Abstract
Glucose transporter-1 (GLUT-1) and PI3K/Akt are known to be closely involved in resistance to chemotherapy. Co-targeted therapy reducing GLUT-1 expression and PI3K/Akt pathway activity may overcome the chemoresistance of human cancers. Apigenin may inhibit the expression of GLUT-1 and the PI3K/Akt pathway. We hypothesized that over-expression of GLUT-1 and p-Akt was associated with the resistance to cisplatin of laryngeal carcinoma Hep-2 cells. We explored whether apigenin inhibited GLUT-1 and p-Akt, resulting in sensitization of laryngeal carcinoma Hep-2 cells to cisplatin. Real-time RT-PCR and Western blotting confirmed the presence of GLUT-1 mRNA, and GLUT-1 and p-Akt proteins in Hep-2 cells. We found that resistance or insensitivity of Hep-2 cells to cisplatin might be associated with such expression. Apigenin markedly enhanced the cisplatin-induced suppression of Hep-2 cell growth. This effect was concentration- and time-dependent. Thus apigenin may significantly reduce the levels of GLUT-1 mRNA, and GLUT-1 and p-Akt proteins, in cisplatin-treated Hep-2 cells, in a concentration- and time-dependent manner. To conclude, overexpression of GLUT-1 mRNA may be associated with the resistance to cisplatin of laryngeal carcinoma Hep-2 cells. Apigenin may enhance the sensitivity to cisplatin of laryngeal carcinoma cells via inhibition of GLUT-1 and p-Akt expression.
Keywords: Glucose transporter-1, pi3k/akt, laryngeal carcinoma, apigenin, cisplatin, chemosensitivity
Introduction
Laryngeal carcinoma is one of the most common head and neck cancers. Current therapeutic strategies for the early stages of laryngeal carcinoma include various types of larynx-conserving surgery, or radiotherapy. For advanced laryngeal carcinoma, combined therapies are often used, including concurrent chemo-radiotherapy or total laryngectomy, with possible adjuvant therapy [1]. However, the survival rate has not improved over the last few decades [2] because of resistance to chemo-radiotherapy and late metastasis [3,4]. Thus, overcoming the resistance to chemo-radiotherapy of laryngeal carcinoma is a challenge in cancer therapy.
Laryngeal carcinoma, like other malignant tumors, is hypoxic in nature and exhibits increased glucose uptake and metabolism [5-7]. Glucose transporter-1 (GLUT-1) has been identified as a hypoxic marker [8] and plays a significant role in malignant glucose metabolism; GLUT-1 may contribute to increased FDG uptake [9]. Our previous studies revealed that GLUT-1 might play a role in the mechanism of radioresistance of laryngeal carcinoma, and we explored methods of improving the radiosensitivity of laryngeal carcinoma [4,10]. Recently, the relationship between GLUT-1 expression and resistance to chemotherapy of some human cancer cell lines has gradually received more attention [11-14]. However, no report has yet explored the possible correlation between GLUT-1 expression and resistance to chemotherapy of laryngeal carcinoma.
Abnormal expression of GLUT-1 is associated with the actions of multiple signal transduction pathways, including the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, which plays an important role in the regulation of GLUT-1 expression. Several studies have confirmed that the PI3K/Akt pathway and GLUT-1 affect glucose metabolism [15,16]. Akt, also called PKB or Rac, is an important downstream serine-threonine regulatory kinase. A variety of molecules can activate Akt; these include insulin, heat shock proteins, and TNFα. Activated Akt plays a central role in PI3K/Akt signal transduction pathways mediating cell growth, survival, and differentiation [17]. Some studies have revealed that PI3K/Akt pathway activity is closely associated with resistance to chemotherapy and, when this pathway is inhibited, the sensitivity to chemotherapy is enhanced [18-20]. Thus, co-targeted therapy that reduces GLUT-1 expression and PI3K/Akt pathway activity may overcome the chemoresistance of human cancers [21,22].
Apigenin is a natural phyto-estrogen flavonoid present in a wide range of fruits, vegetables (especially celery), beans, and tea. In vitro and in vivo studies have demonstrated that apigenin has potential biological effects, including anti-oxidative, anti-inflammatory, and anti-cancer activities [22]. Of these, the anti-tumor effect is the most prominent [22]. Apigenin may inhibit the expression of some biomarkers to enhance the sensitivity to chemotherapy via downregulation of the PI3K/Akt pathway [23-26]. However, only one study has investigated whether apigenin inhibits the expression of GLUT-1 and the PI3K/Akt pathway [23]. Therefore, we further investigated whether apigenin might concurrently inhibit the expression of GLUT-1 and downregulate the PI3K/Akt pathway in human cancers.
In this study, we hypothesized that over-expression of GLUT-1 and p-Akt was associated with resistance to cisplatin of laryngeal carcinoma Hep-2 cells. Next, we explored whether the effect of apigenin on GLUT-1 and p-Akt sensitized laryngeal carcinoma Hep-2 cells to cisplatin.
Materials and methods
Approval
The institutional review board of the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China), approved the present study.
Cells, antibodies, and plasmids
The laryngeal carcinoma Hep-2 cell line was purchased from the Cell Research Institute of the Chinese Academy of Sciences (Shanghai, China). Chloroform, isopropyl alcohol, and anhydrous alcohol were purchased from Hangzhou Changzhen Chemical Plant (Hangzhou, China). Agarose was purchased from Biowest (Spain). TRIzol was purchased from Invitrogen (Carlsbad, CA). Reverse transcriptase MMLV and the TAQ enzyme were purchased from Promega (USA). DNA Marker DL2000, the pcDNA3.1 vector, restriction endonucleases HindIII and XbaI, and T4 DNA ligase were purchased from TaKaRa Co. (Japan). Cisplatin, dimethyl sulfoxide (DMSO), Tween 20, and Ponceau S were purchased from Sigma (St. Louis, MO). Apigenin was purchased from Selleckchem (USA). Primary antibodies against GLUT-1 and p-Akt were purchased from Santa Cruz Biotechnology (CA). The secondary antibodies donkey anti-rabbit and donkey anti-mouse, cell lysis kits, Supersignal West Femto kits, and PMSF, were purchased from Pierce (USA). Primers were synthesized by Invitrogen. The sequence of the entire coding region of GLUT-1 was obtained from GenBank, and primers were designed using the ClustalX and Omega 2.0 Applied Software.
Cell culture
Hep-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT), 2 mM L-glutamine, 100 U/ml penicillin, and 100 g/ml streptomycin, at 37°C in a 5% CO2 atmosphere. Cells were trypsinized and harvested after reaching 80-90% confluence.
Preparation of apigenin and cisplatin
Apigenin (13.5 mg) was dissolved in 1,000-μL DMSO and used at 50 mM in all experiments. Cisplatin (5 mg) was dissolved in 1,000-μL DMSO and used at 5 μg/μl in all experiments.
Proliferation assays of Hep-2 cells using the cell counting Kit-8 (CCK-8) system [27]
Cultured Hep-2 cells were trypsinized using 0.25% trypsin. Cell proliferation was measured using the CCK-8 system (Beyotime, Nanjing, China) according to the manufacturer’s instructions. The 13 experimental groups were as follows: Hep-2 cells, DMSO, Hep-2 cells+DMSO, 10 μM apigenin+Hep-2 cells, 40 μM apigenin+Hep-2 cells, 160 μM apigenin+Hep-2 cells, 2 μg/ml cisplatin+Hep-2 cells, 3 μg/ml cisplatin+Hep-2 cells, 4 μg/ml cisplatin+Hep-2 cells, 5 μg/ml cisplatin+Hep-2 cells, 10 μM apigenin+5 μg/ml cisplatin+Hep-2 cells, 40 μM apigenin+5 μg/ml cisplatin+Hep-2 cells, and 160 μM apigenin+5 μg/ml cisplatin+Hep-2 cells. Cultures proceeded for 24, 48, and 72 h. Briefly, 5 × 103 cells from each group were seeded into wells of 96-well culture plates. Cells were cultured in SFM at 37°C. After 1-6 days, 10 μl of CCK-8 reagent were added to each well, and, after 2 h of incubation at 37°C, the absorbance at 450 nm was measured. OD = ODcell−ODblank. The cell survival rate was calculated as OD/ODcontrol × 100%. All assays were carried out in triplicate.
Detection of GLUT-1 expression in Hep-2 cells by real-time reverse transcription-polymerase chain reaction (RT-PCR)
Cells of each group described above were homogenized in TRIzol reagent (Invitrogen). Total RNA was extracted according to the manufacturer’s protocol. Total RNA concentration was measured by ultraviolet spectrophotometry; and an optical density (OD) 260/280 ratio between 1.8 and 2.0 was deemed to show that the RNA was acceptably pure. Reverse transcription was performed according to the manufacturer’s protocol. Briefly, l μg of total RNA and Moloney murine leukemia virus (MMLV) reverse transcriptase (Fermentas, Canada) were contained in a 20-μl reaction volume with 0.5 μg/μl of oligo d(T) primer solution, 1 μl of random primer solution (0.2 μg/μl), and 10 μl of DEPC·H2O. The reaction mix was first pre-denatured at 65°C for 10 min. After addition of 200 U of MMLV reverse transcriptase, samples were incubated at 42°C for 1 h and annealed at 70°C for 10 min. The above-synthesized cDNAs were used as templates for real-time fluorescence quantitative PCR using the fluorescent dye SYBR Green and the Eppendorf Realplex4 real-time PCR system (Eppendorf Realplex4; Hamburg, Germany). The 20-μl reaction mix consisted of 10 μl of 2 × SYBR Green buffer, 1 μl of template, 1 μl of upstream- and downstream-specific primers, and 8 μl of deionized water. The reaction mix was pre-denatured at 95°C for 2 min, followed by 40 cycles at 95°C for 15 s, 59°C for 20 s, and 72°C for 20 s. Each sample was run in triplicate. The primers used were as follows: GLUT-1 Forward (F): 5’-CCGCAACGAGGAGAACCG-3’; GLUT-1 Reverse (R): 5’-GTGACCTTCTTCTCCCGCATC-3’. GAPDH (control) Forward (F): 5’-TCCTTCCTGGGCATGGAGT-3’; and GAPDH Reverse (R): 5’-CAGGAGGAGCAATGATCTTGAT-3’. The lengths of the PCR products were 123 bp (GLUT-1) and 208 bp (GAPDH).
To distinguish specific and non-specific products and primer dimers, dissociation curve analysis was conducted immediately after amplification by continuous monitoring of the SYBR Green I fluorescence signal at temperatures between 60°C and 95°C. For calculation of differential gene expression levels, the 2-ΔΔCt formula was used.
Analysis of the expression level of GLUT-1 protein by Western blotting
Western blotting was performed as described previously [27]. The levels of the GLUT-1, p-Akt, and GAPDH (control) proteins in each group of Hep-2 cells were assayed using a BAC protein quantitative kit (Wuhan Boster Biological Technology Co. Ltd., Wuhan, China). Briefly, 80-μg amounts of protein were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Millipore, Billerica, MA). Skim milk solution (2%) was used as a blocking solution (room temperature, 1 h). The membrane was incubated with primary antibodies (anti-GLUT-1, 1:250; -p-Akt, 1:250; -GAPDH, 1:3,000) at room temperature for 3 h, and with secondary antibodies (1:1,000, donkey anti-rabbit; 1:3,000, donkey anti-mouse) at room temperature for 1 h. The proteins were detected using an enhanced chemiluminescence system (Santa Cruz Biotechnology, Santa Cruz, CA) by exposure to X-ray film. Protein levels were analyzed semi-quantitatively using the Kodak Gel Logic Analysis System.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL). A P-value less than 0.05 was deemed to indicate statistical significance.
Results
Apigenin enhances the sensitivity of Hep-2 cells to cisplatin
The CCK results showed that the survival rates of Hep-2 cells were significantly reduced with increasing concentrations of apigenin at all timepoints. The survival rates of Hep-2 cells decreased gradually with increasing duration of culture in the presence of 160 μM apigenin (P < 0.01, Figure 1A).
Figure 1.
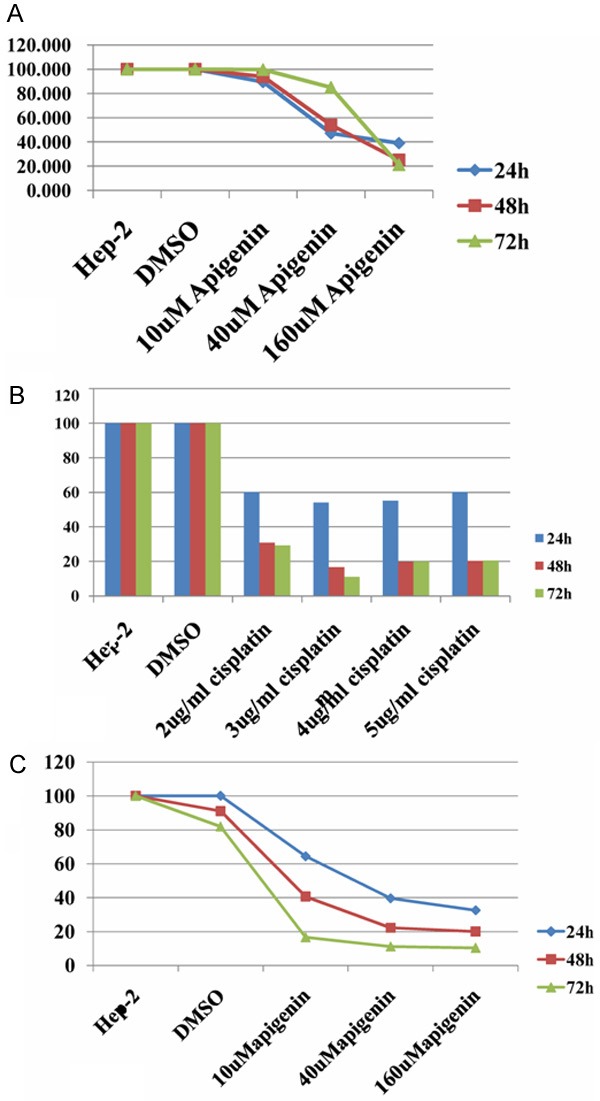
The survival rates of Hep-2 cells by CCK. A: The survival rates of Hep-2 cells were significantly reduced with increasing concentrations of apigenin at all timepoints (P < 0.01). The survival rates of Hep-2 cells decreased gradually with increasing duration of culture in the presence of 160 μM apigenin (P < 0.01). B: The survival rates of Hep-2 cells were reduced significantly in the presence of various concentrations of cisplatin, compared to the control groups (P < 0.01). At 48 or 72 h of culture, the survival rates of Hep-2 cells were lower in the presence of 3, 4, and 5 μg/ml cisplatin than 2 μg/ml cisplatin (P < 0.01); the survival rates of Hep-2 cells were higher in the presence of 4 and 5 μg/ml cisplatin than 3 μg/ml cisplatin (P < 0.05). C: Apigenin markedly enhanced the effect of cisplatin on Hep-2 cells. This effect was apigenin concentration- and time-dependent (P < 0.01).
The survival rates of Hep-2 cells were reduced significantly in the presence of various concentrations of cisplatin, compared to the control groups (P < 0.01, Figure 1B). At 2 and 3 μg/ml cisplatin, the survival rates of Hep-2 cells were significantly reduced with increasing culture duration; however, at 4 and 5 μg/ml cisplatin, the survival rates of Hep-2 cells were not further reduced from 48 to 72 h (P > 0.05). At 24 h of exposure, the survival rates of Hep-2 cells were not significantly different when different concentrations of cisplatin were used (P > 0.05). At 48 or 72 h of culture, the survival rates of Hep-2 cells were lower in the presence of 3, 4, and 5 μg/ml cisplatin than 2 μg/ml cisplatin (P < 0.01, Figure 1B); however, the survival rates of Hep-2 cells were higher in the presence of 4 and 5 μg/ml cisplatin than 3 μg/ml cisplatin (P < 0.05, Figure 1B).
Apigenin markedly enhanced the effect of cisplatin on Hep-2 cells. This effect was apigenin concentration- and time-dependent (P < 0.01, Figure 1C).
Expression of GLUT-1 mRNA, and GLUT-1 and p-Akt Proteins, in Laryngeal Carcinoma Hep-2 cells
The GLUT-1 mRNA and GAPDH mRNA real-time RT-PCR products were of 123 and 208 bp, respectively. Dissociation curve analysis performed at 60-95°C showed only the expected peaks at 87.1°C and 85.1°C for GLUT-1 and GAPDH mRNAs, respectively. Real-time RT-PCR showed that the specific amplified curve for GLUT-1 mRNA and GAPDH. Western blotting confirmed that both GLUT-1 (Figure 2A) and p-Akt (Figure 2B) were expressed in Hep-2 cells.
Figure 2.
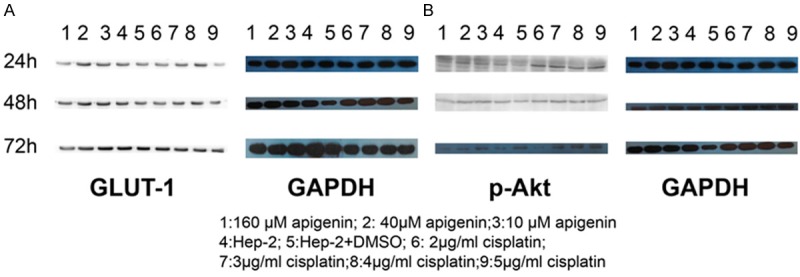
Western blotting confirmed that both GLUT-1 (A) and p-Akt (B) were expressed in Hep-2 cells in different apigenin and cisplatin concentration.
Effects of apigenin and cisplatin on GLUT-1 mRNA and protein levels in Hep-2 cells
At 24 h, 10 and 40 μM apigenin did not decrease the expression level of GLUT-1 in Hep-2 cells compared to controls (P > 0.05); in contrast, 160 μM apigenin did decrease the expression level of GLUT-1 in Hep-2 cells compared to controls (P = 0.005). However, the extent of reduction did not differ significantly among 10, 40, and 160 μM apigenin (P > 0.05). At 48 and 72 h, apigenin downregulated the expression of GLUT-1mRNA in a concentration-dependent manner (P < 0.01, Figure 3A).
Figure 3.
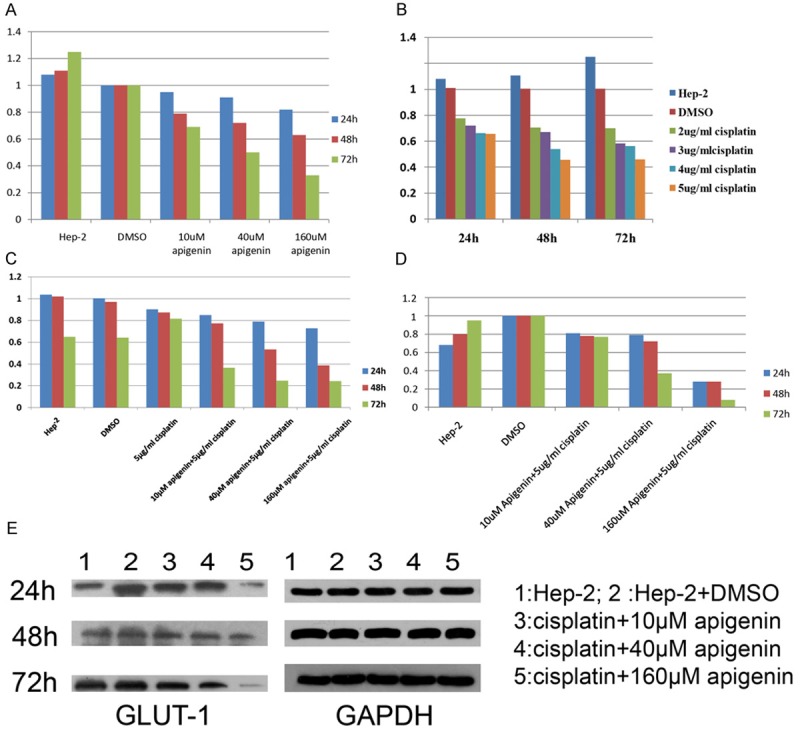
The change of GLUT-1 mRNA and protein expression. A: At 48 and 72 h, apigenin downregulated the expression of GLUT-1mRNA in a concentration-dependent manner (P < 0.01). At the same apigenin concentration, the GLUT-1mRNA level was reduced significantly upon prolongation of culture duration (P < 0.01). B: At 72 h, there was no significant difference in GLUT-1 mRNA level between cultures with 4 and 5 μg/ml cisplatin. The GLUT-1 mRNA level at 72 h of culture was higher than at 48 h of culture in the presence of 4 and 5 μg/ml cisplatin. C: Apigenin significantly reduced the GLUT-1 mRNA level in cisplatin-treated Hep-2 cells in a concentration- and time-dependent manner (P < 0.01). C: Western blotting revealed that apigenin or cisplatin alone did not significantly reduce the GLUT-1 protein levels (P > 0.05). D: Significant decreases in GLUT-1 protein levels were noted after 48 h of exposure to 40 and 160 μM apigenin of cisplatin-treated Hep-2 cells (P <0.01 ). E: The results of Western blotting.
At the same apigenin concentration, the GLUT-1 mRNA level was reduced significantly upon prolongation of culture duration (P < 0.01, Figure 3A).
Real-time RT-PCR showed that cisplatin did not always reduce the level of GLUT-1 mRNA at the same culture duration with increasing cisplatin concentration; similar findings were obtained upon prolongation of culture time using an identical apigenin concentration. At 24 h, there was no significant difference in the GLUT-1 mRNA level in the presence of various concentrations of cisplatin. At 48 h, there were no significant differences in GLUT-1 mRNA levels among cultures with 2, 3, 4, and 5 μg/ml cisplatin. At 72 h, there was no significant difference in GLUT-1 mRNA level between cultures with 4 and 5 μg/ml cisplatin (Figure 3B). At 2 and 3 μg/ml cisplatin, there was no significant difference in the GLUT-1 mRNA level upon prolongation of culture duration (24, 48, and 72 h). At 4 and 5 μg/ml cisplatin, there was no significant difference in the GLUT-1 mRNA level between 48- and 72-h cultures. Although no significant difference was observed, the GLUT-1 mRNA level at 72 h of culture was higher than at 48 h of culture in the presence of 4 and 5 μg/ml cisplatin (Figure 3B).
Apigenin significantly reduced the GLUT-1 mRNA level in cisplatin-treated Hep-2 cells in a concentration- and time-dependent manner (P < 0.01, Figure 3C).
Western blotting revealed that apigenin or cisplatin alone did not significantly reduce the GLUT-1 protein levels (P > 0.05, Figures 2A, 3D). Significant decreases in GLUT-1 protein levels were noted after 48 h of exposure to 40 and 160 μM apigenin of cisplatin-treated Hep-2 cells (P < 0.01, Figure 3D, 3E).
Effects of apigenin and cisplatin on p-Akt protein levels in Hep-2 cells
Western blotting revealed that apigenin or cisplatin alone did not significantly reduce the p-Akt protein level (P > 0.05, Figures 2B, 4A).
Figure 4.
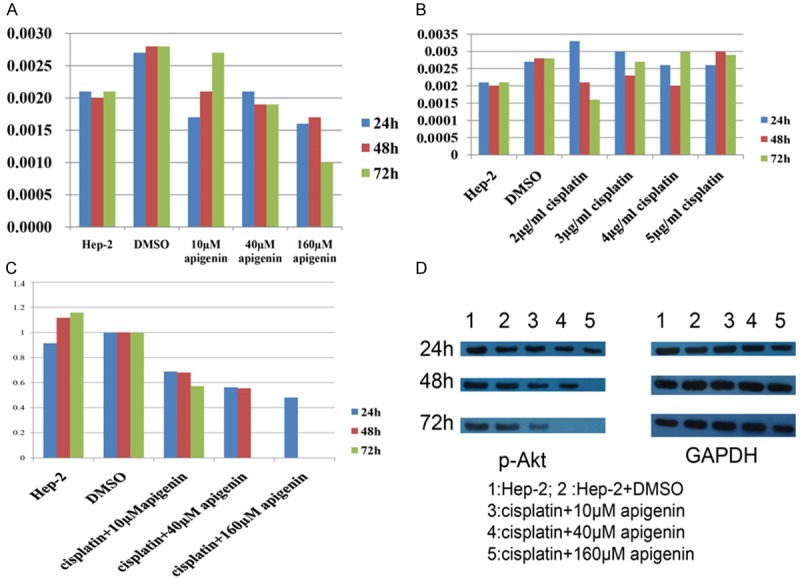
The Effects of Apigenin and Cisplatin on p-Akt Protein Levels in Hep-2 Cells. A, B: Western blotting revealed that apigenin or cisplatin alone did not significantly reduce the p-Akt protein level (P > 0.05). C: Apigenin significantly reduced the p-Akt protein level in cisplatin-treated Hep-2 cells at 48 h of exposure, in an apigenin-concentration-dependent manner (P < 0.01). p-Akt protein was barely detected after 72 h of culture in the presence of 40 and 160 μM apigenin. D: The results of Western blotting.
Apigenin significantly reduced the p-Akt protein level in cisplatin-treated Hep-2 cells at 48 h of exposure, in an apigenin-concentration-dependent manner (P < 0.01, Figure 4B, 4C). p-Akt protein was barely detected after 72 h of culture in the presence of 40 and 160 μM apigenin (Figure 4B, 4C).
Discussion
GLUT-1 over-expression is a possible mechanism of resistance to chemo-radiotherapy of some human cancers [4,11-14]. This may be because elevated GLUT-1 expression provides energy to malignant tumors, allowing development of chemo-radioresistance [4]. GLUT-1 causes chemoresistance by increasing cell turnover [28]. GLUT-1 up-regulates the expression levels of the multidrug resistance-1 (MDR-1) [29] and P-glycoprotein (P-gp) genes [30]. To our knowledge, no work on laryngeal carcinoma has addressed a possible correlation between GLUT-1 expression level and resistance to chemotherapy; factors regulating GLUT-1 expression; or the possibility of inhibiting GLUT-1 expression to enhance sensitivity to chemotherapy.
Cisplatin is one of the most common therapeutic agents used to treat head-and-neck cancers [12-14]. However, development of resistance limits the widespread clinical use of cisplatin [12-14]. In this study, the survival rates of Hep-2 cells treated with cisplatin were not further reduced when cisplatin concentrations increased or culture duration was prolonged. In the presence of 4 or 5 μg/ml cisplatin, the survival rate of Hep-2 cells was paradoxically higher than in 3 μg/ml cisplatin. These results suggest that laryngeal carcinoma Hep-2 cells may become resistant or insensitive to cisplatin treatment. Further, we explored the mechanism underlying this effect. We found that GLUT-1 mRNA and protein were present in laryngeal carcinoma Hep-2 cells. When cisplatin was administrated to Hep-2 cells, the GLUT-1 mRNA levels were not reduced at any timepoint as cisplatin concentrations increased, and we made similar findings upon prolongation of culture time at the same concentrations of apigenin. The effect of cisplatin on the GLUT-1 protein level was similar to that on the GLUT-1 mRNA level. Western blotting revealed that cisplatin alone did not significantly reduce the GLUT-1 protein level. We suggest that the resistance or insensitivity to cisplatin of Hep-2 cells may be associated with enhanced GLUT-1 mRNA and protein levels. We reported similar findings in our previous study of the radioresistance of Hep-2 cells [4]. Therefore, we speculate that GLUT-1 expression plays an important role in the chemo-radioresistance of laryngeal carcinoma Hep-2 cells. Shimanishi et al. also found that proliferation of some oral squamous cell carcinoma cell lines treated with cisplatin was higher under hypoxia than under normoxia; enhanced proliferation was associated with up-regulation of GLUT-1 mRNA and protein levels [14].
Based on the relationship between its over-expression and enhanced chemoresistance to cisplatin, many targeted therapeutic methods aim to inhibit expression of GLUT-1 to enhance sensitivity to chemotherapeutic agents, including cisplatin. Such targeted drugs include small-molecule inhibitors (WZB117, STF-31, CUR+ DOX-loaded micelles, and 2-amino-2-deoxy-D-glucose) [11,13,31,32], and specific molecules targeting GLUT-1 (RNA interference) [12,14].
Several studies have demonstrated that apigenin enhances the sensitivity of carcinomas to cisplatin [33-35]. Apigenin, a natural plant flavone, may have chemopreventative and therapeutic effects as an anti-inflammatory, antioxidant, and anti-cancer agent [22]. The anticancer mechanism may involve induction of apoptosis, inhibition of angiogenesis, reversal of multidrug resistance, and exertion of an anti-proliferative effect. These increase sensitivity to cisplatin by decreasing the levels of various tumor growth factors and hypoxic markers, and by inhibiting signaling pathways, including those triggered by vascular endothelial growth factor (VEGF), tumor necrosis factor receptor (TNF-R), and serine/threonine kinase glycogen synthase kinase-3b (GSK-3b) [22,33-36]. Apigenin may also inhibit expression of GLUT-1 via a mechanism involving PIK/Akt [23]. However, no prior report has shown that apigenin inhibits GLUT-1 expression to chemosensitize laryngeal carcinoma cells to cisplatin. In the present study, we found that apigenin suppressed the survival of Hep-2 cells as the apigenin concentration increased, and cell survival decreased gradually upon prolongation of culture time in the presence of a high concentration (160 μM) of apigenin. We also found that apigenin markedly enhanced the ability of cisplatin to suppress Hep-2 cell growth, in a concentration- and time-dependent manner. Further, we found that the apigenin-mediated potentiation of the effect of cisplatin on laryngeal carcinoma Hep-2 cells may be mediated by inhibition of GLUT-1 expression. Although apigenin did not markedly decrease the expression level of GLUT-1 in Hep-2 cells, apigenin alone downregulated the GLUT-1 mRNA level in a concentration-dependent manner, at 48 and 72 h. Apigenin at all tested concentrations also reduced the GLUT-1 mRNA levels upon prolongation of culture duration. Apigenin combined with cisplatin may significantly reduce the GLUT-1 mRNA level. Therefore, we suggest that apigenin induces sensitization of Hep-2 cells to cisplatin by decreasing the expression level of GLUT-1. However, apigenin alone, or in combination with cisplatin, did not significantly reduce the GLUT-1 protein level. This phenomenon was also noted when antisense oligodeoxynucleotides were used to inhibit GLUT-1 expression, which enhances the radiosensitivity of laryngeal carcinoma [4]. Possible explanations are that GLUT-1 mRNA was degraded when the GLUT-1 protein level peaked, or that the GLUT-1 protein level was increasing when the GLUT-1 mRNA level peaked. Also, transcription and translation may be separated temporally and/or spatially [4]. Our results differed from those of Melstrom et al. [23,34]. These authors found that apigenin concurrently inhibited both GLUT-1 mRNA and protein levels in human pancreatic cancer cells. Thus, the mechanism of action of apigenin should be further investigated.
Melstrom et al. found that apigenin-mediated GLUT-1 down-expression was associated with inhibition of the PI3K/Akt pathway in human pancreatic cancer cells [23]. In the present study, we sought to investigate the p-Akt expression level in laryngeal carcinoma Hep-2 cells and the effect of apigenin on such expression. We found that p-Akt and GLUT-1 proteins were expressed in laryngeal carcinoma Hep-2 cells. Apigenin or cisplatin alone did not significantly reduce the p-Akt protein level. However, apigenin combined with cisplatin markedly decreased the p-Akt expression level in Hep-2 cells, similar to the effect of apigenin on GLUT-1 protein. This finding allows us to speculate that a correlation exists between p-Akt and GLUT-1 expression in laryngeal carcinoma Hep-2 cells. Further work should explore whether apigenin reduces the GLUT-1 expression level to enhance sensitivity to cisplatin via inhibition of the PI3K/Akt pathway in laryngeal carcinoma cells. We plan to investigate the effect of apigenin on GLUT-1 and PI3K/Akt pathway activities in vivo.
In conclusions, GLUT-1 and p-Akt were expressed in Hep-2 cells. Over-expression of GLUT-1 mRNA may be associated with the resistance to cisplatin of laryngeal carcinoma Hep-2 cells. Apigenin may enhance the sensitivity to cisplatin of laryngeal carcinoma via inhibition of GLUT-1 and p-Akt expression.
Acknowledgements
This research was supported by the Department of Education of Zhejiang Province, China (grant no. Y20110426), the National Natural Science Foundation of China (grant nos. 81172562 and 81372903).
Disclosure of conflict of interest
None.
References
- 1.Johung K, Rewari A, Wu H, Judson B, Contessa JN, Haffty BG, Decker RH. Role of excision repair cross-complementation 1 expression as a prognostic marker for response to radiotherapy in early-stage laryngeal cancer. Head Neck. 2013;35:852–857. doi: 10.1002/hed.23041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Li R, Wang R, Zhai R, Dong Z. Targeted inhibition of mammalian target of rapamycin (mTOR) signaling pathway inhibits proliferation and induces apoptosis of laryngeal carcinoma cells in vitro. Tumori. 2011;97:781–786. doi: 10.1177/030089161109700616. [DOI] [PubMed] [Google Scholar]
- 4.Yan SX, Luo XM, Zhou SH, Bao YY, Fan J, Lu ZJ, Liao XB, Huang YP, Wu TT, Wang QY. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci. 2013;10:1375–1386. doi: 10.7150/ijms.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XQ, Chen X, Xie XX, Zhou Q, Li K, Li S, Shen LF, Su J. Co-expression of CD147 and GLUT-1 indicates radiation resistance and poor prognosis in cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:1651–1666. [PMC free article] [PubMed] [Google Scholar]
- 6.Gong L, Zhang W, Zhou J, Lu J, Xiong H, Shi X, Chen J. Prognostic Value of HIFs Expression in Head and Neck Cancer: A Systematic Review. PLoS One. 2013;8:e75094. doi: 10.1371/journal.pone.0075094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J, Li DW, Chen XW, Wang F, Dong P. Expression and significance of hypoxia-inducible factor-1α and MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2 cells. Oncol Lett. 2013;6:232–238. doi: 10.3892/ol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 2012;34:1395–1402. doi: 10.1002/hed.21945. [DOI] [PubMed] [Google Scholar]
- 9.Li LF, Zhou SH, Zhao K, Wang SQ, Wu QL, Fan J, Cheng KJ, Ling L. Clinical significance of FDG single-photon emission computed tomography: Computed tomography in the diagnosis of head and neck cancers and study of its mechanism. Cancer Biother Radiopharm. 2008;23:701–714. doi: 10.1089/cbr.2008.0510. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SH, Fan J, Chen XM, Cheng KJ, Wang SQ. Inhibition of cell proliferation and glucose uptake in human laryngeal carcinoma cells by antisense oligonucleotides against glucose transporter-1. Head Neck. 2009;31:1624–1633. doi: 10.1002/hed.21137. [DOI] [PubMed] [Google Scholar]
- 11.Abouzeid AH, Patel NR, Rachman IM, Senn S, Torchilin VP. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J Drug Target. 2013;21:994–1000. doi: 10.3109/1061186X.2013.840639. [DOI] [PubMed] [Google Scholar]
- 12.Wang YD, Li SJ, Liao JX. Inhibition of Glucose Transporter 1 (GLUT1) Chemosensitized Head and Neck Cancer Cells to Cisplatin. Technol Cancer Res Treat. 2013;12:525–535. doi: 10.7785/tcrt.2012.500343. [DOI] [PubMed] [Google Scholar]
- 13.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimanishi M, Ogi K, Sogabe Y, Kaneko T, Dehari H, Miyazaki A, Hiratsuka H. Silencing of GLUT-1 inhibits sensitization of oral cancer cells to cisplatin during hypoxia. J Oral Pathol Med. 2013;42:382–388. doi: 10.1111/jop.12028. [DOI] [PubMed] [Google Scholar]
- 15.Sommermann TG, O’Neill K, Plas DR, Cahir-McFarland E. IKKβ and NF-κB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291–7300. doi: 10.1158/0008-5472.CAN-11-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167. doi: 10.2217/fon.11.95. [DOI] [PubMed] [Google Scholar]
- 18.Sami A, Karsy M. Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: novel therapeutic agents and advances in understanding. Tumour Biol. 2013;34:1991–2002. doi: 10.1007/s13277-013-0800-5. [DOI] [PubMed] [Google Scholar]
- 19.Patel S. Exploring novel therapeutic targets in GIST: focus on the PI3K/Akt/mTOR pathway. Curr Oncol Rep. 2013;15:386–395. doi: 10.1007/s11912-013-0316-6. [DOI] [PubMed] [Google Scholar]
- 20.Dai C, Zhang B, Liu X, Ma S, Yang Y, Yao Y, Feng M, Bao X, Li G, Wang J, Guo K, Ma W, Xing B, Lian W, Xiao J, Cai F, Zhang H, Wang R. Inhibition of PI3K/AKT/mTOR pathway enhances temozolomide-induced cytotoxicity in pituitary adenoma cell lines in vitro and xenografted pituitary adenoma in female nude mice. Endocrinology. 2013;154:1247–1259. doi: 10.1210/en.2012-1908. [DOI] [PubMed] [Google Scholar]
- 21.Heavey S, O’Byrne KJ, Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat Rev. 2014;40:445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Bao YY, Zhou SH, Fan J, Wang QY. Anticancer mechanism of apigenin and the implications of GLUT-1 expression in head and neck cancers. Future Oncol. 2013;9:1353–1364. doi: 10.2217/fon.13.84. [DOI] [PubMed] [Google Scholar]
- 23.Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37:426–431. doi: 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed] [Google Scholar]
- 24.Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Mao Y, Chen H, Lin Y, Hu Z, Wu J, Xu X, Xu X, Qin J, Xie L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013;13:54. doi: 10.1186/1475-2867-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 27.Chen XH, Bao YY, Zhou SH, Wang QY, Wei Y, Fan J. Glucose transporter-1 expression in CD133+ laryngeal carcinoma Hep-2 cells. Mol Med Rep. 2013;8:1695–1700. doi: 10.3892/mmr.2013.1740. [DOI] [PubMed] [Google Scholar]
- 28.Evans A, Bates V, Troy H, Hewitt S, Holbeck S, Chung YL, Phillips R, Stubbs M, Griffiths J, Airley R. Glut-1 as a therapeutic target: increased chemoresistance and HIF-1-independent link with cell turnover is revealed through COMPARE analysis and metabolomic studies. Cancer Chemother Pharmacol. 2008;61:377–393. doi: 10.1007/s00280-007-0480-1. [DOI] [PubMed] [Google Scholar]
- 29.Vishvakarma NK, Kumar A, Singh V, Singh SM. Hyperglycemia of tumor microenvironment modulates stage-dependent tumor progression and multidrug resistance: implication of cell survival regulatory molecules and altered glucose transport. Mol Carcinog. 2013;52:932–945. doi: 10.1002/mc.21922. [DOI] [PubMed] [Google Scholar]
- 30.Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, Tamaki N, Iwaisako K, Kitamura K, Ikai I, Uemoto S. P-glycoprotein expression affects 18F-fluorodeoxyglucose accumulation in hepatocellular carcinoma in vivo and in vitro. Int J Oncol. 2009;34:1303–1312. [PubMed] [Google Scholar]
- 31.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Cui S, Li S, Du C, Tian J, Wan S, Qian Z, Gu Y, Chen WR, Wang G. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013;73:1362–1373. doi: 10.1158/0008-5472.CAN-12-2072. [DOI] [PubMed] [Google Scholar]
- 33.Chan LP, Chou TH, Ding HY, Chen PR, Chiang FY, Kuo PL, Liang CH. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta. 2012;1820:1081–1091. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Melstrom LG, Salabat MR, Ding XZ, Strouch MJ, Grippo PJ, Mirzoeva S, Pelling JC, Bentrem DJ. Apigenin down-regulates the Hypoxia Response Genes: HIF-1a, GLUT-1, and VEGF in Human Pancreatic Cancer Cells. J Surg Res. 2011;167:173–181. doi: 10.1016/j.jss.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JL, Gonzalez de Mejia E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol. 2013;60:83–91. doi: 10.1016/j.fct.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 36.Luo H, Jiang BH, King SM, Chen CY. Inhibition of cell growth and VEGF expression in ovarian cancer cells by Flavonoids. Nutrit Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]


