Abstract
Hirschsprung’s disease (HSCR) is characterized by the absence of enteric ganglion cells along variable regions of the colon. Established theory demonstrates that HSCR is the consequence caused by the abnormal arrest of the migration and differentiation of neural crest-derived stem cells (NCSCs). And retinoid signaling was considered to be involved. We speculated that, HA117, a retinoid-related transcript of a long noncoding RNA (LncRNA), may be involved in the genesis of HSCR. In current research, colon specimens were collected from 25 HSCR patients and grouped into 3 segments: proximal anastomosis, dilated segment and stenotic segment. Real-Time PCR was used to analyze the expression profiles of HA117 and its neighboring gene DPF3 in different colon segments. Fluorescence in situ hybridization (FISH) was employed to detect the distribution of HA117 in the gut wall. Immunohistochemistry was performed to analyze the protein expression of DPF3 in different colon segments. HA117 expression in stenotic segment was higher compared to proximal anastomosis and dilated segment (p < 0.05). Whereas DPF3b mRNA was lower in stenotic segment than that in two other segments (p < 0.05). FISH detected HA117 was distributed in mucosa and muscle layer, mainly present in stenotic segment. Immunohistochemical staining showed that intensive DPF3 staining occurred in proximal anastomosis and the positive staining was hardly observed in stenotic segment. The results suggested that HA117 may be a factor exerting an anti-differentiation or or anti-maturation role in the genesis of HSCR. This gave us a novel cue for better understanding the etiology of HSCR.
Keywords: HA117, DPF3, long noncoding RNA, Hirschsprung’s disease, enteric ganglion cells
Introduction
Hirschsprung’s disease (HSCR) is a representative congenital gastrointestinal malformation, with a prevalence of 1/2,000~1/5,000, and the ratio of male to female is approximately 4:1. It is well constructed that the primary pathological change of HSCR is the absence of ganglion cells of the myenteric and submucosal plexus in lesion colon portions. In the embryonic phase, embryonic ectoderm neural crest-derived stem cells (NCSCs) being blocked in the process of craniocaudal migration from the rostral to caudal end during gastrulation, and/or NCSCs in the colon not being able to well proliferate or differentiate, were accepted as the main mechanisms of HSCR occurrence. However, the specific etiology and pathogenesis of HSCR remain unclear. Up to now, HSCR is considered as a multiple gene-controlled disease. Mutations or abnormal expressions of the associated genes, such as RET, glial cell line-derived neurotrophic factor (GDNF), are supposed to elicit the migration and/or differentiation of NCSCs to be blocked and consequent malformation.
In recent years, retinoid signaling was considered to take part in the development of the enteric nervous system (ENS) [1]. Recent study reveals that retinoic acid upregulates RET and induces chain migration and population expansion in vagal neural crest cells to colonize the embryonic gut [2]. Defective or excessive intake of vitamin A can lead to congenital malformation or even fetal death [3-5]. After entering into cells, vitamin A is bound by cellular retinol binding proteins (CRABPs) and transformed to retinoic acid through two consecutive oxidation reactions. All-trans retinoic acid (ATRA) is the major active form of retinoid. By the interaction with retinoic acid receptors (RARs), RARα, β, γ, ATRA initiates the target genes transcription [6].
In our previous research, ATRA was used to stimulate leukemia cell line HL-60 by progressively increase of the dosage of ATRA, and finally the multidrug resistance cell line HL-60/ATRA was built. Through suppression subtractive hybridization and microarray analysis of differentially expressed sequences, we screened out a highly expressed sequence in HL-60/ATRA, which was named HA117 (GenBank accession number: CB214920) [7]. It confers multidrug resistance in HL-60/ATRA cells. Sequence alignment identifies the sequence is a transcript of a long non-coding RNA (LncRNA). DPF3 (D4, zinc and double PHD fingers, family 3) is its adjacent upstream gene, which plays a role in neuronal differentiation process and also takes part in the skeletal muscle and heart development [8].
In the past few years, LncRNA has drawn much attention of researchers. It is known to play multifaceted roles in gene expression regulation, like controlling processes of protein synthesis, RNA maturation, and regulating the chromatin structure [9]. And LncRNAs are known implicated in embryonic development [10-12]. In present documents, one mechanism of LncRNAs is regulating the neighboring encoding gene to implement the further function [13-15]. As previous studies suggested, the genes promoting the development of ENS, such as calretinin (CR), RET proto-oncogene, GDNF were highly expressed in ENS well-developed bowel, while lowly expressed in ENS immaturely developed bowel [16,17]. HA117 and its adjacent gene DPF3, as an ATRA-related LncRNA and a neuro-related gene, respectively, their expressions and potential roles in HSCR were uncharacterized. In this study, we sought to describe the expression profiles of HA117 and DPF3 in different portions of HSCR colon. This may provide a clue to better understand the etiology of HSCR.
Bioinformatics analysis
HA117 is located on chromosome 14, in the region of 14q24.2. Its flanking encoding genes are DPF3 and regulators of G protein signaling 6 (RGS6), both of them are involved in nerve system development. And both HA117 and DPF3 are located on the reverse strand. No protein-coding capacity of HA117 was identified with GENESCAN and AUGUSTUS online tools. According to the annotation data (http://genome.ucsc.edu), acetyl-Histone H3 Lysine 27 (H3K27Ac) mark and DNase I binding sites, as signatures of histone modifications, were annotated nearby HA117. HA117 is annotated as one of transcripts of lncRNA lnc-DPF3-1 (http://www.lncipedia.org/). Similar to protein-encoding genes, LncRNAs have 5’ caps and 3’ poly A tails and can be transcribed.
Materials and methods
Patient data collection and colon tissue preparation
Twenty-five cases of HSCR patients’ surgically resected colon tissues were collected randomly in Children’s Hospital of Chongqing Medical University (Chongqing, China) from June 2012 to April 2014, including 16 males and 9 females in an age range from 1 month to 5 years old. Nine cases were long-segment type, 3 cases were short-segment type, 10 cases were common type and 3 cases were total colonic aganglionosis. Seven cases of patients who had colon tissue removed for reasons other than HSCR were set as references. Patients with inflammatory bowel disease were excluded. Clinical diagnosis was supported by the case history, symptoms, and histological identification of the absence of enteric nervous plexuses on the basis of pathological report. According to the resection location and apparent size of bowel, collected colon tissues were divided into 3 groups: proximal anastomosis (proximal anastomosis of resected colon), dilated segment and stenotic segment, corresponding to the normal segment, hypoganglionic segment and aganglionic segment of the resected colon, respectively. All of the cases were recruited after obtaining parental consent and were approved by the Ethics Committee of Children’s Hospital, Chongqing Medical University. Resected colon tissues were processed immediately or stored at -80°C for subsequent Real-Time PCR analysis.
Total RNA extraction and Real-Time PCR analysis
RNA was isolated from tissues using Trizol (Life Technologies, Grand Island, NY, USA). Total RNA was extracted with chloroform, precipitated with isopropyl alcohol, and dissolved in diethyl pyrocarbonate (DEPC)-treated water. And total RNA was reverse transcribed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Otsu, Japan). The expressions of HA117 lncRNA and DPF3b mRNA were determined by the CFX96 Real-Time Detection System (Bio-Rad, Hercules, CA, USA) using SYBR Premix Ex Taq™ II (Takara, Otsu, Japan). Primers were 5’-CAG AGT CAG GGA CTT CAG CCT TAT-3’ and 5’-CTG TTT CCT TCT CAC TCC CAA CCA-3’ for detection of human HA117, 5’-GAC CGA GGC TGT CAA GAC CTA C-3’ and 5’-CAT GTG ATA GCC TCG GTC ACA G-3’ for detection of human DPF3b, and 5’-AGA CCT GTA CGC CAA CAC AG-3’ and 5’-GTA CTT GCG CTC AGG AGG AG-3’ for detection of human β-actin. β-actin was used as an internal reference. cDNA amplification was performed as: 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec, and 59°C for 30 sec. Specificity of each Real-Time PCR product was evaluated with dissociation curve. The expression quantities of RNA relative to β-actin were analyzed using comparative delta-Ct method. Standard curves were employed to correlate the changes in the LncRNA and mRNA levels.
Fluorescence in situ hybridization (FISH)
Freshly resected colon tissue was embedded and frozen in OCT embedding medium according to the different segments of colon. Then tissues were quick-frozen at -20°C, sliced into 15 μm-thick, tiled in anti-off slide, and fixed with 4% paraformaldehyde for 10 min. Slides were digested in 25 mg/ml proteinase K solution at 37°C for 20 min, washed with 2 × SSC (pH 7.0) at room temperature, then dehydrated in graded alcohol solutions at -20°C. Denaturing solution (70% formamide, 2 × SSC, pH 7.0) treated the samples at 78°C for 8 min; Digoxigenin-labeled probe was designed and synthesized according to the sequence analysis and design principles. 10 μl probe was applied onto the slides, added with a coverslip, then incubated at 37°C for 13 h. After washing with a solution of 20 × SSC 4 ml, distilled water 16 ml and formamide 20 ml at 43°C for 15 min, samples were washed by 2 × SSC at 37°C twice and washed with phosphate-buffered saline (PBS). Each slide was added with a mouse anti-digoxin antibody (Sigma, St. Louis, MO, USA) and incubated at 37°C for 20 min. After a triple wash with 1 × PBS, the slides were treated by 30 μl~60 μl of rhodamine-labeled streptavidin at 37°C for 20 min. 30 μl DAPI was used for nuclear staining at room temperature for 8 min. Finally, the results were observed with fluorescence microscope (Olympus, Japan) and analyzed with Image-Pro Plus (Media Cybernetics, Bethesda, MD, USA).
Immunohistochemistry (IHC)
Fresh samples were immediately fixed in 10% neutral formalin and embedded in paraffin, and then cut to 5 μm-thick sections. Slides were de-paraffinized with xylene and rehydrated by immersion in a gradient series of ethanol. Antigen retrieval was carried out by autoclaving in 0.01 M trisodium citrate buffer (pH = 6.0). After incubation with 3% H2O2 for 30 min at room temperature to block endogenous peroxidases, nonspecific sites were blocked with 10% goat serum and incubated with primary antibody to DPF3 (GeneTex, San Antonio, TX, USA, Cat: GTX122249) overnight at 4°C. Then specimens were incubated with biotinylated goat anti-rabbit IgG (Jingmei Biotech, Shenzhen, China) of a 1:100 dilution. After washing with PBS, specimens were incubated with 3, 3’-diaminobenzidine (DAB; Zhongshan Biotech, Beijing, China). All slides were counterstained with hematoxylin, and mounted with neutral gum. Optical microscope was used to evaluate the expression of DPF3. PBS was used in place of the primary antibody or second antibody as the negative control. Intensity of staining was graded as follows: negative (−); low staining (+); moderate staining (++); intensive staining (+++). Calretinin (CR) was set as a reference, and human adrenal gland tissue provided by the manufacturer served as a positive control for CR antibody (Santa Cruz, CA, USA, Cat: sc-365956).
Statistical analysis
SPSS statistical software (version 17.0) was used for statistical analysis. RNA expressions were presented as means ± SEM. An ANOVA analysis was employed for multi group data analysis. The statistical significance of differences between two groups was assessed by the Student’s t test. To facilitate a comparison, the data were normalized. A p value < 0.05 was considered as the minimum level of significance. All reported significance levels represent two-tailed p values.
Results
Expressions of HA117 RNA and DPF3b mRNA in different segments of HSCR
In the proximal anastomosis, dilated segment and stenotic segment of HSCR, the relative expression levels of HA117 RNA were 0.26 ± 0.09, 0.38 ± 0.10, 0.91 ± 0.06, respectively (Figure 1A). Compared with stenotic segment, the expressions of HA117 RNA in dilated segment and proximal anastomosis were significantly lower (p < 0.05), and there was no significant difference between proximal anastomotic segment and dilated segment (p > 0.05). The trend of expression in the three sections showed a progressive decrease. HA117 expression also can be detected in the colon tissue of non-HSCR disease.
Figure 1.
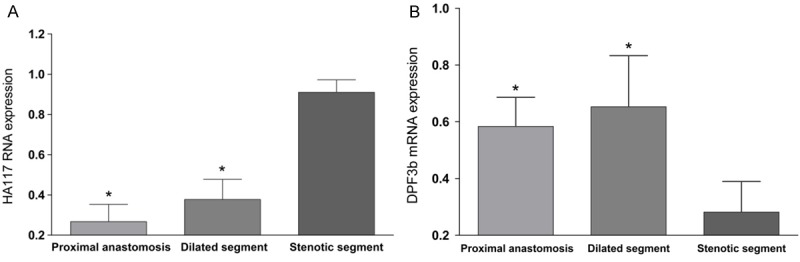
HA117 RNA (A) and DPF3b mRNA (B) expression in different segments of HSCR. *p < 0.05, compared with stenotic segment.
In the proximal anastomosis, dilated segment and the stenotic segment of HSCR, the relative expression levels of DPF3b mRNA were 0.58 ± 0.10, 0.65 ± 0.18 and 0.28 ± 0.11, respectively (Figure 1B). Compared with stenotic segment, the expressions of DPF3b mRNA in dilated segment and proximal anastomosis were significantly higher (p < 0.05), and there was no significant difference between proximal anastomotic segment and dilated segment (p > 0.05). In colon tissue of non-HSCR disease cases, expression of DPF3b mRNA can be detected, too.
Fluorescence expression patterns of HA117 in different segments of HSCR
In the detection result of In situ hybridization with digoxin-labeled nucleic acid probe, HA117 was distributed in both intestinal mucosa and muscle layers. In the colon mucosa layer, HA117 was mostly expressed in the stenotic segment of HSCR, whereas HA117 expression in dilated segment or proximal anastomosis was less than that in stenotic segment (Figure 2). While in muscle layer, the stenotic segment of HSCR was also the segment with the most HA117 fluorescence distribution; in dilated segment the distribution amount of HA117 was less than the amount in stenotic segment; the HA117 distribution in samples from proximal anastomosis was the least among the three segments (Figure 3).
Figure 2.

FISH detected HA117 expression in mucous layer of different segments of HSCR (400 ×). The differential expressions of HA117 in mucous layer of proximal anastomosis (A), dilated segment (B) and stenotic segment (C) of HSCR were observed, mostly expressed in stenotic segment. Red: HA117; Blue: Nucleus. Scale bar = 100 μm.
Figure 3.
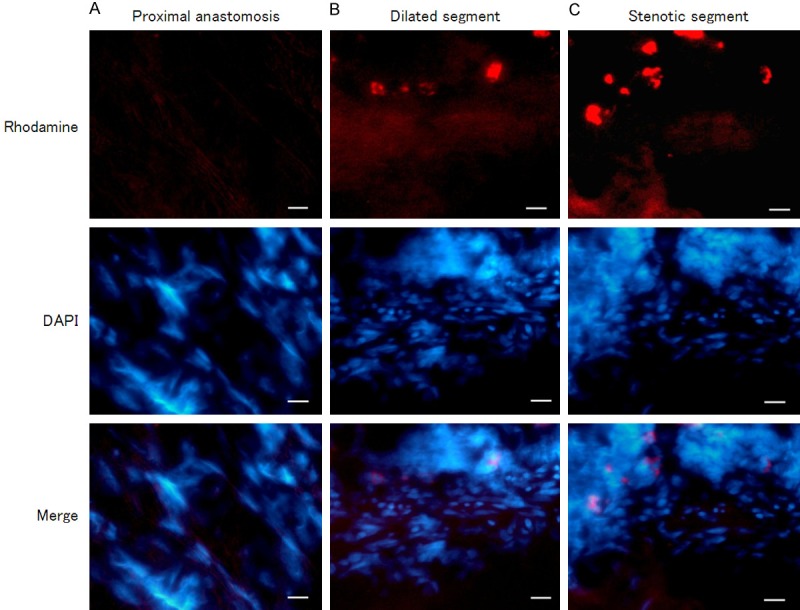
FISH detected HA117 expression in muscle layer of different segments of HSCR (400 ×). Differential expressions of HA117 were demonstrated in muscle layer of proximal anastomosis (A), dilated segment (B) and stenotic segment (C) of HSCR, dominantly distributed in stenotic segment. Red: HA117; Blue: Nucleus. Scale bar = 100 μm.
Immunohistochemical expression manners of DPF3 and CR proteins in different segments of HSCR
As illustrated in Figure 4, CR was aforementionally described as a reference in immunohistochemistry and CR positive-expressed tissue was stained in brown. In the tissue of proximal anastomosis, CR expression was stained in brown and mainly distributed in ganglion cells and myenteric plexus. Whereas in stenotic segment of HSCR, positive staining of CR was hardly observed in the space between the circular muscle and the longitudinal muscle. Correspondingly, DPF3 was mainly present in myenteric plexus between the circular muscle and the longitudinal muscle layer in the tissue of proximal anastomosis. Similar to CR, in stenotic segment, the distribution of DPF3 protein was scarcely observed. There was no brown staining in negative control of CR or DPF3.
Figure 4.
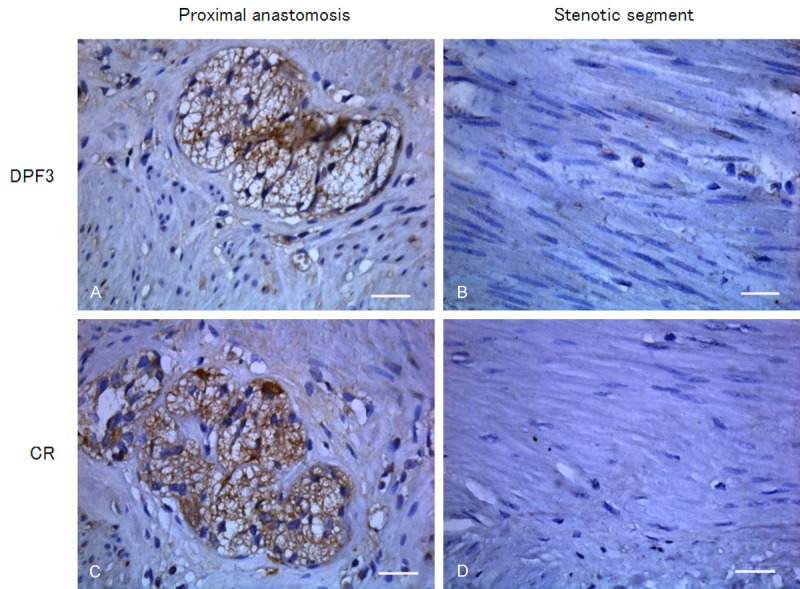
Immunohistochemical staining analyzed the protein expressions of DPF3 and CR in different segments of HSCR (400 ×). Intensive stainings of DPF3 (A) and CR (C) were displayed in ganglion cells and myenteric plexuses of proximal anastomosis. In the stenotic segment of HSCR, positive stainings of DPF3 (B) and CR (D) were not observed between the circular and the longitudinal muscle. Scale bar = 100 μm.
Discussion
HSCR is one of the common gastrointestinal malformations in children, characterized by a congenital absence of enteric ganglion cells in the affected sections of colon. HSCR is the second most neonatal gastrointestinal malformation, only inferior to the incidence of anorectal malformation. Harald Hirschsprung first described HSCR in the year of 1886. In 1948 Swenson made a judgment on the nature of HSCR, that is, the absence of plexus and ganglion cells in the abnormal bowel tube, makes the bowel cannot relax normally and causes obstruction even the loss of bowel motility. Finally secondary expansion and megacolon occurred in the proximal bowel of stenotic segment. It is documented that the main lesion of HSCR is in stenotic segment. Of HSCR cases, about 90% aganglionic regions are located in rectum and distal sigmoid. In individual cases, the entire colon or even terminal ileum is affected, or in some else only terminal rectum is involved.
It is established that abnormal embryonic development and congenital malformations are the consequence of the interaction of multi-gene and environmental factors. After entering into the intestine, NCSCs gradually migrate and proliferate. Affected by intestinal microenvironment and regulated by a series of genes, the NCSCs gather gradually and finally differentiate into ganglion and glial cells, as well as myenteric plexus or submucosal plexus [18]. HSCR-related genes include: RET, GDNF, laminin (LN), sex determining region Y-box 10 (SOX10), nitric oxide synthase (NOS), heme oxygenase-2 (HO-2), Zinc finger homeobox protein 1b (ZFHX1B), endothelin converting enzyme 1 (ECE1), C-fos, Bcl-2, and so on [19]. The positive rates of Nerve growth factor (NGF) and NGFR mRNA in stenotic segment were obviously lower than that in normal colon tissue. This suggested that the reduction of NGF and NGFR mRNA expressions may cause the abnormal development of ganglion cells [20]. CXCR4 plays a crucial role in the migration of NCSCs. In our recent observation, CXCR4 was detected in normal ganglionic and oligoganglionic colon segments of HSCR, but it significantly attenuated in the aganglionic colon segments [21].
In our present research, through the assay of Real-Time PCR and FISH, as a transcript of LncRNA, HA117 is present both in mucosal and muscular layer of the bowel. It is mostly expressed in stenotic segment of HSCR, while least expressed in proximal anastomosis, with the expression level in dilated segment ranking between the other two segments. LncRNAs play a vital biological role in embryonic development [9,10,22,23] and various LncRNAs have potential roles in maintaining the undifferentiated state in embryonic stem cells [24]. LncRNAs are also involved in the development and maturation of nerve tissues [11,25]. For example, homeobox protein Nkx2.2, acting as a transcriptional activator, involves in the formation of the central nervous system. By regulating Nkx2.2, the antisense LncRNA Nkx2.2AS, modulates neural differentiation from neural stem cells into oligodendrocytes [26]. As mentioned above, genes promoting the development of intestinal tissue are usually expressed highly in normal intestinal tissue or in the relatively normal intestinal tissue of HSCR, while lowly expressed in the stenotic region of HSCR. Our data demonstrate that, high expression of HA117 was present in stenotic segment of HSCR, while relatively low expression in dilated segment and proximal anastomosis. This suggests that as a transcript of a LncRNA, HA117 may be an adverse factor for neural or muscle maturation in bowel.
DPF3, also known as CERD4 or BRG1/Brm-Associated Factor 45c (BAF45c), is a member of D4 protein family, which includes DPF1, DPF2 and DPF3. DPF2 is ubiquitously expressed gene. DPF1 and DPF3 are mainly distributed in nerve tissues [27]. DPF3 is a transcription regulator and zinc finger protein binding acetylated histones, and it is a component of the BRG1/Brm-associated factors (BAF) chromatin remodeling complex. In BAF complex, DPF3 acts as a tissue-specific anchor between histone acetylations and methylations and chromatin remodeling. For human, alternative splicing of DPF3 results in two transcript variants, DPF3a and DPF3b. DPF3b can bind to histones H3 and H4, DPF3a cannot bind any histones. Through linking RelA/p50 to chromatin remodeling complex SWI/SNF, DPF3 can induce the transactivation of NF-κB target gene promoters [28]. SWI/SNF and histone acetyltransferases cooperate in retinoid-controlled transcription [29]. When exposed to 1 μM ATRA, DPF3 in zebrafish embryos was reduced [30]. There seems to be some links between ATRA and malformation and DPF3. However, the precise role of retinoid and ATRA in development remains obscure [1].
Our results show that, the adjacent protein-coding gene of HA117, DPF3, also possibly participates in the maturation and differentiation of ganglion cells. In stenotic segment of HSCR, the expression manner of DPF3b mRNA was opposite to that of HA117, with a lowest expression level in stenotic segment. Immunohistochemical assay shows that DPF3 protein is lowly expressed in stenotic segment and highly expressed in proximal anastomosis, consistent with the results of mRNA test. The expression of DPF3 also coincides with the reference protein CR. According to the existing researches, as a specific index reflecting the distribution of ganglion cells, CR can be used for HSCR diagnosis [31-34]. It is expressed in normal ganglion cells and nerve plexus, absence in aganglionic segment or regions without plexus. Thus, we speculate that DPF3 may promote the development of nerve system in colon. And HA117 may participate in an anti-differentiation progress in the genesis of HSCR, and the progress may be ATRA-associated. Whether HA117 plays an adverse effect on differentiation of ENS via inhibiting DPF3 remains to be further investigated.
To sum up, HA117 is highly expressed in stenotic segment of HSCR, lowly expressed in dilated segment and proximal anastomosis. In contrast, its neighboring gene DPF3 is lowly expressed in stenotic segment, highly expressed in in dilated segment and proximal anastomosis. The expression of HA117 was inversed to that of DPF3. The results give us a hint that as an ATRA-related LncRNA, HA117 may contribute to the genesis of HSCR and probably exert an anti-differentiation or anti-maturation action.
Acknowledgements
We greatly thank the patients and their parents involved in this study. This work was supported by the National Natural Science Foundation of China (No. 81370474).
Disclosure of conflict of interest
None.
References
- 1.Butler Tjaden NE, Trainor PA. The developmental etiology and pathogenesis of Hirschsprung disease. Transl Res. 2013;162:1–15. doi: 10.1016/j.trsl.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simkin JE, Zhang D, Rollo BN, Newgreen DF. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One. 2013;8:e64077. doi: 10.1371/journal.pone.0064077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu M, Sato Y, Lyons-Warren A, Zhang B, Kane MA, Napoli JL, Heuckeroth RO. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development. 2010;137:631–640. doi: 10.1242/dev.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Heuckeroth RO. Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro. Dev Biol. 2008;320:185–198. doi: 10.1016/j.ydbio.2008.05.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kam RK, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012;2:11. doi: 10.1186/2045-3701-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng GH, Fu JR, Xu YH, Jin XQ, Liu WL, Zhou JF. Screening and cloning of multi-drug resistant genes in HL-60/MDR cells. Leuk Res. 2009;33:1120–1123. doi: 10.1016/j.leukres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Lange M, Kaynak B, Forster UB, Tonjes M, Fischer JJ, Grimm C, Schlesinger J, Just S, Dunkel I, Krueger T, Mebus S, Lehrach H, Lurz R, Gobom J, Rottbauer W, Abdelilah-Seyfried S, Sperling S. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 2008;22:2370–2384. doi: 10.1101/gad.471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, Macura K, Blass G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martens JA, Wu PY, Winston F. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 2005;19:2695–2704. doi: 10.1101/gad.1367605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens JA, Laprade L, Winston F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature. 2004;429:571–574. doi: 10.1038/nature02538. [DOI] [PubMed] [Google Scholar]
- 15.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM Jr, Milbrandt J. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development. 2004;131:5503–5513. doi: 10.1242/dev.01421. [DOI] [PubMed] [Google Scholar]
- 17.Barshack I, Fridman E, Goldberg I, Chowers Y, Kopolovic J. The loss of calretinin expression indicates aganglionosis in Hirschsprung’s disease. J Clin Pathol. 2004;57:712–716. doi: 10.1136/jcp.2004.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCallion AS, Stames E, Conlon RA, Chakravarti A. Phenotype variation in two-locus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci U S A. 2003;100:1826–1831. doi: 10.1073/pnas.0337540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borrego S, Ruiz-Ferrer M, Fernandez RM, Antinolo G. Hirschsprung’s disease as a model of complex genetic etiology. Histol Histopathol. 2013;28:1117–1136. doi: 10.14670/HH-28.1117. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda T, Ueda M, Nakano M, Saeki M. Altered production of nerve growth factor in aganglionic intestines. J Pediatr Surg. 1994;29:288–292. doi: 10.1016/0022-3468(94)90334-4. discussion 292-283. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Zhao Z, Duan W, Wang S, Jin X, Xiang L. Expression patterns of CXCR4 in different colon tissue segments of patients with Hirschsprung’s disease. Exp Mol Pathol. 2013;95:111–116. doi: 10.1016/j.yexmp.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Liu N, Wang JP, Wang YQ, Yu XL, Wang ZB, Cheng XC, Zou Q. Regulatory long non-coding RNA and its functions. J Physiol Biochem. 2012;68:611–618. doi: 10.1007/s13105-012-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosal S, Das S, Chakrabarti J. Long noncoding RNAs: new players in the molecular mechanism for maintenance and differentiation of pluripotent stem cells. Stem Cells Dev. 2013;22:2240–2253. doi: 10.1089/scd.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tochitani S, Hayashizaki Y. Nkx2.2 antisense RNA overexpression enhanced oligodendrocytic differentiation. Biochem Biophys Res Commun. 2008;372:691–696. doi: 10.1016/j.bbrc.2008.05.127. [DOI] [PubMed] [Google Scholar]
- 27.Nabirochkina E, Simonova OB, Mertsalov IB, Kulikova DA, Ladigina NG, Korochkin LI, Buchman VL. Expression pattern of dd4, a sole member of the d4 family of transcription factors in Drosophila melanogaster. Mech Dev. 2002;114:119–123. doi: 10.1016/s0925-4773(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaka A, Mizutani T, Kobayashi K, Tando T, Sakurai K, Fujiwara T, Iba H. Double plant homeodomain (PHD) finger proteins DPF3a and -3b are required as transcriptional co-activators in SWI/SNF complex-dependent activation of NF-kappaB RelA/p50 heterodimer. J Biol Chem. 2012;287:11924–11933. doi: 10.1074/jbc.M111.322792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flajollet S, Lefebvre B, Cudejko C, Staels B, Lefebvre P. The core component of the mammalian SWI/SNF complex SMARCD3/BAF60c is a coactivator for the nuclear retinoic acid receptor. Mol Cell Endocrinol. 2007;270:23–32. doi: 10.1016/j.mce.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira E, Casado M, Raldua D, Soares A, Barata C, Pina B. Retinoic acid receptors’ expression and function during zebrafish early development. J Steroid Biochem Mol Biol. 2013;138:143–151. doi: 10.1016/j.jsbmb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Hiradfar M, Sharifi N, Khajedaluee M, Zabolinejad N, Taraz Jamshidi S. Calretinin Immunohistochemistery: An Aid in the Diagnosis of Hirschsprung’s Disease. Iran J Basic Med Sci. 2012;15:1053–1059. [PMC free article] [PubMed] [Google Scholar]
- 32.Kannaiyan L, Madabhushi S, Malleboyina R, Are NK, Reddy KR, Rao B. Calretinin immunohistochemistry: A new cost-effective and easy method for diagnosis of Hirschsprung’s disease. J Indian Assoc Pediatr Surg. 2013;18:66–68. doi: 10.4103/0971-9261.109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guinard-Samuel V, Bonnard A, De Lagausie P, Philippe-Chomette P, Alberti C, El Ghoneimi A, Peuchmaur M, Berrebi-Binczak D. Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod Pathol. 2009;22:1379–1384. doi: 10.1038/modpathol.2009.110. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrescu S, Rosenberg H, Tatevian N. Role of calretinin immunohistochemical stain in evaluation of Hirschsprung disease: an institutional experience. Int J Clin Exp Pathol. 2013;6:2955–2961. [PMC free article] [PubMed] [Google Scholar]


