Abstract
Nuclear expression of β-catenin has been suggested as an independent prognostic marker in a variety of cancers. The objective of this study was to investigate the clinicopathologic significance of nuclear β-catenin expression in patients with cervical squamous cell carcinoma (CSCC). In this original research article, we detected nuclear β-catenin expression in 29/171 CSCC tissues (17.0%). Patients without nuclear β-catenin expression had a significantly better outcome than patients with nuclear β-catenin expression (93.7% versus 82.7% P = 0.027). Furthermore, nuclear β-catenin expression was predictive of prognosis in CSCC patients with early stage disease (FIGO stage I or tumor size ≤ 4 cm), with well/moderately differentiated tumors, or lymph node metastasis. Interestingly, nuclear β-catenin expression correlated with poor outcome in patients who received postoperative chemotherapy or radiotherapy. Multivariate analysis suggested that nuclear β-catenin expression is an independent prognostic indicator in CSCC. Our findings suggest that nuclear β-catenin expression may be used as a prognostic biomarker in CSCC, especially for patients with early stage disease, well/moderately differentiated tumors, or lymph node metastasis. Moreover, nuclear β-catenin expression has potential as a predictive marker of chemoresistance and radioresistance in CSCC.
Keywords: β-catenin, Wnt signaling pathway, cervical squamous cell carcinoma, chemoresistance, radioresistance
Introduction
Cervical cancer is one of the most common gynecologic malignancies worldwide, and is one of the primary causes of cancer-related death for women in developing countries [1]. One of the most complicated challenges in the treatment of cervical cancer is tumor recurrence [2]. Although the cure rate for early-stage cervical cancer is more than 80%, 20% of patients with cervical cancer develop pelvic recurrence [3]. The reported 5-year survival rates for recurrent cervical cancer range from 10.1% to 22.3% [4,5]. Resistance to chemotherapy and radiotherapy are the major reasons for cervical cancer recurrence; however, there is a lack of predictive markers or therapeutic targets to overcome chemo-/radioresistance in cervical cancer.
The Wnt signaling pathway is a highly conserved, intercellular signaling mechanism which plays an important role in a number of key cellular processes. β-catenin is a key mediator of Wnt signaling; the levels of β-catenin are regulated by glycogen synthase kinase 3-β, which functions by phosphorylating β-catenin and targeting it for degradation. Activation of Wnt signaling inhibits GSK-3β, and allows unphosphorylated β-catenin to translocate into the nucleus where it interacts with Tcf/Lef transcription factors [6]. Continuous activation of Wnt signaling is highly implicated in the uncontrolled self-renewal of cancer cells [7], and the Wnt/β-catenin signaling pathway contributes significantly to the development of many types of cancer [8]. In addition, activation of Wnt/β-catenin signaling promotes tumor metastasis and invasion properties [9,10]. Moreover, an association between β-catenin nuclear localization, which is indicative of Wnt signaling activation, and poor clinical outcome has been reported in several human cancers, such as colon cancer [11], gliomas [12], breast cancers [13], and thyroid cancers [14]. Remarkably, Wnt/β-catenin signaling has been shown to be involved in the regulation of cancer stem cells and chemo-/radioresistance [15-17]. Collectively, these studies indicate the important role of Wnt/β-catenin signaling in oncogenesis and therapeutic sensitivity of malignancies.
Recently, attention has been drawn to the role of Wnt signaling in cervical cancer. Uren et al. hypothesized that cervical carcinogenesis is a multi-step process, in which the activation of Wnt/β-catenin signaling was considered as an initial hit [18]. Ramachandran et al. discovered that Wnt inhibitory factor 1, a Wnt signaling antagonist, inhibits cervical cancer cell proliferation in vitro [19]. During the investigation of β-catenin localization in cervical cancer tissues, it was frequently observed that β-catenin accumulated in both the cytoplasm and the nucleus [18,20], demonstrating the possibility of using nuclear β-catenin as an indicator of cancer development or progression. However, there has been no report of whether nuclear expression of β-catenin correlates with the clinical outcome or therapeutic sensitivity of cervical cancer.
In this study, we report for the first time the correlation of nuclear β-catenin expression and poor clinical outcome in human CSCC. We also found that nuclear β-catenin expression correlated with poor prognosis in patients receiving postoperative chemotherapy or radiotherapy. Our results strongly suggest that nuclear expression of β-catenin might be used as a valuable prognostic marker in CSCC, and also indicate that inhibitors of Wnt/β-catenin signaling may have therapeutic potential for controlling chemo-/radioresistant CSCC.
Materials and methods
Patient information and tissue specimens
This study was conducted on a total of 171 paraffin-embedded CSCC samples which were histopathologically and clinically diagnosed at the Sun Yat-sen University Cancer Center between 2001-2005. For the use of these clinical materials for scientific purposes, the patients’ informed consent and approval from the Institutional Research Ethics Committee were obtained. None of the patients had received chemotherapy or radiotherapy before surgery. Postoperative chemotherapy was administered to 94 patients. Platinum-based chemotherapy was initiated within two weeks after surgery and then repeated for four cycles at four-week intervals. Postoperative radiotherapy was given to 59 patients. The whole total pelvic dose was 50.4 Gy, started within four weeks postoperatively, and administered five times a week.
Primary cancers of the cervix were classified according to the FIGO guidelines for clinical staging ref. In total, 171 patients with CSCC up to stage IIB, including three patients with well differentiated tumors, 65 patients with moderately differentiated tumors and 103 patients with poorly differentiated tumors were investigated (Table 1). The mean follow-up time was 59.75 months (range, 0.53-131.63 months). The median age of the patients was 43 years (range, 25-68 years).
Table 1.
Nuclear β-catenin expression in CSCC according to the patients’ clinicopathologic characteristics
| Characteristic | No. of Patients | Nuclear | β-catenin | P value |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Age (y) | ||||
| ≤ 50 | 132 | 25 | 107 | |
| > 50 | 39 | 4 | 35 | .072 |
| FIGO Stage | ||||
| I | 107 | 24 | 83 | |
| II | 64 | 5 | 59 | .019 |
| Differentiation | ||||
| Well | 3 | 1 | 2 | |
| Moderate | 65 | 11 | 54 | |
| Poor | 103 | 17 | 86 | .746 |
| Tumor Size | ||||
| ≤ 4 cm | 125 | 24 | 101 | |
| > 4 cm | 46 | 5 | 41 | .254 |
| Nodal Metastasis | ||||
| + | 27 | 6 | 21 | |
| - | 144 | 23 | 121 | .411 |
| Total No. of Patients | 171 | 29 | 142 | |
Immunohistochemistry
Immunohistochemical analysis was performed to study β-catenin expression in the 171 human CSCC tissues. Formalin-fixed, paraffin-embedded specimens were cut into 4 μm sections and mounted onto poly-L-lysine-coated slides. The sections were deparaffinized, rehydrated, boiled for 10 min in 10 μmol/L citrate buffer solution at pH 6.0, and then cooled in the same buffer. Endogenous peroxidase activity was quenched using 0.3% H2O2 for 30 min, and non-specific staining was blocked by treating the slides with 1% fish skin gelatin for 30 min at room temperature. Tissue sections were incubated overnight with monoclonal rabbit antibody against β-catenin (Cell Signaling Technology, MA, USA; 1:100), and then incubated with prediluted anti-rabbit secondary antibody (Dako, Denmark; 1:200). Diaminobenzidine (DAB) was used as a chromagen, and then the sections were counterstained with hematoxylin and mounted. For negative controls, the rabbit anti-β-catenin antibody was replaced with normal non-immune serum.
All stained slides were separately evaluated by two pathologists. β-catenin staining was classified into three patterns: (1) a membranous pattern, if staining was present only at the cell membrane; (2) a cytoplasmic pattern, if staining was mainly present in the cytoplasm but not in the nucleus; and (3) a nuclear pattern, if immunoreactivity was present in the nuclei. The nuclear pattern was regarded as a positive signal. The cutoff value for positive and negative nuclear expression was selected on the basis of a measure of heterogeneity using the log-rank test with respect to overall survival and recurrence-free survival.
Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 16.0, SPSS Inc, Chicago, IL, USA). Overall survival was defined as the time between the date of primary surgery to the date of death or the end point. Recurrence-free survival was defined as the time between the date of primary surgery to the date of relapse or end point. Overall and recurrence-free survival rates were estimated using Kaplan-Meier analysis; differences were compared using the log-rank test. A Cox proportional hazards multivariate regression model was used to select significant independent prognostic factors. The correlations between nuclear β-catenin expression and clinicopathologic characteristics were analyzed using the χ2 test. In all cases, P < 0.05 was considered statistically significant.
Results
Expression of β-catenin in CSCC tissues
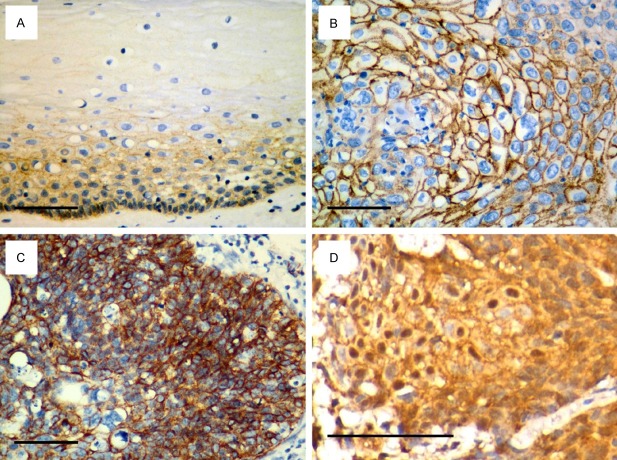
To verify whether Wnt signaling is activated in cervical cancer, we determined the expression and subcellular localization of β-catenin in 171 CSCC tissues. In normal cervical epithelium, β-catenin staining was only detected in the membrane and cytoplasm of the basal and suprabasal layers (Figure 1A). In most of the tumor tissues, the β-catenin expression pattern was membranous (75%; Figure 1B) and/or cytoplasmic (89%; Figure 1C); however, 17.0% of the tumor tissues had positive nuclear β-catenin expression (Figure 1D). Nuclear β-catenin expression was observed in a large number of patients with stage I tumors (63%), with a tumor size no greater than 4 cm (83%), or without lymph node metastasis (79%). More grade 3 tumors (59%) had positive nuclear β-catenin expression than grade 1 (3%) and grade 2 tumors (38%; Table 1).
Figure 1.
Representative immunohistochemical analysis of β-catenin expression. (A) Normal cervical epithelial cells displayed membranous and cytoplasmic expression of β-catenin in the basal and suprabasal layers. (B) Membranous, (C) membranous and cytoplasmic and (D) nuclear patterns of β-catenin expression were observed in cervical squamous cell cancer (CSCC). Scale bar, 100 μm.
Nuclear expression of β-catenin is associated with CSCC clinical features
To investigate the clinical significance of β-catenin in cervical cancer, we examined the correlation between nuclear expression of β-catenin and the clinical characteristics of CSCC. There was no significant relationship between nuclear expression of β-catenin and patient age, tumor differentiation, tumor size or nodal metastasis. In contrast, there was a significant difference in nuclear β-catenin expression between FIGO stage I and stage II (P = 0.019, Table 1). These results suggest that activation of Wnt/β-catenin signaling may be associated with disease progression in early stage CSCC.
Relationship between nuclear β-catenin expression and the prognosis of patients with CSCC
On the basis of the results above, we further analyzed the correlation between nuclear β-catenin expression and clinical prognosis in CSCC patients. Kaplan-Meier survival analysis revealed an inverse correlation between nuclear β-catenin expression and the overall survival time of CSCC patients. The log-rank test indicated that patients with positive nuclear β-catenin expression had a shorter overall survival time. However, there was no significant difference between the recurrence-free survival time of the positive and negative nuclear β-catenin groups. The cumulative 10-year survival rates of patients with nuclear β-catenin positive and negative tumors were 93.7% and 82.7%, respectively (P = 0.027, Figure 2A).
Figure 2.
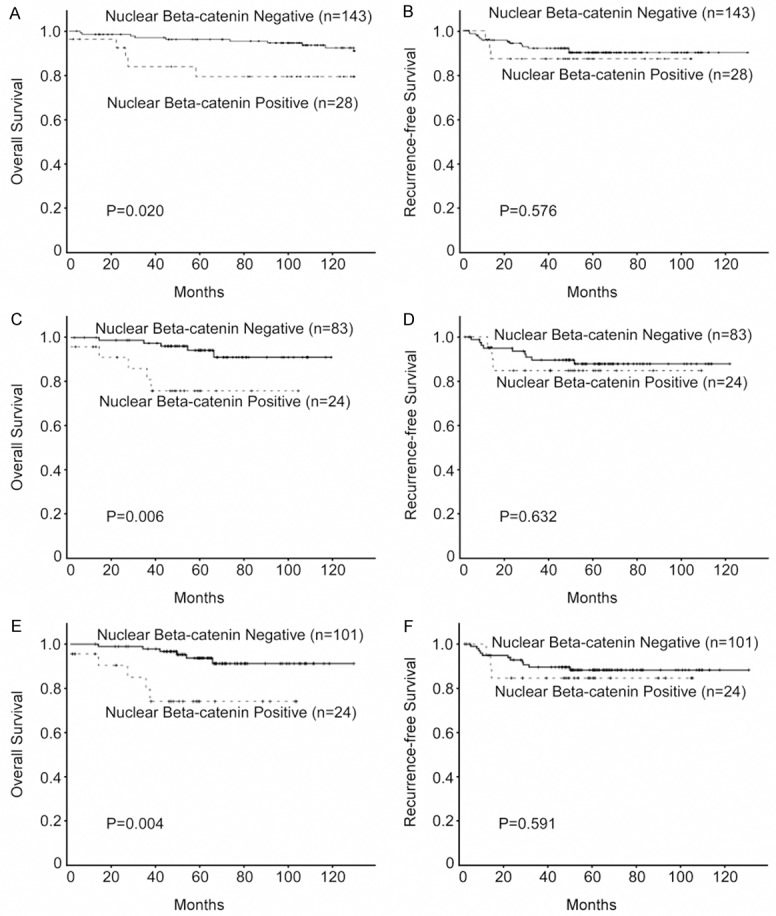
Kaplan-Meier analysis of overall survival and recurrence free survival in relation to nuclear β-catenin expression. (A, B) Analysis of all 171 cervical squamous cell cancer (CSCC) patients; (C, D) The 107 patients with FIGO stage I CSCC; (E, F) and the 125 patients with small tumors (≤ 4 cm).
Relationship between nuclear β-catenin expression and the prognosis in different CSCC patient subgroups
We further analyzed the prognostic value of nuclear β-catenin expression in selected patient subgroups, when stratified according to FIGO stage, tumor size, tumor differentiation or lymph node status. Patients with tumors exhibiting positive nuclear β-catenin expression had a significantly shorter overall survival, compared to patients with nuclear β-catenin negative tumors in the FIGO stage I subgroup (n = 107; P = 0.006; Figure 2C), the small (≤ 4 cm) tumor subgroup (n = 125; P = 0.004; Figure 2E), and the grade 1/2 subgroup (n = 67; P = 0.044; Figure 3A). In contrast, no significant differences in overall survival were observed between patients with tumors exhibiting positive or negative nuclear β-catenin expression in the subgroups of patients with advanced carcinomas (FIGO stage II or tumor > 4 cm) or grade 3 tumors (data not shown). Nuclear β-catenin expression and recurrence-free survival correlated significantly in the subgroup of patients with lymph node metastasis (LN+; n = 67; P = 0.022; Figure 3D). There was no significant difference with respect to recurrence-free survival in the subgroups of patients with FIGO stage I, small tumors or grade 1/2 tumors (Figures 2B, 2D, 2F and 3B). Multivariate Cox regression analysis indicated that nuclear β-catenin expression was an independent prognostic factor for overall survival in patients with CSCC (Table 2).
Figure 3.
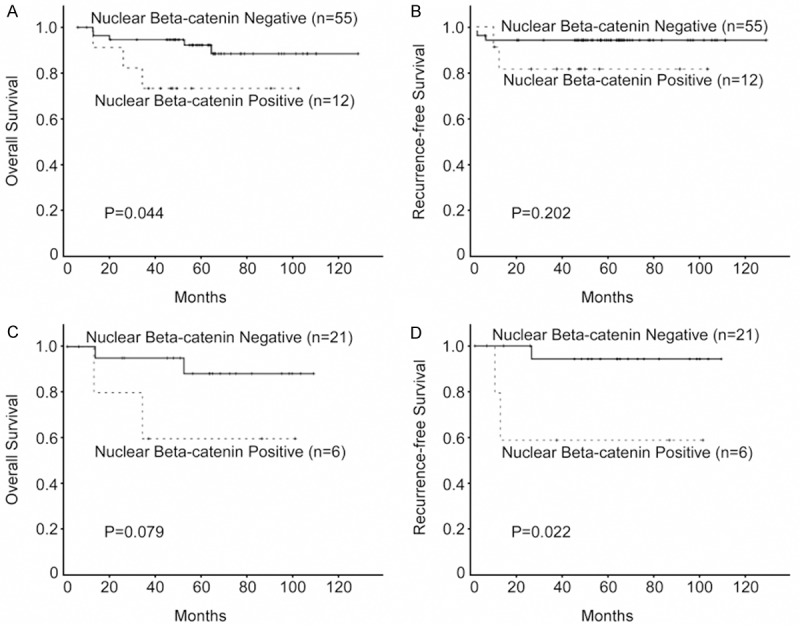
Kaplan-Meier analysis of overall survival and recurrence free survival in relation to nuclear β-catenin expression. (A, B) Analysis of the 67 cervical squamous cell cancer (CSCC) patients with grade 1/2 tumors; (C, D) and the 27 CSCC patients with lymph node metastasis.
Table 2.
Multivariate analyses of different prognostic parameters in cervical squamous cell cancer using a Cox regression analysis model
| Prognostic variables | Overall Survival | Recurrence-free Survival | ||
|---|---|---|---|---|
|
|
|
|||
| Risk ratio (95% CI) | P value | Risk ratio (95% CI) | P value | |
| Age (> 50 vs. ≤ 50) | 1.063 (1.007-1.122) | 0.028 | 1.028 (0.969-1.090) | 0.359 |
| Nuclear β-catenin (+ vs. -) | 4.109 (1.342-12.580) | 0.013 | 1.281 (0.358-4.583) | 0.703 |
Relationship between nuclear β-catenin expression and the prognosis of patients with postoperative adjuvant treatment
To examine the predictive value of Wnt signaling in chemo-/radioresistance of CSCC, we evaluated the correlation between nuclear β-catenin expression and prognosis according to the patients’ postoperative treatment modality. Notably, nuclear β-catenin expression correlated with poor overall survival (n = 94; P = 0.006; Figure 4A) and recurrence-free survival (n = 94; P = 0.039; Figure 4B) in patients who received postoperative adjuvant chemotherapy. Moreover, nuclear β-catenin expression was associated with unfavorable overall survival (n = 59; P = 0.005; Figure 4C, but not with recurrence-free survival (n = 59; P = 0.198; Figure 4D) in patients who received postoperative radiotherapy. These results suggest that nuclear β-catenin expression may potentially serve as a prognostic marker and therapeutic target for chemo-/radioresistance in CSCC patients.
Figure 4.
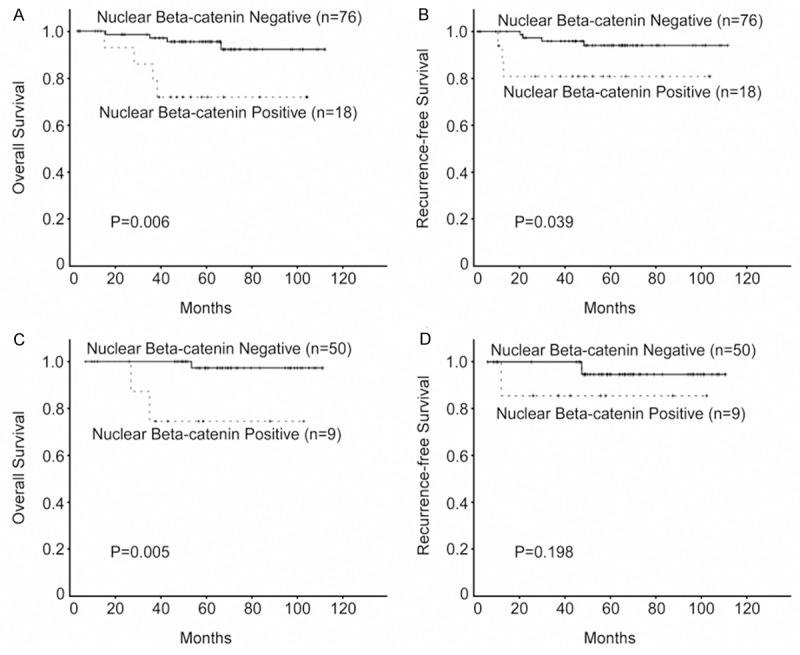
Kaplan-Meier analysis of overall survival and recurrence free survival in relation to nuclear β-catenin expression. (A, B) Analysis of the 94 cervical squamous cell cancer (CSCC) patients who received postoperative chemotherapy, (C, D) and the 59 CSCC patients who received postoperative radiotherapy.
Discussion
In the current study, we report for the first time that CSCC patients with nuclear β-catenin expression have a shorter survival time than patients without nuclear β-catenin expression. In addition, nuclear β-catenin expression was associated with poor survival in the subgroups of patients with FIGO stage I tumors, grade 1/2 tumors, or lymph node metastasis. Importantly, we also demonstrated that nuclear accumulation of β-catenin could be a prognostic factor for shorter survival in CSCC patients receiving postoperative therapies. This study suggests that nuclear β-catenin represents a novel prognostic indicator for CSCC, and that targeting the Wnt/β-catenin pathway may be a promising strategy for treating recurrent CSCC.
Numerous studies have documented that the Wnt/β-catenin signaling pathway regulates a variety of processes essential for tumor pathogenesis and progression. Stabilization or abnormal phosphorylation of β-catenin can promote cancer in numerous tissues [21,22]. Inactivation of Wnt signaling inhibits cancer cell proliferation [23], while failure to inhibit Wnt signaling can lead to excessive proliferation of tumor cells [24]. One may speculate that accumulation of β-catenin in the nucleus, and the subsequent binding of β-catenin to Tcf transcription factors, results in increased cancer cell proliferation and tumor development [25]. Collectively, these studies indicate that Wnt/β-catenin signaling plays a crucial role during several steps of carcinogenesis.
Interestingly, the present study shows that nuclear β-catenin expression is associated with poor prognosis in CSCC, which strongly suggests that activation of Wnt/β-catenin signaling may be associated with disease progression in cervical carcinoma. Varying correlations between Wnt/β-catenin signaling and clinical outcomes have been reported in several malignancies. Norwood et al. reported that nuclear β-catenin was a positive prognostic factor for overall survival in gastrointestinal adenocarcinoma [26]. Chen et al. demonstrated that nuclear accumulation of β-catenin was a poor prognostic marker in colon cancer [27]. These findings imply that the role of β-catenin may be tissue specific, and also indicate that β-catenin has diverse effects in different cancers. However, in contrast to our study, none of these studies described the subcellular localization of β-catenin.
We further analyzed the correlation between nuclear β-catenin expression and prognosis in different subgroups of CSCC patients. We found that nuclear β-catenin expression was predictive of poor overall survival in patients with early-stage CSCC, but not in patients with advanced-stage CSCC, indicating that Wnt signaling may be one potential mechanism which facilitates the progression of early stage cervical cancer. This hypothesis is supported by Fadare and colleagues [28], who proposed that Wnt/β-catenin signaling, may be associated with the pathogenesis of very early stage cervical cancer, based on a tissue microarray study. We also observed that nuclear β-catenin expression correlated with a poor prognosis in patients with grade 1/2 tumors, which concurs with a previous study which reported that Wnt/β-catenin signaling plays a pivotal role in the promotion of cancer cell differentiation [29]. Moreover, β-catenin nuclear localization correlated with a poor prognosis in patients with lymph node metastasis. In accordance with this observation, a recent analysis of gene expression patterns in cervical cancer suggested Wnt/β-catenin pathway is involved in promoting the high proliferative rate of local tumor cells, which expand rapidly and escape from the immune system, spreading regionally to nearby lymph nodes [30].
Most importantly, we found that nuclear β-catenin expression was associated with shorter overall and recurrence-free survival in CSCC patients who received postoperative chemotherapy. Similarly to our study, Sivula et al. reported that cyclooxygenase-2, a downstream target of the Wnt signaling pathway, correlates with poor overall and recurrence-free survival in bladder cancer patients receiving chemotherapy [31]. Jang et al. also revealed that β-catenin and cyclin D1 could be used as prognostic indicators in colorectal carcinoma patients receiving adjuvant chemotherapy [32]. Thus, we can infer that Wnt/β-catenin signaling is associated with resistance to chemotherapy in several malignancies. This assumption is supported by recent studies which reported that activation of Wnt/β-catenin signaling by the Wnt receptor frizzled family receptor 1 can induce overexpression of multidrug resistance gene 1, which in turn leads to chemoresistance [33]. Conversely, inhibition of Wnt signaling can induce chemosensitivity in cancer cells [34].
It is also particularly noteworthy that nuclear β-catenin expression was associated with shorter overall survival in CSCC patients who received postoperative radiotherapy. However, nuclear β-catenin expression did not have a significant effect on recurrence-free survival in patients who received postoperative therapies, probably due to the relatively small sample size in this study. Our results demonstrate that nuclear β-catenin expression may predict a poor response to radiotherapy in CSCC patients. In line with these results, Watson et al. recently demonstrated that stabilization of β-catenin results in radioresistance in pancreatic cancer [35]. Using in vitro radiation resistance assay, Chen and Woodward [36] found that a subpopulation of Sca1 positive cells expressed high levels of β-catenin and were resistant to radiation in breast cancer, suggesting that Wnt signaling mediates the radioresistance of mammary gland progenitors. Taken together, our results indicate that Wnt signaling may be involved in the chemoresistance, radioresistance and ultimately the recurrence of cervical cancer. Further studies on the molecular mechanisms by which Wnt/β-catenin signaling promotes chemoresistance and radioresistance are required to improve the efficacy of postoperative treatments in cervical cancer.
In conclusion, we demonstrated that nuclear β-catenin expression can be regarded as a potential prognostic marker for CSCC. In addition, accumulation of nuclear β-catenin may be a potentially useful marker for chemoresistance and radioresistance in CSCC. Understanding the mechanisms by which Wnt/β-catenin signaling is involved in the progression and chemo-/radioresistance of cervical carcinoma will not only increase our understanding of the biology of cervical cancer, but may also enable the discovery of potential therapeutic targets for recurrent CSCC.
Acknowledgements
We thank Jiehua He who helped with the immunostudies.
Disclosure of conflict of interest
None.
References
- 1.Rogers LJ, Eva LJ, Luesley DM. Vaccines against cervical cancer. Curr Opin Oncol. 2008;20:570–574. doi: 10.1097/CCO.0b013e328303e2a1. [DOI] [PubMed] [Google Scholar]
- 2.Chiva LM, Lapuente F, Gonzalez-Cortijo L, Gonzalez-Martin A, Rojo A. Surgical treatment of recurrent cervical cancer: state of the art and new achievements. Gynecol Oncol. 2008;110:S60–66. doi: 10.1016/j.ygyno.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Duenas-Gonzalez A, Lizano M, Candelaria M, Cetina L, Arce C. Epigenetics of cervical cancer. An overview and therapeutic perspectives. Mol Cancer. 2005;4:38. doi: 10.1186/1476-4598-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto T, Kino N, Shirai T, Fujimura M, Takahashi M. Late recurrence of invasive cervical cancer: twenty years’ experience in a single cancer institute. J Obstet Gynaecol Res. 2005;31:514–519. doi: 10.1111/j.1447-0756.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang CJ, Lai CH, Huang HJ, Hong JH, Chou HH. Recurrent cervical carcinoma after primary radical surgery. Am J Obstet Gynecol. 1999;181:518–524. doi: 10.1016/s0002-9378(99)70486-2. [DOI] [PubMed] [Google Scholar]
- 6.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 7.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher SJ, Rambow F, Kumasaka M, Champeval D, Bellacosa A. Beta-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene. 2012;32:2230–8. doi: 10.1038/onc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi CS, Huang NN, Kehrl JH. Regulator of G-Protein Signaling 3 Isoform 1 (PDZ-RGS3) Enhances Canonical Wnt Signaling and Promotes Epithelial Mesenchymal Transition. J Biol Chem. 2012;287:33480–33487. doi: 10.1074/jbc.M112.361873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401–8. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 12.Shi Z, Qian X, Li L, Zhang J, Zhu S, Zhu J, Chen L, Zhang K, Han L, Yu S, Pu P, Jiang T, Kang C. Nuclear Translocation of beta-catenin is Essential for Glioma Cell Survival. J Neuroimmune Pharmacol. 2012;7:892–903. doi: 10.1007/s11481-012-9354-3. [DOI] [PubMed] [Google Scholar]
- 13.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbosh PH, Nephew KP. Multiple signaling pathways converge on beta-catenin in thyroid cancer. Thyroid. 2005;15:551–61. doi: 10.1089/thy.2005.15.551. [DOI] [PubMed] [Google Scholar]
- 15.Janikova M, Skarda J. Differentiation pathways in carcinogenesis and in chemo- and radioresistance. Neoplasma. 2012;59:6–17. doi: 10.4149/neo_2012_002. [DOI] [PubMed] [Google Scholar]
- 16.Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, Katano M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30:2041–8. [PubMed] [Google Scholar]
- 17.Zhang M, Atkinson RL, Rosen JM. Selective targeting of radiation-resistant tumor-initiating cells. Proc Natl Acad Sci U S A. 2010;107:3522–7. doi: 10.1073/pnas.0910179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uren A, Fallen S, Yuan H, Usubutun A, Kucukali T, Schlegel R, Toretsky JA. Activation of the canonical Wnt pathway during genital keratinocyte transformation: a model for cervical cancer progression. Cancer Res. 2005;65:6199–206. doi: 10.1158/0008-5472.CAN-05-0455. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, Queimado L. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene. 2012;31:2725–37. doi: 10.1038/onc.2011.455. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara A, Yokoyama Y, Wan X, Takahashi Y, Mori Y, Takami T, Shimokawa K, Tamaya T. Cytoplasmic/nuclear expression without mutation of exon 3 of the beta-catenin gene is frequent in the development of the neoplasm of the uterine cervix. Gynecol Oncol. 2001;82:450–5. doi: 10.1006/gyno.2001.6298. [DOI] [PubMed] [Google Scholar]
- 21.Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CH, Hung HW, Hung PH, Shieh YS. Epidermal growth factor receptor regulates beta-catenin location, stability, and transcriptional activity in oral cancer. Mol Cancer. 2010;9:64. doi: 10.1186/1476-4598-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q, He B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/beta-catenin signaling. Int J Oncol. 2012;41:292–8. doi: 10.3892/ijo.2012.1423. [DOI] [PubMed] [Google Scholar]
- 24.Senda T, Iizuka-Kogo A, Onouchi T, Shimomura A. Adenomatous polyposis coli (APC) plays multiple roles in the intestinal and colorectal epithelia. Med Mol Morphol. 2007;40:68–81. doi: 10.1007/s00795-006-0352-5. [DOI] [PubMed] [Google Scholar]
- 25.van Noort M, Clevers H. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev Biol. 2002;244:1–8. doi: 10.1006/dbio.2001.0566. [DOI] [PubMed] [Google Scholar]
- 26.Norwood MG, Bailey N, Nanji M, Gillies RS, Nicholson A, Ubhi S, Darnton JJ, Steyn RS, Womack C, Hughes A, Hemingway D, Harrison R, Waters R, Jankowski JA. Cytoplasmic beta-catenin accumulation is a good prognostic marker in upper and lower gastrointestinal adenocarcinomas. Histopathology. 2010;57:101–11. doi: 10.1111/j.1365-2559.2010.03587.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu P, Ni C, Zhang Z, Ye J, Xu J, Huang J. beta-catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: a meta-analysis. PLoS One. 2013;8:e63854. doi: 10.1371/journal.pone.0063854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadare O, Reddy H, Wang J, Hileeto D, Schwartz PE, Zheng W. E-Cadherin and beta-Catenin expression in early stage cervical carcinoma: a tissue microarray study of 147 cases. World J Surg Oncol. 2005;3:38. doi: 10.1186/1477-7819-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–97. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 30.Hsu NY, Chow KC, Chen WJ, Lin CC, Chou FF, Chen CL. Expression of nm23 in the primary tumor and the metastatic regional lymph nodes of patients with gastric cardiac cancer. Clin Cancer Res. 1999;5:1752–7. [PubMed] [Google Scholar]
- 31.Wulfing C, Eltze E, von Struensee D, Wulfing P, Hertle L, Piechota H. Cyclooxygenase-2 expression in bladder cancer: correlation with poor outcome after chemotherapy. Eur Urol. 2004;45:46–52. doi: 10.1016/j.eururo.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Saito S, Tsuno N, Nagawa H, Sunami E, Zhengxi J, Osada T, Kitayama J, Shibata Y, Tsuruo T, Muto T. Expression of platelet-derived endothelial cell growth factor correlates with good prognosis in patients with colorectal carcinoma. Cancer. 2000;88:42–9. [PubMed] [Google Scholar]
- 33.Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, Beckmann JS, Joseph JM, Muhlethaler-Mottet A, Gross N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2245–56. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 34.Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2005;62:61–8. doi: 10.1002/pros.20117. [DOI] [PubMed] [Google Scholar]
- 35.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER, Hammer GD, Lawrence TS, Ben-Josef E. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12:357–65. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–77. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]