Abstract
Patients with two types of primary cancers are rare. In this study, we investigated the expression of p53, cyclin D1, and Ki-67 in the second primary malignancy. Tissue samples were obtained from the second primary cancer site of 43 patients who met the diagnostic criteria for double primary cancer. p53, cyclin D1 and Ki-67 were determined using immunohistochemistry. Categorical variables were compared using the Chi-squared test; correlation between data scores and histology was calculated using the Spearman’s rank-order correlation. The expression rates of p53, cyclin D1 and Ki-67 in the second primary malignancy site were 60.5%, 30.2% and 65.1% respectively. p53 expression showed statistically significant association with tumor occurrence interval, pathological grading and nodal metastasis (p < 0.05). Positive correlation was detected between the expression of cyclin D1 and Ki-67 and the expression of p53 (r = 0.313, p = 0.041; r = 0.319, p = 0.037, respectively). High-expressing p53 or cyclin D second primary malignancies were associated with decreased overall survival (p = 0.040 and p = 0.043, respectively). Ki-67 expression levels did not exhibit statistically significant differences in survival. In conclusion, elevated protein expression of p53, cyclin D1 and Ki-67 in the second primary malignancy is an indicator of more aggressive malignant behavior of the secondary tumor. These markers may have prognostic value in the clinical setting.
Keywords: Double primary malignancies, second primary malignancy, immunohistochemistry, p53, cyclin D1, Ki-67
Introduction
Second Primary Malignances (SPM) are newly developed malignant neoplasms present either synchronously or metachronously in a patient with known malignant disease, as first studied and reported by Warren and Gates and later refined by the Surveillance, Epidemiology, and End Results (SEER) program [1]. The reported incidence of SPM varies among different nationalities and ethnic groups. Wang et al. calculated that 2.0% of 103,381 patients diagnosed with malignant diseases developed a second primary malignant neoplasm (2011 cases) [2]. Using incidence and follow-up data from the SEER program, Angela et al. estimated that almost 8% of the cancer survivor population in the United States (756,467 patients) had been affected by cancer more than once between 1975 and 2001 [3]. It is well known that patients living with cancer are at increased risk for subsequent tumors [4]. Early diagnosis and highly effective treatment have resulted in substantial gains in long-term survival with moderate to good quality of life. As a result, occurrence of second primary malignancies is expected to increase. However, very few studies have focused on the etiology and clinicopathological behavior of second primary malignancies.
In the present study, we investigated the expression of p53, cyclin D1 and Ki-67 in SPM, and their association with the malignancies clinical-pathological behavior, thereby providing groundwork data towards establishing indicators of poor cancer prognosis.
Materials and methods
Patient and study design
Malignant tissue samples were collected from 43 cases of SPM using the diagnostic criteria developed by Warren and Gates, and the SEER program (Tables 1 and 2). The majority of samples (n = 36) were obtained by surgical resection; a smaller number of samples were obtained by endoscopic or transthoracic biopsy (n = 7). Of the 43 SPM cases, 10 were synchronous and 33 were metachronous. The median time of occurrence after the first malignancy was diagnosed was 41 months. Tissue samples of SPM were fixed with 10% formaldehyde and embedded in paraffin. Immunohistochemistry analysis was then carried out. All tests were carried out in the pathology departments of USNTCM Teaching Hospital and Yanbian University. The primary objective is to investigate the expression of p53, cyclin D1 and Ki-67 in SPM and their correlation with the SPM’s clinicopathological behavior.
Table 1.
Summary of 43 Double Primary Malignancies
| No | Sex | Primary malignancy | Secondary malignancy | Time interval (months) | No | Sex | Primary malignancy | Secondary malignancy | Time interval (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Adenocarcinoma of the breast | Malignant glioma | 5 | 23 | F | Invasive ductal carcinoma | Tubular adenocarcinoma of the colon | 27 |
| 2 | F | Squamous cell carcinoma of the skin of the ear canal | Breast carcinoma | 42 | 24 | M | Adenocarcinoma of the lung | Esophageal squamous cell carcinoma | 5 |
| 3 | M | Adenocarcinoma of the lung | Papillary carcinoma of the thyroid | 13 | 25 | F | Esophageal leiomyosarcoma | Chest wall fibrosarcoma | 5 |
| 4 | M | Mucinous adenocarcinoma of the rectum | Gastric adenocarcinoma | 18 | 26 | M | Gastric adenocarcinoma | Well-differentiated Esophageal squamous cell carcinoma | 108 |
| 5 | F | (right) Breast carcinoma | (left) Sarcomatoid metaplastic ductal carcinoma of the breast | 3 | 27 | F | Basal cell carcinoma of the skin | Mixed ductal/sebaceous carcinoma of the breast | 46 |
| 6 | F | Gastric adenocarcinoma | Ovarian mucinous adenocarcinoma | 28 | 28 | F | (right) Infiltrating ductal carcinoma of the breast | (left) Mixed ductal carcinoma in situ/acne clear cell carcinoma | 1 |
| 7 | M | Gastric Cardia Adenocarcinoma | Esophageal squamous cell carcinoma | 8 | 29 | M | Bulge-like skin fibrosarcoma | Adenocarcinoma of the rectum | 54 |
| 8 | F | Ovarian serous adenocarcinoma | Adenocarcinoma of the colon | 43 | 30 | M | Well-differentiated squamous cell carcinoma of the tonsil | Hepatocellular carcinoma | 35 |
| 9 | F | Infiltrating ductal carcinoma | Squamous cell carcinoma of the lung | 56 | 31 | F | Chondrosarcoma | Ovarian serous adenocarcinoma | 120 |
| 10 | F | Malignant sweat gland tumor | Papillary carcinoma of the thyroid | 39 | 32 | F | Clear-cell carcinoma of the ovary | Medullary carcinoma of the breast | 61 |
| 11 | F | Malignant thymoma | Papillary carcinoma of the thyroid | 3 | 33 | M | Adenosquamous carcinoma of the lung | Low grade adenocarcinoma of the stomach | 21 |
| 12 | F | Gastric adenocarcinoma | Infiltrating ductal carcinoma | 146 | 34 | F | Laryngeal squamous cell carcinoma | Infiltrating ductal carcinoma | 5 |
| 13 | F | squamous cell carcinoma of tongue | Adenocarcinoma of the lung | 86 | 35 | F | Malignant mixed tumor of parotid | Infiltrating adenocarcinoma of the breast | 32 |
| 14 | F | Well-differentiated squamous cell carcinoma of the cervix | Adenocarcinoma of the colon | 91 | 36 | F | Tubular Adenocarcinoma of the ascending colon | Mucinous adenocarcinoma of the sigmoid colon | 25 |
| 15 | M | Papillary carcinoma of the thyroid | Adenocarcinoma of the lung | 43 | 37 | M | Follicular thyroid carcinoma | Squamous cell carcinoma of the lung | 76 |
| 16 | F | Invasive lobular carcinoma | Endometrial cancer | 61 | 38 | M | Esophageal squamous cell carcinoma | Adenosquamous cell carcinoma of the esophageal gastric junction | 0 |
| 17 | F | Adenocarcinoma of the stomach | Ovarian serous adenocarcinoma | 23 | 39 | F | Cervical squamous cell carcinoma | Signet-ring carcinoma of the colon | 34 |
| 18 | M | Hepatocellular carcinoma | Diffuse large B-cell lymphoma of the Antrum | 44 | 41 | F | Mid-differentiated Cutaneous melanoma differentiation | Squamous cell carcinoma of the lung | 47 |
| 19 | F | Perineal skin squamous cell carcinoma | Undifferentiated carcinoma of the colon | 89 | 41 | M | Stromal tumor of the colon | Tubular adenocarcinoma of the colon | 0 |
| 20 | F | Renal clear cell carcinoma | Supraclavicular mid-differentiated lymphoma | 77 | 42 | F | Cervical carcinoma in situ | Follicular carcinoma of the thyroid | 76 |
| 21 | F | Malignant gliomas | Ovarian serous cystadenocarcinoma | 5 | 43 | M | Medium Differentiated squamous cell carcinoma of the nasopharyngeal cavity | Adenosquamous cell carcinoma of the lung | 21 |
| 22 | F | High grade Adenocarcinoma Of the gallbladde | Tubular adenocarcinoma of the colon | 68 |
Table 2.
Demographic data for the secondary primary malignancies studied
| Gender | No. | Age (years) | Average age (years) | Synchronous | Metachronous | Average Occurrence Interval (months) |
|---|---|---|---|---|---|---|
| Male | 14 | 51-79 | 65.6 | 3 | 13 | 39.2 |
| Female | 29 | 37-68 | 51.3 | 7 | 20 | 62.4 |
| Total | 43 | 37-77 | 56.1 | 10 | 33 | 53.7 |
Immunohistochemistry
Mouse anti-human cyclin D1 monoclonal antibody (Santa Cruz: CAT# SC-20044) was purchased from Beijing BOAOSEN Bio-tech ltd, China. Rabbit anti-human p53 (Santa Cruz: CAT# SC-126) and Ki-67 (Santa Cruz: CAT# SC-23900) monoclonal antibodies and other reagents used in immunohistochemistry process were purchased from Beijing Zhongshan Goldenbridge Company, China. The expression of p53, cyclin D1 and Ki-67 was studied in deparaffinized sections using the streptavidin-peroxidase-biotin (SP) immunohistochemical method. Briefly, 4 μm paraffin embedded tissue sections were deparaffinized with xylene and re-hydrated using alcohol gradients. Antigens were retrieved from the deparaffinized sections in sodium-citrate buffer using a pressurized-cooker. Endogenous peroxidase was blocked with 3% peroxide-methanol. Tissue sections were incubated overnight with mouse anti-human cyclin D1 and rabbit anti-human p53 antibody or rabbit anti-human Ki-67 antibody diluted 1:100 at 4 degrees Celsius. Sections were then incubated at 37 degrees Celsius for 40 min with a secondary antibody (anti-mouse or anti-rabbit IgG) according to the source of the primary antibody used. Diaminobenzidine tetrahydrochloride was used as the chromogen, and hematoxylin was used for counterstaining. Known positive sections of malignant tissues served as positive controls.
Evaluation of immunohistochemical staining
Two pathologists independently blinded to clinical data evaluated all slides under a light microscope at 400 x magnification, and the final scores were calculated by averaging the two independent scores. Figure 1 shows representative photographs of immunohistochemistry staining for p53, Ki-67 and cyclin D1. Cells staining positive for p53 had brown granules inside their nuclei, cells staining positive for cyclin D1 had brown-yellow granules inside and around their nuclei, and cells staining positive for Ki-67 had brown granules inside nuclei. Five high magnification (400 x) fields were studied for every slide, and the fields used to calculate the percentage of immune-reactive nuclei were selected based on tissue preservation and absence of artifacts. Staining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). If two or more staining intensities were present in a single slide, the staining intensity was graded according to the strongest immunoreactivity. Percentages of positive staining nuclei were estimated by counting at least 500 nuclei. A percentage score based on rate of positive staining nuclei was given: 0 (< 5%), 1 (5-9%), 2 (10-19%), 3 (20-49%), 4 (> 50%). The percentage score of positive staining was multiplied by the staining intensity score to obtain a final score of 0 to 12 and expression intensity categories: less than 2 as negative (-), 3~5 as low expression (+), 6~8 as moderate expression (++) and greater than 9 as high expression (+++). When conducting survival analysis, we defined a score between 0 and 5 as low expression group and a score between 6 and 12 as high expression group.
Figure 1.
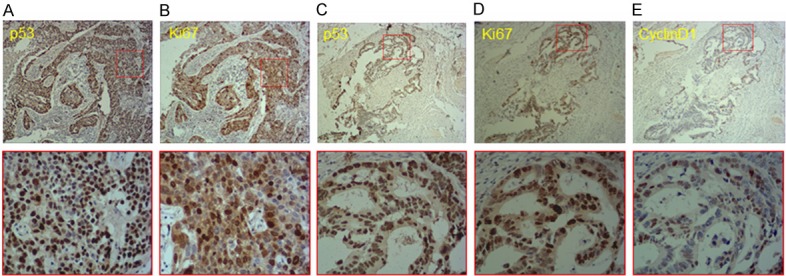
Immunohistochemistry staining for p53, cyclin D1 and Ki-67 in tissue sections of second primary malignancies. Representative photographs of immunohistochemistry staining for p53, Ki-67 and cyclin D1 in second primary breast adenocarcinoma and rectal adenocarcinoma. A. p53 expression in breast adenocarcinoma. B. Ki-67 expression in breast adenocarcinoma. C. p53 expression in rectal adenocarcinoma. D. Ki-67 expression in rectal adenocarcinoma. E. Cyclin D1 expression in rectal adenocarcinoma. 200 x magnification (upper panels) and 400 x magnification of boxed area (lower panels).
Statistical analysis
Comparisons among categorical variables were conducted using the Chi-squared test, and correlations were calculated using the Spearman’s rank-order correlation. Survival curves were estimated using the Kaplan-Meier method, and statistical analyses were performed using the log-rank test. A p < 0.05 was considered statistically significant. All statistical analysis were performed using SPSS 19.0 (IBM Inc. Armonk, New York, USA).
Results
Expression of p53, cyclin D1 and Ki-67 in tissue sections of secondary malignancy
Figure 1 depicts representative photographs of immunohistochemistry staining for p53, Ki-67 and cyclin D1 in breast and rectal adenocarcinoma. The overall positive expression rates of p53, cyclin D1 and Ki-67 in tissue sections of secondary malignancy were 60.5%, 30.2% and 65.1% respectively. In this group of patients, clinicopathological variables included age, time to occurrence interval, TNM stage, differentiation grade, lymph node metastasis, whether the patients had previously been treated with chemotherapy and/or radiation therapy, and family history of malignant disease. A detailed summary of the data is illustrated in Table 3.
Table 3.
Clinicopathological features and the expression of p53, cyclin D1 and Ki-67 protein in the second primary malignancy
| Variable | Cases | Positive cases (%) | ||
|---|---|---|---|---|
|
| ||||
| p53 | cyclin D1 | Ki-67 | ||
| Gender | ||||
| Male | 14 | 10 (71.4) | 5 (35.7) | 10 (71.4) |
| Female | 29 | 16 (55.2) | 8 (27.6) | 18 (62.1) |
| Age | ||||
| < 50 y | 30 | 18 (60.0) | 7 (23.3) | 18 (60.0) |
| ≥ 50 y | 13 | 8 (61.5) | 6 (46.2) | 10 (76.9) |
| Occurrence Interval | ||||
| < 6 m | 10 | 9 (90.0)* | 3 (30.0) | 5 (55.6) |
| ≥ 6 m | 33 | 18 (54.5) | 10 (29.4) | 23 (67.6) |
| Differentiation Degree | ||||
| I-II | 16 | 6 (37.5)* | 1 (6.2)* | 7 (43.8)* |
| III-IV | 27 | 20 (74.1) | 12 (44.4) | 21 (77.8) |
| TNM Stage | ||||
| I/II | 21 | 14 (66.7) | 4 (19.0) | 13 (61.9) |
| III | 22 | 17 (77.3) | 9 (40.9) | 15 (68.2) |
| Lymph node metastasis | ||||
| Positive | 25 | 11 (44.0)* | 11 (44.0)* | 20 (80.0)* |
| Negative | 18 | 15 (83.3) | 2 (11.1) | 8 (44.4) |
| Chemotherapy | ||||
| Yes | 39 | 24 (61.5) | 12 (30.8) | 26 (66.7) |
| No | 4 | 2 (50.0) | 1 (25.0) | 2 (50.0) |
| Radiotherapy | ||||
| Yes | 31 | 15 (48.4) | 7 (22.6) | 21 (67.7) |
| No | 12 | 11 (91.7) | 6 (50.0) | 7 (58.3) |
| Family History | ||||
| Yes | 5 | 2 (40.0) | 2 (40.0) | 3 (60.0) |
| No | 38 | 24 (76.3) | 11 (28.9) | 25 (65.9) |
p<0.05.
Positive staining for p53 was observed in 90% (9/10) of SPM diagnosed within 6 months of the first primary malignancy and in 54.5% (18/33) of SPM diagnosed 6 months or more after the first primary malignancy (p < 0.05). p53 staining was associated with advanced histological grade, with detection rates of 37.5% (6/10) in SPM with grades I-II and of 74.1% (20/27) in SPM with grades III-IV (p < 0.05). Positive staining for p53 was also observed in 44% (11/25) of SPM with at least 1 lymph node metastasis, and 85.3% (15/18) of SPM with no lymph node involvement (p < 0.05). No statistically significant association was detected between positive staining for p53 and the other categorical variables tested (gender, age, TNM staging, and previous treatment).
Cyclin D1 staining was also associated with advanced histological grade, with detection rates of 6.2% (1/16) in SPM with grades I-II SPM and of 44.4% (12/27) in SPM with grades III-IV (p < 0.05). SPM stained positive for cyclin D1 in 44% (11/25) of the cases with nodal metastasis and in 11% (2/18) of cases with no lymph node metastasis (p < 0.05). No statistically significant association was detected between positive staining for cyclin D1 and the other categorical variables tested.
Similarly to p53 and cyclin D1, Ki-67 staining was associated with advanced histological grade, with detection rates of 43.8% (7/16) in SPM with grades I-II and of 77.8% (21/27) in SPM with grades III-IV (p < 0.05). SPM stained positive for Ki-67 in 80% (20/25) of cases with nodal metastasis, and 44.4% (8/18) of cases with no lymph node metastasis (p < 0.05). No statistically significant association was detected between positive staining for Ki-67 and the other categorical variables tested.
Correlation between the expression of p53, cyclin D1 and Ki-67 in tissue sections of secondary malignancy
Expression of p53 and cyclin D1 in the SPM had a low but statistically significant correlation (r = 0.313, p = 0.041) (Table 4), as did p53 and Ki-67 (r = 0.319, p = 0.037) (Table 5). No correlation was observed between cyclin D1 and Ki-67 (Table 6).
Table 4.
Correlation between the p53 and cyclin D1 protein expression in the second primary malignancy
| Cases | cyclin D1 | r-value | P-value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Low-expression | High-expression | |||||||
|
|
|
|||||||
| - | + | ++ | +++ | |||||
| p53 | - | 17 | 15 | 2 | 0 | 0 | 0.313 | 0.041 |
| + | 9 | 5 | 2 | 1 | 1 | |||
| ++ | 11 | 6 | 1 | 3 | 1 | |||
| +++ | 6 | 4 | 0 | 0 | 2 | |||
Table 5.
Correlation between p53 and Ki-67 protein expression in the second primary malignancy
| Cases | Ki-67 | R-value | P-value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Low-expression | High-expression | |||||||
|
|
|
|||||||
| - | + | ++ | +++ | |||||
| p53 | - | 17 | 7 | 8 | 2 | 0 | 0.319 | 0.037 |
| + | 9 | 3 | 0 | 5 | 1 | |||
| ++ | 11 | 4 | 2 | 3 | 2 | |||
| +++ | 6 | 1 | 1 | 3 | 1 | |||
Table 6.
Correlation between the cyclin D1 and Ki-67 protein expression in the second primary malignancy
| Cases | Ki-67 | R-value | P-value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Low-expression | High-expression | |||||||
|
|
|
|||||||
| - | + | ++ | +++ | |||||
| cyclin D1 | - | 30 | 7 | 10 | 9 | 4 | 0.271 | 0.079 |
| + | 5 | 3 | 0 | 1 | 1 | |||
| ++ | 4 | 3 | 0 | 1 | 0 | |||
| +++ | 4 | 2 | 1 | 1 | 0 | |||
Positive expression of p53, cyclin D1 and Ki-67 and association with outcome
Follow-up time after the diagnosis of SPM varied between 6 and 67 months (median = 41 months). The survival rate was 20.9% (9/43), and no cases were lost to follow-up. The overall high expression rates of p53, cyclin D1 and Ki-67 in tissue sections of secondary malignancy were 39.5% (17/43), 18.6% (8/43) and 39.5% (7/43). Patients whose SPM had high p53 expression exhibited decreased survival time compared those with low p53 expression (p = 0.040) (Figure 2), and similar results were observed when comparing overall survival between patients whose SPM had high and low cyclin D1 expression (p = 0.043) (Figure 3). Overall survival was longer in patients with low Ki-67 expression [mean (95% CI) = 25.7 (19.6-31.9)] compared with high Ki-67 expression [mean (95% CI) = 29.6 (21.0-38.3)], but that difference was not statistically significant (p = 0.572) (Figure 4).
Figure 2.
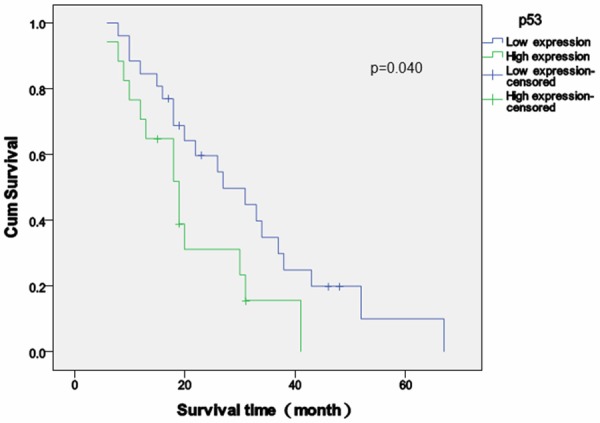
Survival analysis of p53 expression in second primary malignancies. Survival time (months) relative to the expression score of p53 (high versus low). Patients with high p53 expression exhibited decreased overall survival time compared those with low p53 expression. P = 0.040, log-rank test.
Figure 3.

Survival analysis of cyclin D1 expression in second primary malignancies. Survival time (months) relative to the expression score of cyclin D1 (high versus low). Patients with high cyclin D1 expression exhibited decreased overall survival time compared those with low cyclin D1 expression. P = 0.040, log-rank test.
Figure 4.
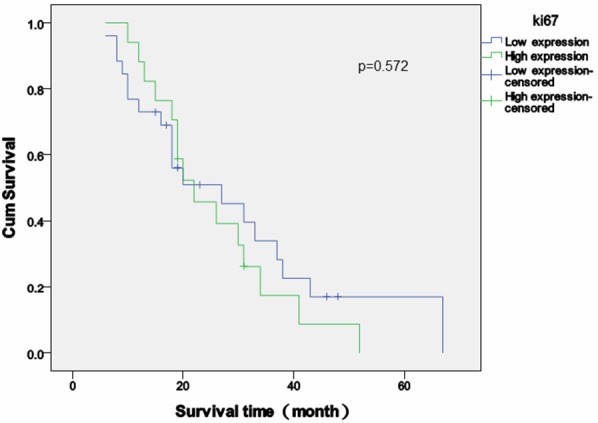
Survival analysis of Ki-67 expression in second primary malignancies. Survival time (months) relative to the expression score of cyclin D1 (high versus low). Patients with high Ki-67 expression exhibited a trend towards decreased overall survival time compared those with low Ki-67 expression, but the difference was not statistically significant (p = 0.572, log-rank test).
Discussion
Etiology of SPM
Once considered a very rare disease, the number of patients who go on and develop an SPM is on a rise. Advances in early detection and treatment led to an unprecedented increased in the survival rate of cancer patients, but may also have contributed to a rise in the number of cases of SPM. The etiology of SPM is not very well understood. Several causal factors have been attributed to the development of SPM, including genetic predisposition and environmental exposures (both of which contribute to family history) and the carcinogenic risk associated with prior cancer therapy (chemotherapy, radiation therapy, hormonal therapy) [5-8].
Various most common malignancy sites have been reported for double malignancies. Outside mainland China, the most common sites for the primary malignancy are lung, colon and rectum, and the most common SPM sites are lung, breast, esophagus, and nasopharyngeal cavity, in order of incidence [9]. Based on our data, however, the most common primary sites were breast, skin and stomach, and the most common SPM sites were colon and rectum, breast, and lung, in order of incidence. We hypothesize that such discrepancies between our study and the literature are due to regional epidemiologic factors for each cancer.
Expression and clinical significance of p53 in SPM
As an important tumor suppressor protein, p53 is encoded by the Tp53 gene, located on chromosome 17p13. The name p53 refers to its apparent molecular mass: it runs as a 53kDa protein on SDS-PAGE. Wild-type p53 plays a role in apoptosis, genomic stability, and inhibition of angiogenesis. p53’s anti-oncogenic properties are related to its activation of DNA repair proteins when DNA has sustained damage. p53 can arrest growth by holding the cell-cycle at the G1/S checkpoint by regulating p21 downstream. p21 then binds to the G1-S/CDK (CDK2) and S/CDK complexes, inhibiting their activity. p53 can also hold the cell-cycle at the G2 phase by binding cyclin B1. Additionally, p53 can initiate apoptosis by either activating apoptotic genes such as Puma, Noxa, p53AIPI and Bax, or regulating the mitochondrial pathway via Bcl proteins. Wild-type p53 can’t be tested by IHC, since it is expressed at relatively low levels. If the Tp53 gene is damaged, the mutant p53 will no longer bind DNA effectively and, as a consequence, the p21 protein will not be able to act as the “stop signal” for cell division and tumor suppression becomes severely impaired. More than 50 percent of human tumors contain a mutation or deletion of the Tp53 gene [10]. Mutant p53 has a longer half-life, facilitating its detection by IHC.
In our study, a positive expression was recognized in 60.5% of tissue sections, of which 14.0% were (+++) positive and 25.6% were (++) positive, indicating that p53 is over expressed in SPM. We discovered that increased expression of p53 is associated with early detection of SPM, more advanced histological grade, and lymph node metastasis (p < 0.05). Other current studies suggested the high expression of p53 is mostly seen in lung cancer, breast cancer and colorectal cancer, in order of incidence. In our data, breast cancer, colorectal cancer and lung cancer were the top three p53-expressing malignancies. We consider this difference in the malignancies expressing p53 to be accounted for by differences between first and second primary malignancies. The overall survival was considerably longer for patients with low p53 expression [mean (95% CI) = 31.3 (23.9-38.7) months], compared with patients with high p53 expression [mean (95% CI) = 20.8 (15.1-26.6) months)], suggesting that p53 expression could become a useful prognostic factor for SPM.
90% of synchronous and 54.5% of metachronous SPM expressed p53. Four synchronous SPM were (+++) positive, accounting for 66.7% of all samples with (+++) p53 expression. The association of shorter onset interval with increased expression of p53 reinforces p53’s potential to be used as a prognostic marker. We observed ultra-high expression in two SPM cases: one gastric cancer and one spindle cell (sarcomatoid) carcinoma of the breast, raising the possibility that p53 somatic mutations may play an important role in the development of SPM. However, the present study was not powered or designed to address this issue.
Expression and clinical significance of cyclin D1 in SPM
Cyclin D1 is an important regulator of G1/S transition in the cell-cycle, and is encoded by the CCND1 gene on chromosome 11q13 in humans [11]. Cyclin D1 is also a well-recognized proto-oncogene [12], and its deregulated expression occurs in several types of human cancer. Mutation, amplification and overexpression of this gene are frequently observed in a variety of malignancies, and may contribute to oncogenesis, as they may shorten G1/S transition and accelerate cell-cycle progression.
In our study, 30.2% of SPM expressed cyclin D1, 61.5% of which had high expression (++ or +++). We discovered that high expression of cyclin D1 was highly associated with histological grade and lymph node metastasis (p < 0.05), suggesting that the high cyclin D1-expressing SPM had increased invasiveness and metastatic potential. We also recognized that 3 of 4 SPM with high cyclin D1 expression were synchronous, (diagnosis interval less than six months). That being said, no statistically significant association was detected between cyclin D1 expression and the onset interval time. Diagnosis of the first and second malignancies was carried out in different institutions and, therefore, we could not determine whether cyclin D1 was overexpressed in the first malignancy. The overall survival of patients with low cyclin D1 expression was 29.5 (23.3-35.7) months, while that of patients with high cyclin D1 expression was only 17.6 (10.5-24.8) months [mean (95% CI)]. Therefore, high cyclin D1 expression indicates a worse prognosis. However, only 8 patients expressed high levels of cyclin D1, and type I error could not be excluded. A COX regression study of a larger sample size will be needed to confirm this finding.
Expression and clinical significance of Ki-67 in SPM
The Ki-67 protein, encoded by the MKI67 gene, is a prototypic cell-cycle nuclear protein expressed in G1, S, G2, and peaking at M phase, but not in the interphase (G0). Therefore, Ki-67 has been widely used as a marker for cell proliferation. The Ki-67 antigen is a large basic protein found in two forms with molecular weights of 345 and 395 kDa. High expression of Ki-67 has recently been found in several malignancies and tumor cells. Rhee et al. have found Ki-67 to be overexpressed in 35.1% of triple-negative breast cancer [13]; Wang et al. have reported high expression rate in 61.8% of oral cavity squamous cell carcinoma [14]; and Soichi Oka et al. have reported Ki-67 expression in 22.4% of 183 patients with adenocarcinoma of the lung [15]. The variation of expression rate appears to be due to the different origin of each malignancy, but high expression of Ki-67 often coincided with a worse prognosis, particularly for breast and colon cancer.
Our study showed that 65.1% of SPM had positive expression of Ki-67, 4 of which had ultra-high expression (+++). We discovered that high expression of Ki-67 was associated with histological grade and lymph node metastasis, suggesting that Ki-67 expression may be able to predict invasiveness and metastatic potential. Patients with SPM with high expression of Ki-67 had lower survival rates, but the difference in survival was not statistically significant.
Association between expression of cyclin D1, p53 and Ki-67 and SPM
Spearman’s rank-order correlation showed positive correlation between p53 and cyclin D1, as well as p53 and Ki-67. Positive expression of p53, cyclin D1 and Ki-67 in double primary malignancy suggests poor differentiation, more aggressive invasiveness and early metastasis, and it’s likely to be a clinical significant prognosis factor. The prognostic use of p53, cyclin D1 and Ki-67 expression should be further confirmed in an adequately-powered prospective cohort.
Conclusion
Recent studies suggest that proteins regulating cell-cycle are closely related to those affecting malignant transformation. Oncogene and anti-oncogene dysregulation, and dysfunction of cell-cycle mediators play an important role in the process of malignant transformation, invasion, and metastasis. Investigating the expression of these genes is important to reveal the mechanisms leading to second primary malignancies. High expression rate of p53, cyclin D1 and Ki-67 proteins was highly associated with malignant behavior of the second primary malignancy, and their detection has the potential to become a useful clinical prognostic marker. Further prospective studies are required to determine their prognostic ability.
Disclosure of conflict of interest
All authors state no conflict of interest.
References
- 1.Howe HL, editor. A Review of the Definition for Multiple Primary Cancers in the United States. Workshop Proceedings; 2002 December 4-6; Princeton, New Jersey. Springfield (IL): North American Association of Central Cancer Registries; 2003. May, [Google Scholar]
- 2.Chengfeng W, Yongfu S, Haizeng Z. Chinese Journal of Clinical Oncology. 2000:27–30. [Google Scholar]
- 3.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 4.Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 5.Zumsteg ZS, Spratt DE, Pei I, Zhang Z, Yamada Y, Kollmeier M, Zelefsky MJ. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Huang XY, Huang ZL, Huang J, Wang ZG, Zheng Q. A case of multiple primary malignancies and investigation of family history. Oncol Lett. 2012;4:931–934. doi: 10.3892/ol.2012.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen D, Wang S, Zhang L, Wei L, Zhou W, Peng Q. Early onset, multiple primary malignancies, and poor prognosis are indicative of an inherited predisposition to esophageal squamous cell carcinoma for the familial as opposed to the sporadic cases--an update on over 14-year survival. Eur J Med Genet. 2009;52:381–5. doi: 10.1016/j.ejmg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw HD, Smith A, Rungruang B, Kelley JL Jr, Beriwal S, Krivak TC, Sukumvanich P, Olawaiye AB. The risk of subsequent malignancies in women with uterine papillary serous or clear cell endometrial cancers. Int J Gynecol Cancer. 2013;23:1044–9. doi: 10.1097/IGC.0b013e3182959053. [DOI] [PubMed] [Google Scholar]
- 9.Yang JG, Li XX, Kong FM. Clinical features analysis of multiple primary carcinoma. Modern Oncol. 2011;19:2251–2253. [Google Scholar]
- 10.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 11.Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A. “A novel cyclin encoded by a bcl1-linked candidate oncogene”. Nature. 1991;350:512–5. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 12.Bates S, Peters G. cyclin D1 as a cellular proto-oncogene. Semin Cancer Biol. 1995;6:73–82. doi: 10.1006/scbi.1995.0010. [DOI] [PubMed] [Google Scholar]
- 13.Rhee J, Han SW, Oh DY, Kim J, Im SA, Han W, Ae Park I, Noh DY, Bang YJ, Kim TY. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8:307. doi: 10.1186/1471-2407-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlos de Vicente J, Herrero-Zapatero A, Fresno MF, López-Arranz JS. Expression of Fascin and ki-67 in squamous cell carcinoma of the oral cavity: clinicopathological and prognostic significance. Oral Oncol. 2002;38:301–8. doi: 10.1016/s1368-8375(01)00060-4. [DOI] [PubMed] [Google Scholar]
- 15.Oka S, Uramoto H, Shimokawa H, Iwanami T, Tanaka F. The expression of ki-67, but not proliferating cell nuclear antigen, predicts poor disease free survival in patients with adenocarcinoma of the lung. Anticancer Res. 2011;31:4277–4282. [PubMed] [Google Scholar]


