor microenvironment contains immune cells in addition to the cancer cells and their surrounding stromal cells, and the interactions of the diverse cell subsets in the tumor microenvironment play an important role in the clinical prognosis of human cancer patients [21]. Hypoxia has been defined as a common feature in tumor microenvironments. It was reported that under low oxygen conditions, a series of genes related to tumor cell proliferation, angiogenesis and immune cell functions and recruitment are overexpressed in tumor microenvironments through the regulation of HIF-1α protein [22,23]. In this study, we examined the expression pattern of hypoxia-induced protein HIF-1α in different cell populations in tumor tissues from 136 patients with ESCC.
The HIF-1α protein is a good intrinsic marker of tumor hypoxia. Our results suggest that HIF-1α is located in the nucleus and cytoplasm of both tumor cells and TILs (Figure 1). The increased level of HIF-1α in tumor cells was significantly associated with high T status and N status in ESCC patients, and increased levels of HIF-1α in both tumor cells and TILs were significantly correlated with poor DFS and OS in patients with ESCC, according to the univariate Kaplan-Meier analysis (Figure 2). However, only HIF-1α expression in TILs, but not in tumor cells, was an independent prognostic marker for ESCC in a multivariate Cox model analysis that included gender, T status and N status, which were significantly associated with DFS and OS in ESCC patients in the univariate analysis (Table 2). Interestingly, we did observe that high expression of HIF-1α in tumor cells was an independent predictor of DFS and OS in patients with locoregional ESCC (Figure 3A), whereas HIF-1α expression in TILs was an independent predictor of DFS and OS in patients with metastatic ESCC, but not in locoregional tumors. These results suggest that increased expression of HIF-1 in tumor cells has an important effect on the progression of malignant ESCC cells at early disease stage, while the expression of HIF-1α in TILs has an important effect on the clinical prognosis of ESCC patients in the whole disease stage. Previous reports have also indicated that HIF-1 expression was upregulated in the malignant cells of cancers, including ESCC, to promote tumor cell proliferation and survival, EMT and angiogenesis [24-26]. Recent studies about the impact of HIF-1α expression on the survivals of ESCC patients were contrast in different research groups, discrepancies from these studies may arise from different populations and not to distinguish the HIF-1α positive cell populations in tumor microenvironments [27-31]. We know that under hypoxic conditions, immune cells can switch to glycolysis by regulating HIF-1α protein expression to alter the balance of Th1 and Th2 helper cells and by regulating the activities of immune cells to alter immune responses [32]. Some studies determined that the overexpression of HIF-1α in lymphocytes could upregulate the expression of Foxp3, increasing the suppressive function of regulatory T cells and impairing the function of cytotoxic T cells [33,34]. These results also support our finding that high HIF-1α expression in TILs was significantly associated with poor survival in total ESCC patients and in ESCC patients with late disease stage. Furthermore, to the best of our understanding, there is no other report about the correlation between HIF-1 expression level in TILs and cancer patients’ survival.
In summary, our results revealed for the first time that the hypoxia-induced protein HIF-1α is overexpressed in both tumor cells and TILs within the tumor microenvironment of ESCCs. The expression of HIF-1α in tumor cells or in TILs was all significantly associated with poor survivals in ESCC patients. Interestingly, the expression of HIF-1α in tumor cells was an independent predictor of survival for locoregional ESCCs, whereas the expression levels of HIF-1 in TILs was an ideal survival predictive factor for total ESCC patients and metastatic ESCC patients. More importantly, patients with the combined high expression of HIF-1α in both tumor cells and in TILs showed the worst survival in all ESCC patients in this retrospective study.
Taken together, these data suggest that the expression levels of HIF-1α in different cell populations of tumor microenvironment have different clinical relevance and prognostic values in patients with ESCC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 81172164, JL], the Guangdong Province Natural Science Foundation [grant number 10151008901000156, JL], the Key Program of Guangzhou City Science Foundation [grant number 2011Y100036, JL], and the Education Administration Starting Foundation for scientists returning to China from overseas [grant number 43, JL].
Disclosure of conflict of interest
None.
References
- 1.Yan W, Wistuba II, Emmert-Buck MR, Erickson HS. Squamous cell carcinoma - similarities and differences among anatomical sites. Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 2.He LR, Liu MZ, Li BK, Jia WH, Zhang Y, Liao YJ, Chen YC, Zhang LJ, Guan XY, Zeng YX, Kung HF, Xie D. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer. 2010;127:138–147. doi: 10.1002/ijc.25031. [DOI] [PubMed] [Google Scholar]
- 3.Khoshbaten M, Naderpour M, Mohammadi G, Alipoor SH, Estakhri R, Fazeli Z. Epidemiology of esophageal lesions in patients with head and neck squamous cell carcinoma. Asian Pac J Cancer Prev. 2010;11:863–865. [PubMed] [Google Scholar]
- 4.Gan SY, Zhong XY, Xie SM, Li SM, Peng H, Luo F. Expression and significance of tumor drug resistance related proteins and beta-catenin in esophageal squamous cell carcinoma. Chin J Cancer. 2010;29:300–305. doi: 10.5732/cjc.009.10599. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35:e91–99. doi: 10.1016/j.canep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghcheli K, Marjani HA, Nasrollahzadeh D, Islami F, Shakeri R, Sotoudeh M, Abedi-Ardekani B, Ghavamnasiri MR, Razaei E, Khalilipour E, Mohtashami S, Makhdoomi Y, Rajabzadeh R, Merat S, Sotoudehmanesh R, Semnani S, Malekzadeh R. Prognostic factors for esophageal squamous cell carcinoma--a population-based study in Golestan Province, Iran, a high incidence area. PLoS One. 2011;6:e22152. doi: 10.1371/journal.pone.0022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Hu Z, He Z, Jia W, Wang F, Zhou Y, Liu Z, Zhan Q, Liu Y, Yu D, Zhai K, Chang J, Qiao Y, Jin G, Shen Y, Guo C, Fu J, Miao X, Tan W, Shen H, Ke Y, Zeng Y, Wu T, Lin D. Genome-wide association study identifies three new susceptibility loci for esophageal squamous-cell carcinoma in Chinese populations. Nat Genet. 2011;43:679–684. doi: 10.1038/ng.849. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Chen Z, Cheng J, Zhu X, Guo W, Hu A, Du Y, Zhou Y, Wang Y. The high incidence of esophageal cancer in parts of China may result primarily from genetic rather than environmental factors. Dis Esophagus. 2010;23:392–397. doi: 10.1111/j.1442-2050.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 9.Akita H, Doki Y, Miyata H, Hirao T, Yano M, Takachi K, Miyashiro I, Sasaki Y, Ishikawa O, Ohigashi H, Imaoka S. Clinical significance of the second cycle response to cisplatin-based chemotherapy as preoperative treatment for esophageal squamous cell carcinoma. J Surg Oncol. 2006;93:401–409. doi: 10.1002/jso.20501. [DOI] [PubMed] [Google Scholar]
- 10.He LR, Liu MZ, Li BK, Rao HL, Liao YJ, Guan XY, Zeng YX, Xie D. Prognostic impact of H3K27me3 expression on locoregional progression after chemoradiotherapy in esophageal squamous cell carcinoma. BMC Cancer. 2009;9:461. doi: 10.1186/1471-2407-9-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim W, Cho J, Kwon HY, Park Y, Rhyu MR, Lee Y. Hypoxia-inducible factor 1 alpha activates and is inhibited by unoccupied estrogen receptor beta. FEBS Lett. 2009;583:1314–1318. doi: 10.1016/j.febslet.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 13.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1 alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 14.Gort EH, Groot AJ, Derks van de Ven TL, van der Groep P, Verlaan I, van Laar T, van Diest PJ, van der Wall E, Shvarts A. Hypoxia-inducible factor-1 alpha expression requires PI 3-kinase activity and correlates with Akt1 phosphorylation in invasive breast carcinomas. Oncogene. 2006;25:6123–6127. doi: 10.1038/sj.onc.1209643. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier RJ, Chung DC. HIF-1alpha and HIF-2alpha have divergent roles in colon cancer. Int J Cancer. 2009;124:763–771. doi: 10.1002/ijc.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Groep P, Bouter A, Menko FH, van der Wall E, van Diest PJ. High frequency of HIF-1alpha overexpression in BRCA1 related breast cancer. Breast Cancer Res Treat. 2008;111:475–480. doi: 10.1007/s10549-007-9817-z. [DOI] [PubMed] [Google Scholar]
- 18.Nakai H, Watanabe Y, Ueda H, Hoshiai H. Hypoxia inducible factor 1-alpha expression as a factor predictive of efficacy of taxane/platinum chemotherapy in advanced primary epithelial ovarian cancer. Cancer Lett. 2007;251:164–167. doi: 10.1016/j.canlet.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura H, Dhar DK, Kohno H, Kubota H, Fujii T, Ueda S, Kinugasa S, Tachibana M, Nagasue N. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30–42. doi: 10.1002/ssu.10019. [DOI] [PubMed] [Google Scholar]
- 21.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21. doi: 10.3389/fimmu.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 24.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monti E, Gariboldi MB. HIF-1 as a target for cancer chemotherapy, chemosensitization and chemoprevention. Curr Mol Pharmacol. 2011;4:62–77. doi: 10.2174/1874467211104010062. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharyya A, Chattopadhyay R, Hall EH, Mebrahtu ST, Ernst PB, Crowe SE. Mechanism of hypoxia-inducible factor 1 alpha-mediated Mcl1 regulation in Helicobacter pylori-infected human gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1177–1186. doi: 10.1152/ajpgi.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, Millard P, Fuggle S, Harris AL. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. [PubMed] [Google Scholar]
- 28.Kimura S, Kitadai Y, Kuwai T, Tanaka S, Hihara J, Yoshida K, Toge T, Chayama K. Expression of p53 protein in esophageal squamous cell carcinoma: relation to hypoxia-inducible factor-1alpha, angiogenesis and apoptosis. Pathobiology. 2005;72:179–185. doi: 10.1159/000086787. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Lu Y, Lu C, Zhang L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res. 2009;15:487–493. doi: 10.1007/s12253-008-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzao C, Lee SC, Tung HJ, Hsu HS, Hsu WH, Sun GH, Yu CP, Jin JS, Cheng YL. Expression of hypoxia-inducible factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers. 2008;25:141–148. doi: 10.1155/2008/468323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of hypoxia-inducible-factor 1 alpha (HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer. 2003;89:1042–1047. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palazon A, Martinez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, Perez-Gracia JL, Penuelas I, Hervas-Stubbs S, Rouzaut A, Landazuri MO, Jure-Kunkel M, Aragones J, Melero I. The HIF-1 alpha Hypoxia Response in Tumor-Infiltrating T Lymphocytes Induces Functional CD137 (4-1BB) for Immunotherapy. Cancer Discov. 2012;2:608–623. doi: 10.1158/2159-8290.CD-11-0314. [DOI] [PubMed] [Google Scholar]
- 33.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1 alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]


