Abstract
Overexpression of aquaporins (AQPs) has been reported in several human cancers. Extracellular signal-regulated kinases 1/2 (Erk1/2) are associated with tumorigenesis and cancer progression and may upregulate AQPs expression. In this study, we examined cervical tissue samples to establish the relationship between Erk1/2 and AQPs in cervical carcinoma by RT-PCR, Western blot and immunohistochemistry. We also examined the relationship between AQP8, Erk1/2 and clinicopathological variables in patients with cervical cancer. Our results showed that Erk1/2 was differentially expressed at the level of transcription and was most highly expressed in CIN samples (P < 0.05). At the level of translation, significant differences were seen in the expression of AQP8, Erk1/2 and P-Erk1/2 (P < 0.05). Expression was highest in CIN samples, where 80.9%, 76.6%, and 66% of samples were positive for AQP8, Erk1/2 and P-Erk1/2, respectively. Expression in cervical carcinoma samples was higher than in normal cervical tissues (P < 0.01). AQP8 expression was associated with the depth of invasion of cervical cancer cells, and the expression of Erk1/2 and P-Erk1/2 was increased in earlier clinical stages and in lymphatic metastasis. AQP8 expression was positively correlated with Erk1/2 expression in cervical cancer. In conclusions, increased AQP8, Erk1/2 and P-Erk1/2 expression may play a role in transformation of CIN into cervical cancer, and in early invasion and lymphatic metastasis of cervical cancer. These proteins could potentially be used as molecular markers for early diagnosis of cervical carcinoma.
Keywords: Aquaporin 8, Erk1/2 phosphorylation, cervical intraepithelial neoplasia, cervical carcinoma
Introduction
Cervical cancer is the most common cancer among women in developing countries and the second most common cancer among women globally. In 2008, it accounted for 9% (529,800) of new cancer cases and 8% (275,100) of all cancer deaths among women. More than 85% of cases and deaths occurred in developing countries, including China [1,2]. In Xinjiang, China, especially southern Xinjiang, the incidence of cervical carcinoma in women of Uygur ethnicity is very high, with a prevalence of 527 per 100,000 females. Furthermore, approximately 80% of cervical carcinoma patients present at an advanced stage [3]. Although early diagnosis and the survival rate for patients with cervical carcinoma have improved in recent years, the efficacy of treatment and prognosis for patients with advanced cervical cancer remains poor. Further research on the pathogenesis of cervical carcinoma and the identification of molecular biomarkers for early diagnosis, especially for women of Uygur ethnicity is, therefore, essential.
Aquaporins (AQPs) increase cell plasma membrane water permeability 5-50 times compared to membranes where water moves primarily through the lipid bilayers. In mammals, 13 members of the aquaporin gene family (AQP0 through AQP12) have been identified [4]. AQPs are expressed in a variety of epithelial tissues where they are responsible for regulating rapid water movement across epithelial barriers driven by osmotic gradients. Malignant tumor cells have vigorous life cycles and active metabolism, and thus have an increased demand for water. Recent evidences suggest that AQPs are also involved in cell migration [5], angiogenesis [6], and tumor growth [7]. They are strongly expressed in tumor cells of different origins, particularly aggressive tumors. In contrast to the pro-tumorigenic effects resulting from enhanced AQP expression in some tumors, reduced expression of AQP8 in hepatocellular carcinoma is associated with increased resistance to apoptosis [8]. AQPs are involved in cell migration induced by extracellular signal-regulated protein kinases 1 and 2 (Erk1/2). The Erk1/2 pathway is a critical signal transduction pathway in the mitogen-activated protein kinase (MAPK) family, and is closely related with tumorigenesis and tumor progression [9,10].
To gain insight into the role of AQPs and Erk1/2 in human cervical carcinoma, we probed the effects of AQP8 expression and phosphorylation of Erk1/2 on cervical carcinogenesis at the levels of transcription and translation, and analyzed the clinical significance.
Materials and methods
Human cervical tissues and reagents
Expression of AQP8 and Erk1/2 in cervical tissues was examined by real-time (RT)-PCR using a one-step RT-PCR kit (QIAGEN, Germany). Thirty fresh tissue samples were collected from gynecological procedures at the Department of Gynecology, Third Affiliated Hospital of Xinjiang Medical University. Samples were collected between January 2012 and March 2013. Ten samples were obtained of normal tissue, ten samples of cervical intraepithelial neoplasia (CIN), and ten samples of early cervical cancer. Immunohistochemistry was performed on paraformaldehyde-fixed and paraffin-embedded tissues obtained from the Department of Pathology, Third Affiliated Hospital of Xinjiang Medical University, including 47 CIN samples (grade 2-3; age range 30 to 51 years, median age 38.5 years), 108 cervical cancer samples (age range 25 to 68 years, median age 46 years; 95 cases for grade 1-2, 13 cases for grade 3; 17 cases for stage 1, 90 cases for stage 2, 1 cases for stage 3) and 79 normal tissue samples (age range 38 to 60 years, median age 39.5 years). Tumor distribution was defined according to the International Federation of Gynecology and Obstetrics stages.
Inclusion and exclusion criteria
Patients with abnormalities in the uterine cervix were included in the study. Clinical stage was determined by two gynecologists, while two pathologists confirmed diagnoses of squamous cell carcinoma. Patients had not undergone chemoradiotherapy prior to surgery. All patients provided informed consent for this study.
Real-time PCR
Total RNA from human cervical tissues was extracted using TRIzol Reagent (Invitrogen, USA). The RT-PCR conditions were as follows: reverse transcription 50°C, 30 min; PCR initial activation step 95°C, 15 min; denaturation 94°C, 15 s; annealing 55°C, 30 s; extension 72°C, 30 s. Steps were repeated for 40 cycles. Melting curve analysis: 95°C, 1 min; 55°C, 30 s; 95°C, 30 s. PCR products were analyzed by 1.5% agarose gel electrophoresis. Gene expression of AQP8 and ERK1/2 was calculated using the 2-ΔΔCt method. β-actin expression was used as a control. Primers (Table 1) were purchased from QIAGEN (German).
Table 1.
Primer sequences for amplification of AQP8, Erk1/2 and the house keeping gene β-actin
| Primer | Cat. no. | Size of product (bp) |
|---|---|---|
| β-actin | QT00095431 | 146 |
| AQP8 | QT00039123 | 87 |
| Erk1/2 | QT00065933 | 118 |
Western blot analysis
Tissue lysates were prepared in 1 mL ice-cold radio-immunoprecipitation assay (RIPA) buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with the addition of a 1 × concentration of the protease inhibitor phenylmethanesulfonyl fluoride (PMSF). Crude protein lysates were collected in microcentrifuge tubes and centrifuged for 15 min at 15,000 rpm at 4°C. Supernatants were transferred to clean microcentrifuge tubes and kept at -80°C until use. Protein (60 μg of each sample) was mixed with an equal volume of 5 × loading buffer, heated at 100°C for 5 min and cooled on ice for 3 min before loading. Protein samples were resolved on a 7.5% SDS-polyacrylamide gel and transferred onto polyvinylidene fluoride membranes (Millipore), and blocked for 1 hour with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20. AQP8 primary rabbit polyclonal antibody (sc-28624, Santa-Cruz), Erk1/2 primary rabbit monoclonal antibody (4695, Cell Signaling Technology, USA), P-Erk1/2 (Thr202/Thr204; Cell Signaling Technology, USA) at 1:200, 1:800, and 1:1000, dilutions respectively, were incubated overnight at 4°C. Incubation with the secondary goat antibody ready to use (PV-9000, Zhongshan Golden Bridge, Beijing, China) was performed for 1 h at room temperature. Protein bands were detected by chemiluminescence using LumiGlo reagent A/B (Cell Signaling Technology, MA, USA). β-actin was used as a loading control.
Immunohistochemistry
Four percent paraformaldehyde-fixed cervical lesion tissues were prepared in 2 μm thick sections on slides. The sections were labeled using anti-AQP8, Erk1/2, P-Erk1/2 primary antibodies at 1:100, 1:100, and 1:200 dilutions, respectively, at 4°C overnight. Secondary antibody ready to use was then added for 30 min at room temperature. Following DAB (3,3’-diaminobenzidine) staining, hematoxylin counterstaining, dehydration, and mounting were performed. AQP8, Erk1/2 and P-Erk1/2 immunolabeling was reviewed by expert pathologists. Staining intensity and tumor cell proportion were scored. Immunolabeling intensity was scored as negative (-), dilute brown (score 1), brown (score 2), and deep brown (score 3). The proportion was the approximate number of AQP8, ERK1/2 and P-ERK1/2 positive cells among total tumor cells. Scoring was as follows: 0-4% (-), 5-25% (score 1), 26-50% (score 2), 51%-75% (score 3), and > 75% (score 4). The intensity plusing proportion was scored as - (score 0-1), + (score 2-3), ++ (score 4-5), and +++ (score 6-7).
Statistical analysis
Data were analyzed using SPSS 20.0 statistical software. Measurement data were expressed as the x̅ ± s. Statistical differences among groups were compared using one-sample T-test for measurement data and x 2 test for numeration data. P < 0.05 was considered to be statistically significant.
Results
Expression differences in Erk1/2 are statistically significant across groups at the level of transcription
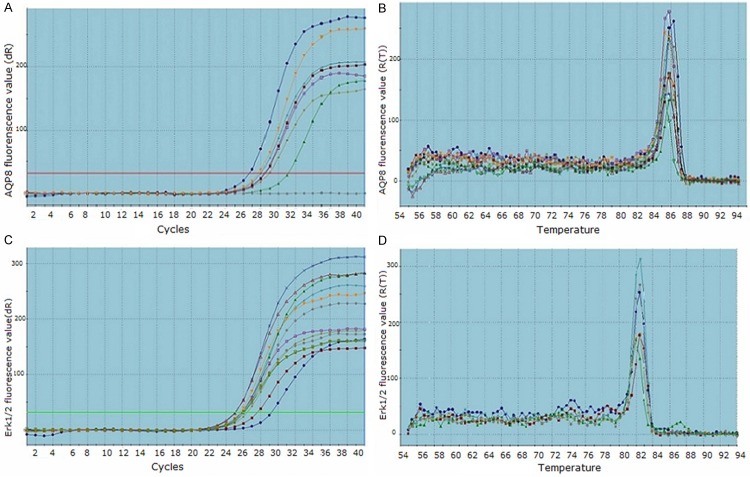
Expression of AQP8 and Erk1/2 was detected by RT-PCR (Figure 1A-D). The data were showed with 2-ΔΔCT value. Expression levels of Erk1/2 in normal tissue, CIN and cervical cancer tissue were significantly different, expression level of Erk1/2 in CIN was highest (P < 0.05; Table 2). Expression levels of AQP8 in normal tissue, CIN and cervical cancer tissue were not significantly different (P > 0.05; Table 2).
Figure 1.
Amplification plots and dissociation curves by RT-PCR. (A) Amplification plot for AQP8 gene, (B) Dissociation curve for AQP8 gene; (C) Amplification plot for Erk1/2 gene, (D) Dissociation curve for Erk1/2 gene. X axis of amplification plots: cycles, Y axis of amplification plots: fluorescence value; X axis of dissociation curves: temperature, Y axis of dissociation curves: fluorescence value.
Table 2.
AQP8 and Erk1/2 mRNA expression (x̅ ± s)
| Group | AQP8 2-ΔΔCT | t value | P value | Erk1/2 2-ΔΔCT | t value | P value |
|---|---|---|---|---|---|---|
| Cervical carcinoma | 2.372 ± 2.436 | 1.781 | 0.109 | 2.348 ± 3.682 | 1.158 | 0.277** |
| CIN | 1.104 ± 1.383 | 0.239 | 0.817 | 7.109 ± 6.745 | 2.864 | 0.019*** |
| Normal tissue | 1.834 ± 2.128 | 1.239 | 0.247 | 5.806 ± 4.491 | 3.384 | 0.008* |
Cervical carcinoma vs Normal tissue;
Cervical carcinoma vs CIN;
CIN vs Normal tissue.
Expression and localization of AQP8, Erk1/2, and P- Erk1/2 proteins in CIN and cervical carcinoma
Western blotting and immunohistochemistry assays were performed. AQP8, Erk1/2, P-Erk1/2 proteins were found to be expressed in cervical lesion tissues. AQP8 localized to the cytoplasm and nuclei of CIN and carcinoma cells. In normal tissues, it localized to the membrane and cytoplasm of basal and proliferative cells (Figure 2A-D). AQP8 expression was highest in CIN samples, and expression in cervical carcinoma samples was higher than in normal tissues (Figure 2E-G, P < 0.01). Erk1/2 mainly localized to the cytoplasm, with some protein present in the nuclei of CIN and carcinoma cells. Expression in normal tissues occurred in the cytoplasm of basal and proliferative cells (Figure 3A-D). Erk1/2 expression was highest in CIN samples, and expression in cervical carcinoma was higher than in normal tissues (Figure 3E-G, P < 0.01). P-Erk1/2 localized to the nuclei in CIN samples, cervical carcinoma samples and proliferative cells of normal tissues (Figure 4A-D). P-Erk1/2 expression was highest in CIN samples, and expression in cervical carcinoma samples was higher than in normal cervical tissues (Figure 4E-G, P < 0.01).
Figure 2.
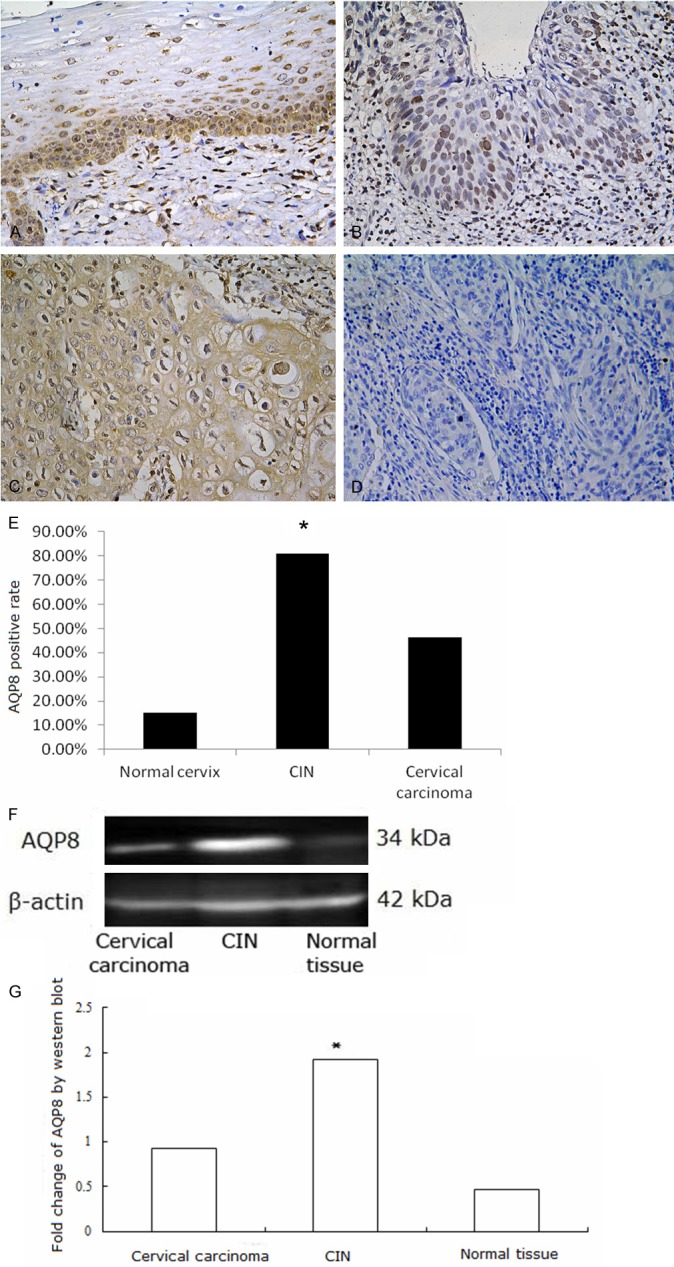
AQP8 expression in cervical tissues. A: AQP8 expressed in the membrane and cytoplasm of basal and proliferative prickle cells in normal cervical tissue; B: AQP8 expressed in the nuclei in CIN; C: AQP8 expressed in the cytoplasm and membrane in cervical cancer; D: Negative control of AQP8 expression in cervical cancer; E: Fraction of AQP8 positive cells in each group. A-E: AQP8 expression detected by Immunohistochemistry. Magnification × 200. *P < 0.05. F, G: AQP8 expression detected by Western blot was higher in CIN than in cervical carcinoma or normal tissue samples *P < 0.05.
Figure 3.
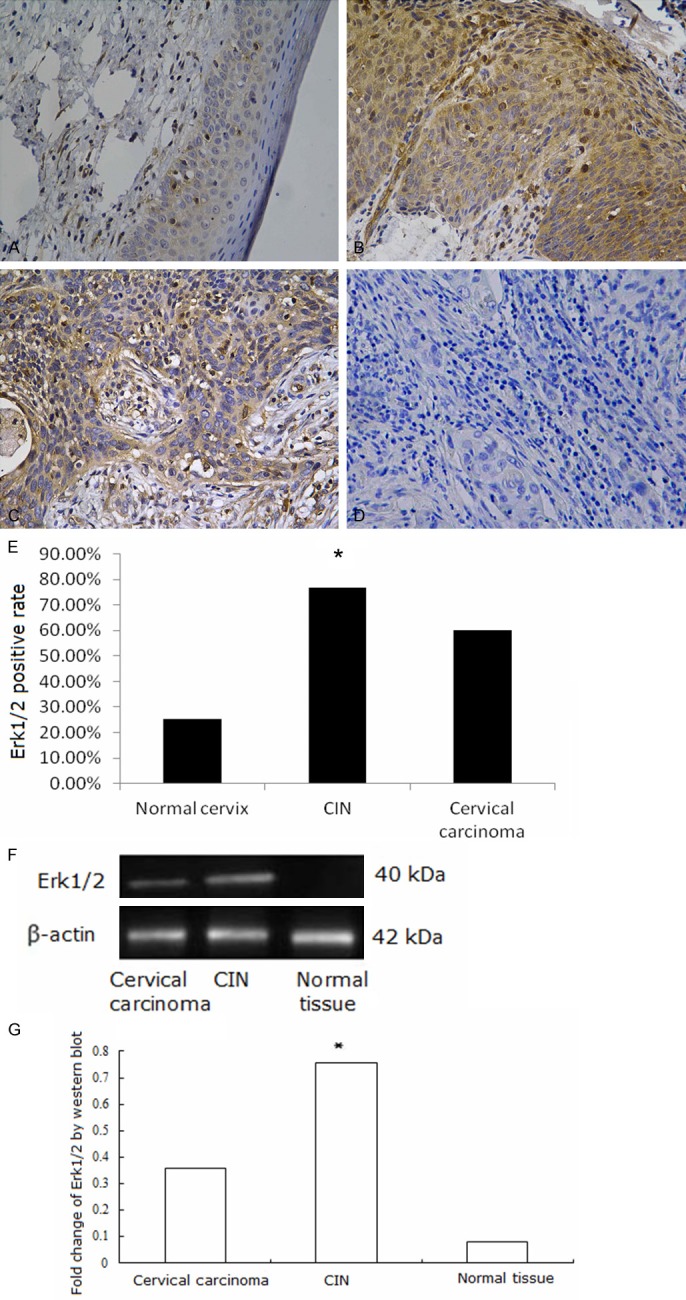
Erk1/2 expression in cervical tissues. A: Erk1/2 expressed in the cytoplasm in normal cervical tissue; B: Erk1/2 expressed in the cytoplasm in CIN; C: Erk1/2 expressed in the cytoplasm in cervical cancer; D: Negative control of Erk1/2 expression in cervical cancer; E: Fraction of Erk1/2 positive cells in each group. A-E: Erk1/2 expression detected by Immunohistochemistry. Magnification × 200. *P < 0.05. F, G: Erk1/2 expression detected by Western blot was higher in CIN than in cervical carcinoma or normal tissue samples *P < 0.05.
Figure 4.
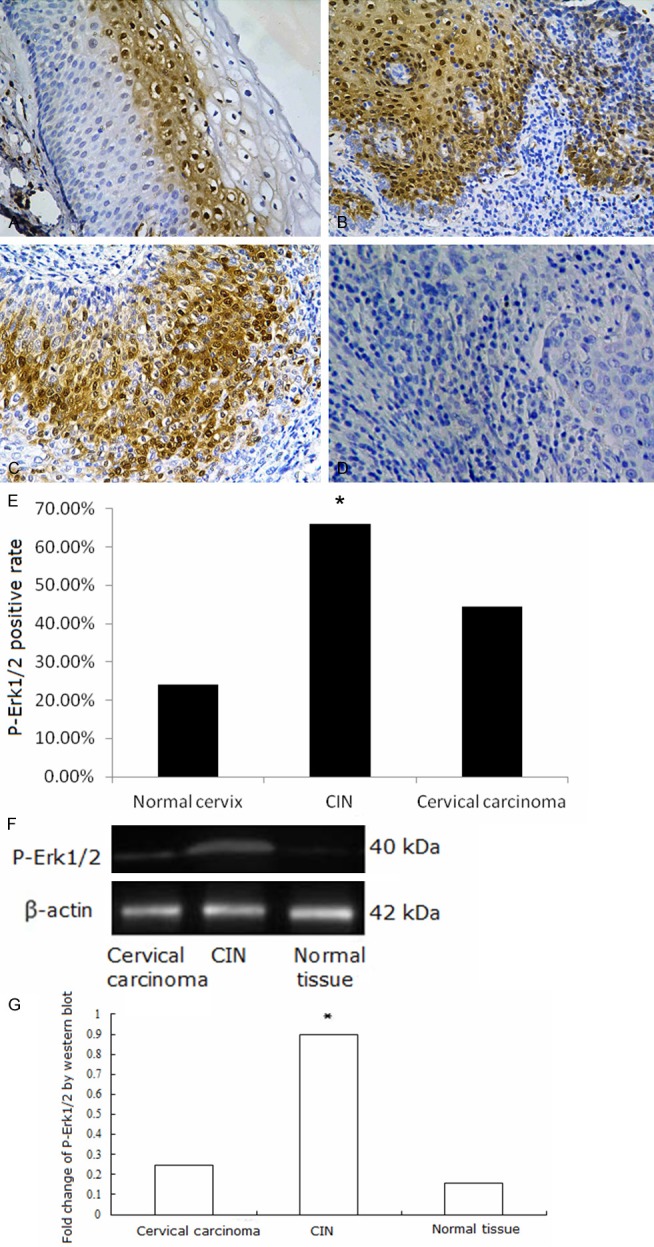
P-Erk1/2 expression in cervical tissues. A: P-Erk1/2 mainly expressed in the nuclei in normal cervical tissue; B: P-Erk1/2 expressed in the nuclei in CIN; C: P-Erk1/2 expressed in the nuclei in cervical cancer; D: Negative control of P-Erk1/2 expression in cervical cancer; E: Fraction of P-Erk1/2 positive cells in each group. A-E: P-Erk1/2 expression detected by Immunohistochemistry. Mmagnification × 200. *P < 0.05. F, G: P-Erk1/2 expression detected by Western blot was higher in CIN than in cervical carcinoma or normal tissue samples. *P < 0.05.
AQP8, Erk1/2 and P-Erk1/2 expression in cervical carcinoma is correlated with clinicopathological parameters
Correlation of AQP8, Erk1/2 and P-Erk1/2 expression in cervical carcinoma with clinicopathological variables was analyzed. AQP8 expression significantly increased with deeper tumor infiltration (P < 0.05). Erk1/2 and P-Erk1/2 expression were significantly higher in early clinical stages and metastatic lymph nodes (P < 0.05). AQP8, Erk1/2 and P-Erk1/2 expression in cervical carcinoma was not correlated with pathological grade, tumor size or patient age (P > 0.05; Table 3).
Table 3.
Correlation of AQP8, Erk1/2 and P-Erk1/2 expression in cervical carcinoma with clinicopathological parameters
| Clinicopathological parameter | n | AQP8 | Erk1/2 | P-Erk1/2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| + | x 2 value | P value | + | x 2 value | P value | + | x 2 value | P value | ||
| Stage | 0.369 | 0.544 | 8.275 | 0.004 | 5.108 | 0.024 | ||||
| I-IIa | 43 | 16 | 30 | 24 | ||||||
| IIb~VI | 65 | 28 | 27 | 22 | ||||||
| Grade | 0.050 | 0.822 | 1.555 | 0.212 | 0.042 | 0.838 | ||||
| G1-G2 | 95 | 47 | 56 | 41 | ||||||
| G3 | 13 | 6 | 10 | 6 | ||||||
| Infiltrating depth | 9.111 | 0.003 | 0.170 | 0.680 | 0.899 | 0.343 | ||||
| ≤ 1/2 | 31 | 7 | 18 | 11 | ||||||
| > 1/2 | 77 | 42 | 48 | 35 | ||||||
| Age | 3.620 | 0.057 | 0.164 | 0.685 | 0.032 | 0.858 | ||||
| ≤ 40 | 50 | 21 | 30 | 25 | ||||||
| > 40 | 58 | 35 | 37 | 30 | ||||||
| Tumor diameter | 0.036 | 0.850 | 2.218 | 0.136 | 0.606 | 0.436 | ||||
| ≤ 4 cm | 97 | 50 | 58 | 56 | ||||||
| > 4 cm | 11 | 6 | 4 | 5 | ||||||
| Metastatic lymph node | 0.238 | 0.626 | 10.699 | 0.001 | 8.682 | 0.003 | ||||
| + | 41 | 17 | 37 | 26 | ||||||
| - | 67 | 31 | 41 | 23 | ||||||
AQP8 expression in cervical carcinoma is correlated with the presence of Erk1/2
Correlation of AQP8 expression in cervical carcinoma with expression of Erk1/2 and P-Erk1/2 was analyzed. AQP8 expression was increased with higher expression of Erk1/2 in cervical carcinoma (r = 0.286, P < 0.05; Table 4).
Table 4.
Correlation analysis of AQP8 and Erk1/2, P-Erk1/2 expression in cervical carcinoma
| AQP8 | |||||
|---|---|---|---|---|---|
|
| |||||
| + | - | r value | P value | ||
| Erk1/2 | + | 40 | 29 | 0.286 | 0.003 |
| - | 11 | 28 | |||
| P-Erk1/2 | + | 21 | 21 | 0.074 | 0.445 |
| - | 28 | 38 | |||
Discussion
Tumor growth, invasion and metastasis depends on nutrition provision and metabolism, and water molecules play an important role in maintaining the microenvironment and metabolism of tumor cells. AQPs are a family of hydrophobic, small, and integrated transmembrane glucoproteins (30 kDa monomer) that are involved in multiple physiological and pathological processes in the human body, and are closely associated with many types of human tumors [11,12]. High expression of both AQP3 and AQP5 is a poor prognostic factor in esophageal squamous cell carcinoma patients [13]. The AQP8 gene, situated in chromosome 16 p12 [14], encodes a 261 amino acid protein that participates in water metabolism. The protein is mainly distributed in gastrointestinal epithelial cells (especially in the colon), the liver, pancreas, and male and female reproductive organs. AQP8 may contribute to the proliferation of astrocytomas, and may be a biomarker and candidate therapy target for patients with astrocytomas [15]. However, some experiments show controversial results about AQP8. Wang et al. [16] reported that expression of AQP8 was low in colorectal carcinoma cells. The pro-tumorigenic and/or anti-tumorigenic functions of AQPs, however, are poorly understood.
In this study, we detected AQP8 expression in cervical tissues of Uyghur women from the Xinjiang region of China at the level of transcription and translation. We analyzed the correlation between expression and clinicopathological variables in cervical carcinoma patients. We found that AQP8 exhibited significantly differential expression across samples of cervical carcinoma, CIN and normal tissues at the level of translation. AQP8 expression was highest in CIN, and increased markedly with deeper infiltration. These results agree with our previous study [17], showing that AQP8 may play a role in the transformation of precancerous CIN lesions into cervical cancer and deeper infiltration of cervical carcinoma. Similar findings were reported elsewhere. It was reported that AQP8 was over-expressed in human astrocytoma and cervical carcinoma, and its expression boosted with higher pathological grade [15,18]. Expression of other AQP subtypes was also upregulated in different tumor types. AQP5 expression in colorectal carcinoma cells was closely associated with differentiation, tumor node metastasis stage and distant lymph node metastasis [19]. Epidermal growth factor enhanced MPC-83 pancreatic cancer cell migration thr-ough the upregulation of AQP3 [20]. AQPs are small, transmembrane proteins that facilitate osmotically driven water transport. We propose that AQP8 overexpression in CIN and early cervical cancer may enhance tumor cell permeability to water, altering tumor cell volume and shape, and promoting infiltration of cervical carcinoma. Other groups [19,21] have reported that AQP8 expression is downregulated in human colorectal carcinoma and mouse hepatocellular carcinoma models, findings that are not consistent with ours. It is necessary to study whether AQP8 differential expression in tumor tissues is associated with specific tumor tissues. We also found that expression of AQP8 was not significantly different among the three groups studied at transcription level. AQP8 protein expression could be regulated by interactions between AQP8 and other proteins. Zhang et al. [22] reported that AQP5 protein overexpression resulted in enhanced activation of the epidermal growth factor receptor (EGFR), Erk1/2 pathway in cancer cells.
The Erk1/2 signaling pathway plays a critical role in regulating cell proliferation, differentiation, survival, and apoptosis of various cancer types such as bone, prostate, breast, liver, and lung [23,24]. It has been estimated that Erk1/2 targets more than 180 different molecules that are responsible for cell growth, survival, and differentiation; thus, aberrant regulation greatly affects cell growth. Erk1/2 kinase couples the signals from cell surface receptors to molecules that transmit cell proliferative signals. Following a cascade reaction, Erk1 phosphorylates Erk1/2, leading to the upregulation of various transcription factors such as Ets-1, c-Jun, c-Myc, and HIF1α [25]. Our research demonstrated that Erk1/2 and P-Erk1/2 expression is significantly higher in CIN than cervical carcinoma, and lowest in normal tissues at both transcription and translation levels. Additionally, expression is associated with earlier clinical stages and metastatic lymph nodes. We also found that Erk1/2 was mainly localized to the cytoplasm, while P-Erk1/2 was mainly localized to the nuclei. AQP8 protein expression was positively correlated with Erk1/2 expression. Our results suggested that synergy between AQP8 and the Erk1/2 signaling pathway may be involved in the transformation of precancerous lesions into cervical cancer, and favor infiltration and lymph node metastasis of cervical cancer. Other studies support these findings. Kang et al. [26] reported that induction of AQP5 expression during colorectal carcinogenesis occurred through the interaction of AQP5 with the Ras/Erk1/2/retinoblastoma protein signaling pathway.
In summary, AQP8, Erk1/2 and P-Erk1/2 expression increased in CIN and cervical carcinoma samples from Uyghur women in China. The expression of these proteins facilitated infiltration and lymph node metastasis of cervical carcinoma, suggesting that they could be candidate molecular markers for early diagnosis of cervical carcinoma. Future studies will probe the interactions and functions of AQP8 and the Erk1/2 signaling pathway by investigating AQP8 gene expression.
Acknowledgements
The authors thank Cuicui Wang for helpful advice in the process of RT-PCR. This study was supported by the Natural Science Foundation of Xinjiang Uyghur Autonomous Region (Grant Number: 2012211A041) and the Key Laboratory Open Issue of Xinjiang Uyghur Autonomous Region (Grant Number: XJDX02082011-05).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Farivar TN, Johari P, Shafei S, Najafipour R. Lack of association between herpes simplex virus type 2 infection and cervical cancer--Taq Man realtime PCR assay findings. Asian Pac J Cancer Prev. 2012;13:339–42. doi: 10.7314/apjcp.2012.13.1.339. [DOI] [PubMed] [Google Scholar]
- 3.Peng YH, La-lai SZK, Zhou K, Wang ZH, Fang XZ, Wang L. Clinical analysis for 4505 cases of cervical cancer. Zhonghua Fu Chan Ke Zazhi. 2003;38:764–65. [Google Scholar]
- 4.Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–27. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008 Jul;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou LB, Shi S, Zhang RJ, Wang TT, Tan YJ, Zhang D, Fei XY, Ding GL, Gao Q, Chen C, Hu XL, Huang HF, Sheng JZ. Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis of vascular endothelial cells. J Clin Endocrinol Metab. 2013;98:E672–82. doi: 10.1210/jc.2012-4081. [DOI] [PubMed] [Google Scholar]
- 7.Nico B, Ribatti D. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp Cell Res. 2011;317:2391–96. doi: 10.1016/j.yexcr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH, McKillop IH. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasioudi KE, Saetta AA, Sakellariou S, Levidou G, Michalopoulos NV, Theodorou D, Patsouris E, Korkolopoulou P. pERK activation in esophageal carcinomas: clinicopathological associations. Pathol Res Pract. 2012;208:398–404. doi: 10.1016/j.prp.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 10.McCubrey JA, Steelman LS, Chappel WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistence. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung HJ, Park JY, Jeon HS, Kwon TH. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS One. 2011;6:e28492. doi: 10.1371/journal.pone.0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishimoto S, Wada K, Usami Y, Tanaka N, Aikawa T, Okura M, Nakajima A, Kogo M, Kamisaki Y. Differential expression of aquaporin 5 and aquaporin 3 in squamous cell carcinoma and adenoid cystic carcinoma. Int J Oncol. 2012;41:67–75. doi: 10.3892/ijo.2012.1445. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Zhang S, Jiang H, Yang Y, Jiang Y. Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Med Oncol. 2013;30:636. doi: 10.1007/s12032-013-0636-2. [DOI] [PubMed] [Google Scholar]
- 14.Viggiano L, Rocchi M, Svelto M, Calamita G. Assignment of the aquaporin-8 water channel gene (AQP8) to human chromosome 16p12. Cytogenet Cell Genet. 1999;84:208–10. doi: 10.1159/000015260. [DOI] [PubMed] [Google Scholar]
- 15.Zhu SJ, Wang KJ, Gan SW, Xu J, Xu SY, Sun SQ. Expression of aquaporin8 in human astrocytomas: correlation with pathologic grade. Biochem Biophys Res Commun. 2013;440:168–72. doi: 10.1016/j.bbrc.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li Q, Yang T, Bai G, Li D, Li Q, Sun H. Expression of AQP5 and AQP8 in human colorectal carcinoma and their clinical significance. World J Surg Oncol. 2012;10:242. doi: 10.1186/1477-7819-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi YH, Chen R, Talafu T, Nijiati R, Lalai S. Significance and Expression of Aquaporin 1, 3, 8 in Cervical Carcinoma for Xinjiang Uygur Women of China. Asian Pac J Cancer Prev. 2012;13:1971–75. doi: 10.7314/apjcp.2012.13.5.1971. [DOI] [PubMed] [Google Scholar]
- 18.Yao JF, Zhou CY, Wei LF, Wang SY, Shi YF. Expression of aquaporin-8 and bcl-2 protein in human cervical carcinoma and their correlations. Zhonghua Fu Chan Ke Za Zhi. 2008;43:205–8. [PubMed] [Google Scholar]
- 19.Wang W, Li Q, Yang T, Bai G, Li D, Li Q, Sun H. Expression of AQP5 and AQP8 in human colorectal carcinoma and their clinical significance. World J Surg Oncol. 2012;10:242. doi: 10.1186/1477-7819-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Wang K, Gong K, Li X, Luo K. Epidermal growth factor enhances MPC-83 pancreatic cancer cell migration through the upregulation of aquaporin 3. Mol Med Rep. 2012;6:607–10. doi: 10.3892/mmr.2012.966. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH, McKillop IH. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen Z, Song Y, Zhang P, Hu J, Bai C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol. 2010;221:210–20. doi: 10.1002/path.2702. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Qiu H, Ke S, Hu S, Yu S, Zou S. The fibroblast growth factor receptor 2-mediated extracellular signal-regulated kinase 1/2 signaling pathway plays is important in regulating excision repair cross-complementary gene 1 expression in hepatocellular carcinoma. Biomed Rep. 2013;1:604–08. doi: 10.3892/br.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma B, Wells A. The Mitogen-activated Protein (MAP) Kinases p38 and Extracellular Signal-regulated Kinase (ERK) Are Involved in Hepatocyte-mediated Phenotypic Switching in Prostate Cancer Cells. J Biol Chem. 2014;89:11153–61. doi: 10.1074/jbc.M113.540237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chetram MA, Hinton CV. PTEN regulation of Erk1/2 signaling in cancer. J Recept Signal Transduct Res. 2012;32:190–5. doi: 10.3109/10799893.2012.695798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang SK, Chae YK, Woo J, Kim MS, Park JC, Lee J, Soria JC, Jang SJ, Sidransky D, Moon C. Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173:518–25. doi: 10.2353/ajpath.2008.071198. [DOI] [PMC free article] [PubMed] [Google Scholar]