Abstract
Increasing evidences reveal that Aurora-B may be involved in metastasis of malignant tumor. In this study, we investigated the inhibitory effect of Aurora-B on invasion and migration of OS cells and the activity of PI3K/Akt/NF-κB signaling pathway in vitro. The expression of Aurora-B and p-Akt (Ser473) proteins was detected by immunohistochemistry in OS tissues from 24 patients with pulmonary metastatic disease, and the relationship between Aurora-B and p-Akt was investigated. The results showed that there was a positive correlation between Aurora-B and p-Akt protein expression. Furthermore, we down-regulated the expression of Aurora-B through a recombinant lentivirus (Lv-shAURKB). Migration and invasion of cells were investigated by wound healing and transwell invasion assays. Results showed that silencing Aurora-B inhibited cell migratory and invasive ability of OS cells in vitro. Finally, knockdown of Aurora-B suppresses the activity of PI3K/Akt/NF-κB signaling pathway in OS cells. Our results indicated that knockdown of Aurora-B suppresses OS cells migratory and invasive ability via modulating the “PI3K/Akt/NF-κB” signaling pathway in vitro. The Aurora-B blocker may be a new therapeutic strategy in OS management.
Keywords: Osteosarcoma, Aurora-B, invasion and migration, PI3K/Akt/NF-κB signaling pathway
Introduction
Osteosarcoma (OS) is the most common human primary malignant bone tumor among children and adolescents. With the advent of effective chemotherapy, the five-year survival rate for OS patients has been reported at 55-80% [1-4]. However, numerous studies report that the five-year survival rate of patients with metastatic diseases was less than 20% [5-7]. The pulmonary metastasis is the leading cause of death for the patients with extremities OS [8]. Therefore, making clear the molecular mechanism of metastasis of OS to improve the curative effect is necessary for the management of OS.
Aurora-B is one of the major protein kinases that ensures the proper execution and fidelity of mitosis. As one of member of the chromosomal passenger complex, Aurora-B has been implicated in various mitotic functions, including chromosome-microtubule interactions, sister chromatid cohesion, the spindle-assembly checkpoint and cytokinesis. Recently, increasing studies reveal that nuclear Aurora-B are strongly associated with metastasis in tumor and is believed to be an important anti-tumor target [9,10]. However, whether Aurora-B is involved in OS metastasis and the potential molecular mechanism are still uncertain.
PI3K/Akt plays a crucial role in the cell- extracellular matrix (ECM) and cell-cell adhesion. Due to lack of correct adhesion, the adhesion-dependent signals are interrupted, resulting in adhesion-related apoptosis, namely anoikis. Recently studies show that the inhibitor of Aurora-B could decrease Akt phosphorylation at Ser473 and its substrates GSK3alpha/beta phosphorylation at Ser21 and Ser9 in cancer cells [11]. The phosphorylation and activation of Akt has been recognized as an important regulatory factor in NF-κB signaling pathway. Substantial studies reveal that activation of the NF-κB gene, the upstream regulator of MMPs, promotes the tumor cell invasion and migration [12,13]. Matrix metalloproteinases (MMPs) are involved in the degradation of the basement membrane and epimatrix, among which MMP-2 and 9 markedly correlate with tumor invasion. MMP-2 and 9 are increased in OS cells and promote OS cells migration and invasion by degrading components of the basement membrane and epimatrix [14]. Therefore, we hypothesize that inhibition of Aurora-B could suppresses OS cell invasion and migration via decreasing the activity of PI3K/Akt/ NF-κB signaling pathway.
In this study, we investigated the effect of inhibition Aurora-B on the activity of PI3K/Akt/ NF-κB signaling pathway, invasion and migration in OS cells.
Materials and methods
Antibodies
Rabbit monoclonal Aurora-B, NF-κB (p65), PI3K, p-PI3K (Tyr199), p-Akt (Ser473), goat monoclonal Akt and mouse monoclonal β-actin were purchased from Cell Signaling Technology Inc.
Patient specimens
A total of 24 samples of OS tissues were obtained from patients with pulmonary metastatic disease who underwent surgery in our hospital (The First Hospital Affiliated to Nanchang University, China) from 2005 to 2012. The pulmonary metastasis survey was performed with plain films and chest CT scans at first diagnosis. All the patients have no history of prior therapies with anticancer drugs or radiotherapy. The samples were fixed with 10% formalin and embedded in paraffin, and then, were cut into 4 μm sections. In all cases, informed consent was taken from related departments and persons, and the study had the approval from the Institute Ethics Committee.
Immunohistochemical analysis
Immunoperoxidase procedure (S-P procedure) and hematoxylin and eosin (H&E) staining were performed on paraffin-embedded sections. Antigen retrieval was performed with heating the sections in 10 mmol/L citrate buffer (pH 6.0) for 20 min. Anti-Aurora-B (1:500) and p-Akt (Ser473) (1:1000) antibodies were used as the primary antibody at a final dilution as corresponding product specifications. Then the sections were chemiluminescence stained and counterstained using hematoxylin. Stained sections were evaluated and scored by two pathologic doctors in a blind manner without prior knowledge of the clinical pathological features of patients. According to the staining intensity by examining at least 500 cells in five representative areas, the expression level of Aurora-B and p-Akt protein was judged and the intensity scores were recorded as follows: none, 0; weak, 1; moderate, 2; and intense, 3. According to the percentage of tumor cells with positive expression of p-Akt and Aurora-B protein, the percentage scores were recorded: 0% (score 0); less than 10% (score 1), 11-50% (score 2), 51-80% (score 3), and 81-100% (score 4). The final score was averaged with the scores from the two pathologic doctors; these scores were calculated by multiplying the intensity score to the percentage score.
Construction of AURKB-shRNA-expressing lentiviral vector
The shRNAs targeting Aurora-B mRNA were designed online (Hwww.invitrogen.com/rnaiH): homo-shRNA-AURKB-1, 5’-AGAGCTGCACATTTGACGA-3’; homo-shRNA-AURKB-2, 5’-TGCGTCTCTACAACTATTT-3’; homo-shRNA-AURKB-3, 5’-ACCTCCTCCTTTGTTTAAT-3’; shRNA-NC, 5’-TTCTCCGAACGTGTCACGT-3’, was used as control. The lentivirus expression plasmid (pGC-LV-AURKB or pGC-LV-control vector), together with pHelper 1.0 and pHelper 2.0 plasmids that contained the imperative elements for virus packaging, were co-infected into 293T cells with lipofectamine 2000, according to the manufacturer’s instructions for the generation of AURKB-shRNA lentivirus (LV-AURKB-1, LV-AURKB-2 and LV-AURKB-3) or control lentivirus (LV-NC). Lentivirus was harvested at 48 h post-infection, centrifuged to get rid of cell debris, and then filtered through 0.45 μm cellulose acetate filters followed by ultracentrifugation.
Cell culture and infection
The human OS cell line U2-OS and HOS were purchased from American Type Culture Collection (Manassas, VA), and routinely cultured in RPMI-1640 (HyClone) supplemented with 10% fetal bovine serum (Sigma) in a humidified 37°C incubator containing 5% CO2. The U2-OS and HOS cells were grown to 30-40% confluence and infected with LV-AURKB or LV-NC at MOI of 12.5 and 25. In order to determine the infection efficiency, cells expressing GFP protein were observed using fluorescence microscopy (ECLIPSE-TS-100, Nikon, Japan) 3 days after infection.
qRT-PCR
The OS cell total RNA was isolated after inflected 72 h with Trizol reagent (Invitrogen, USA). MMLV Reverse Transcriptase and oligo (dT) were used to primer Reverse transcription Relative .Levels of Aurora-B mRNA were examined using SYBR green real-time quantitative reverse transcription-PCR (qRT-PCR) (Applied Biosystems) and normalized with β-actin. All amplifications were performed in the final reaction mixture (20 μl). Primer sequences used to amplify the containing were as follows: Aurora-B sense 5’-AGAAGGAGAACTCCTACCCCT-3’, Aurora-B antisense 5’-CGCGTTAAGATGTCGGGTG-3’; β-actin sense 5’-CGGGAAATCGTGCGTGAC-3’, β-actin antisense 5’-TGGAAGGTGGACAGCGAGG-3’. The amplification reaction was performed using MJ real-time PCR (Bio-Rad, Hercules, CA, USA) for 40 cycles. Relative expression was calculated using the 2-ΔΔCt method.
Western blot assay
Total protein from the cells was extracted using RIPA lysis buffer containing 60 μg/ml PMSF. Protein concentrations were determined by BCA protein assay kit (Boster, China). The protein samples were denatured at 100°C for 10 min and then preserved at -20°C for later use. The protein samples were separated by 8% SDS-polyacrylamide gels and transblotted onto Nitrocellulose blotting membrane (0.22 μm). Membranes were blocked with 5% skim milk for 1 h at room temperature, and probed with primary antibodies (rabbit anti-Aurora-B IgG, 1:5000; rabbit anti-PI3K, anti-p-PI3K (Tyr199), anti-NF-κB (p65) and anti-p-Akt (Ser473) IgG, 1:1000; goat anti-AKT IgG, 1:1000; mouse anti-β-actin, 1:2000) overnight at 4°C. After incubation with the appropriate anti-rabbit, anti-goat or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000, Boster, China) for 1.5 hours at room temperature, immunoreactive bands were visualized by the chemiluminescence dissolvent (Thermo, USA) and exposed to the X-ray film (Kodak, USA). The determination of grayscale value was processed by Image J. All experiments were repeated by six times over multiple days.
Transwell assay
Invasion of OS cells was measured using the BD BioCoatTM BD MatrigelTM Invasion Chamber (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. The medium in the lower chamber contained 5% fetal calf serum as a source of chemo attractants. Cultures were rinsed with PBS and replaced with fresh quiescent medium alone or containing 10% FBS, following which the cells were incubated at 37°C for 24 h. Cells that passed through the Matrigel-coated membrane were stained with Diff-Quik (Sysmex, Kobe, Japan) and photographed under a microscope (ECLIPSE-TS-100, Nikon, Japan; magnification, 400×). Cell migration was quantified by direct microscopic visualization and counting. The values for invasion were obtained by counting three fields per membrane and represented as the average of six independent experiments done over multiple days.
Wound healing assay
We assessed cell migration by determining the ability of the cells to move into a cellular space in a two-dimensional in vitro “wound healing assay”. In brief, cells were grown to confluence in 6-well tissue culture plastic dishes to a density of approximately 5×106 cells/well. The cells were denuded by dragging a rubber policeman (Fisher Scientific, Hampton, NH, USA) through the center of the plate. Cultures were rinsed with PBS and replaced with fresh quiescent medium alone or containing 10% FBS, following which the cells were incubated at 37°C for 24 h. Images were captured under a microscope (ECLIPSE-TS-100, Nikon, Japan; magnification, 200×) at 0 and 24 h, and the migrated distance was measured using Image J (NIH, Bethesda, MD, USA). The cells migration rate was obtained by counting three fields per area and represented as the average of six independent experiments done over multiple days.
Statistical analysis
The correlation of Aurora-B with p-Akt protein in OS tissues was evaluated using the Wilcoxon rank Sum Test. All measurement data were presented as x̅ ± SD and analyzed by one-way ANOVA. A value of P<0.05 was considered as a significant difference. All analysis was performed with SPSS Version 13.0 (SPSS Inc, Chicago, IL, USA).
Results
Positive correlation between Aurora-B and p-Akt (Ser473) protein expression in tissues of OS with pulmonary metastasis
In order to investigate the relationship between Auror-B and p-Akt in OS tissues which exist pulmonary metastatic disease, the Aurora-B and p-Akt protein in 24 samples from patients with pulmonary metastatic disease was detected by immunohistochemistry. Aurora-B was expressed in the nucleus and the p-Akt protein was expressed in the nucleus and cytoplasm (Figure 1A-C). There was a significant positive relationship between Aurora-B and p-Akt expression (R = 0.726, P = 0.02). These data suggest that a possible connection between Aurora-B expression and the phosphorylation of Akt exists in OS.
Figure 1.
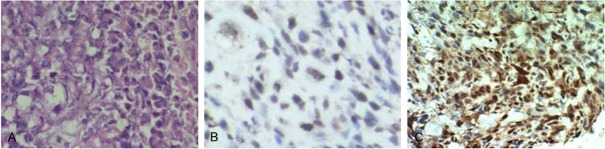
Representative images of HE staining and immunohistochemical staining of Aurora-B or p-Akt protein in OS. A: Cells in osteosarcoma (OS) tissues with pulmonary metastasis were polygonal and short spindle, with large pleomorphic nuclei and abundant cytoplasm. B: Aurora-B protein was showed brownish-yellow particle deposition and expressed in the nucleus. C: p-AKT protein was shown as brownish-yellow particle deposition and expressed in the cytoplasm and nucleus.
Specific shRNA lentivirus inhibited Aurora-B expression in OS cells
In order to investigate effect of inhibition Aurora-B in the subsequent experiments, the LV-AURKB was used to suppress Aurora-B expression in U2-OS and HOS cells. The result of qRT-PCR and western blot assays show that the Aurora-B protein and mRNA was significantly lower in cells infected by LV-AURKB than in those infected by LV-NC (Figure 2A, 2B).
Figure 2.

The specific shRNA lentivirus suppressed the expression of Aurora-B in OS cells. A: The expression of Aurora-B protein was measured by western blot. The result showed that Aurora-B was significantly blocked in positive groups compared with control group. B: The mRNA level of Aurora-B was detected by qRT-PCR. The data indicated that the Aurora-B mRNA was also inhibited both in U2-OS and HOS infected by LV-AURKB compared by LV-NC. Columns, mean (n = 6); bars, SD; *P<0.05 VS LV-NC group.
Silencing Aurora-B decreased PI3K/Akt/NF-κB signaling pathway in OS cells
In order to investigate the effect of inhibiting Aurora-B on the activity of PI3K/Akt/NF-κB signaling pathway in OS cells, the U2-OS and HOS cells were infected with LV-AURKB or LV-NC for 8 h, and then cultured for 72 h. The protein of p-PI3K (Tyr199), PI3K, AKT, p-Akt (Ser473) and NF-κB (p65) was measured using Western blot analysis. Results revealed that PI3K, p-PI3K (Tyr199), and p-Akt (Ser473), NF-κB (p65) protein expression in cells infected with LV-AURKB was significantly lower than cells treated with LV-NC (Figure 3).
Figure 3.

Inhibiting Aurora-B suppressed the expression of p-PI3K, PI3K, p-Akt and NF-κB proteins in U-2 OS and HOS cells. The OS cells were infected with LV-AURKB or LV-NC for 8 h, cultured for 72 h, and then lysed. The protein was quantified, separated with 8% SDS-PAGE and assayed by Western-Blot. This is a representative image of the six experiments shown for each groups. It show that with the silencing of Aurora-B, the p-PI3K, PI3K, p-Akt and NF-κB proteins were blockaded in varying degrees, which indicated that Aurora-B may down-regulate the PI3K/Akt/NF-κB signaling pathway.
Inhibiting Aurora-B blocked OS cells invasion and migration
Transwell assays was used to examine the effect of inhibiting Aurora-B on the invasion of OS cells. In transwell invasion assays, the number of invaded cells infected by LV-AURKB was significantly lower than those of cells infected by LV-NC (P<0.05) (Figure 4A). Those data suggested that inhibiting Aurora-B could suppress OS cell invasion in vitro. The migration was measured by “wound healing” assay and the results showed that the migrated rate of OS cells infected by LV-NC, LV-AURKB-1, LV-AURKB-2 and LV-AURKB-3 was as follows: 90 ± 4.5%, 40 ± 7.5%, 42 ± 6.0% and 35 ± 5.6% (U2-OS); 93 ± 4.0%, 60 ± 7.0%, 53 ± 6.5% and 46 ± 5.4% (HOS). The difference was significant (P<0.05) (Figure 4B). These data showed that inhibiting Aurora-B could suppress the migration of OS cell in vitro.
Figure 4.
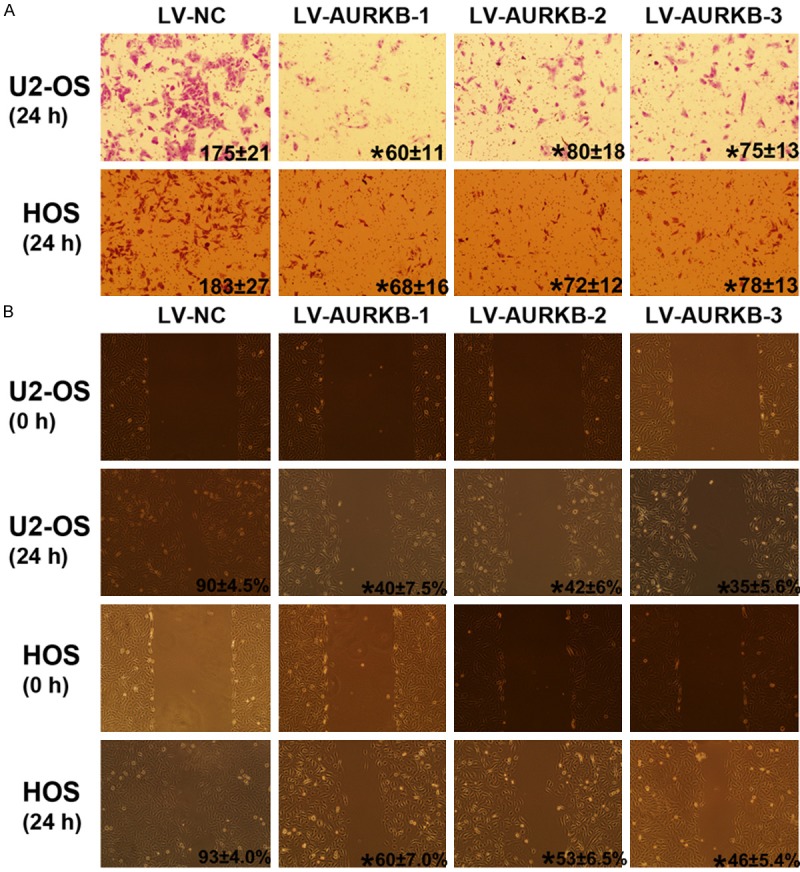
Inhibiting Aurora-B blocked OS cells invasion and migration. A: Cellular invasion through Matrigel-coated transwells is shown for each group. The number of cells invading through the membrane was on the lower right corner. It indicated that cell invasion was significantly inhibited by Silencing Aurora-B. *P<0.05 versus LV-NC group. B: A representative image of the wound healing assays is shown for each group. The migration rate on the lower right corner of micrograph (24 h) indicated the migration of OS cells is significantly inhibited by Silencing Aurora-B. *P<0.05 VS LV-NC group.
Discussion
Aurora kinases are serine/threonine kinases essential for cell cycle control and mitosis. Mammals have three Aurora kinase family members (A, B and C), and these kinases are expressed at maximum levels during mitosis. Aurora B, part of the chromosome passenger complex (CPC), is located on the chromosome arms during prophase and at the centromeres during prometaphase and metaphase, and subsequently localizes to the midbody during cytokinesis [15]. Aurora-B has been shown to be over-expressed in many types of tumors [16,17]. Various studies reveal that inhibition Aurora-B could block cell proliferation and induce cell apoptosis in varieties tumors [18,19]. These findings have led to an interest in Aurora-B as molecular targets for cancer treatment. Interesting, recently studies show that the up-regulated expression was associated with tumor cells metastasis, and down-regulating Aurora-B could inhibit cell invasion and migration in various tumor [19,20]. However, the effect of inhibition Aurora-B on the metastatic behaviors of OS cells remains to be lack of fully elucidation. In this study, to explore the effect of inhibition Aurora-B on OS cells migration and invasion, the plasmid targeting Aurora-B was used to inhibit Aurora-B express in U2-OS and HOS cells, and the results showed that the migrated and invaded cells was significantly lower in cells infected by LV-AURKB than those infected by LV-NC. It indicated that knockdown of Aurora-B could suppress OS cell migration and invasion in vitro.
In this study, the potential molecular mechanism associated with Aurora-B inhibiting OS cells migration and invasion was also analyzed. The role of the PI3K/Akt/NF-κB signaling pathway in tumors cell survival, angiogenesis, differentiation, growth and metastasis was confirmed [21]. Yao JE showed that Aurora-A kinase, via activating Akt, stimulated nuclear factor-kappaB signaling pathway to promote cancer cell survival [22]. To investigate the potential connection between Aurora-B and Akt activity, the Aurora-B and p-Akt protein expression in 24 samples from OS patients with pulmonary metastatic disease was detected by IHC. Unfortunately, with the emergence of new adjuvant chemotherapy, the number of OS tissue samples meeting the requirement of this research, which had not received chemotherapy, is very limited, and only 24 samples were collected in this study. The results showed that the positive expression of Aurora-B was observed in nucleus and p-Akt protein was located in cytoplasm and nucleus. There was a significant relationship between Aurora-B and p-Akt protein expression. It suggested that a possible connection between Aurora-B expression and the phosphorylation of Akt exists in OS.
Therefore, we speculated that knockdown of Aurora-B could decrease activation of Akt in OS cell. To define our hypothesis, the Aurora-B expression in OS cells was inhibited by RNAi. The results show the protein of p-Akt was significant decreased in cells transfected by LV-AURKB when compared with those in cell infected by LV-NC. The phosphorylation of Akt has been recognized as an important regulatory factor in regulation NF-κB activation. Specifically, activation of Akt has been identified to be essential for degradation of an inhibitor of NF-κB, inhibitor of κB (IκB) and NF-κB activation mediated by IκB kinases (IKKs). NF-κB is composed of DNA-binding subunits (p50 and p52) and subunits with transcriptional activity (p65 and RelB or c-Rel), which dimerize in various combinations. The primary form of NF-κB is a heterodimer of the p50 and p65 subunits and is localized mainly to the cytoplasm in an inactive form bound to IκB. Substantial studies reveal that activation of the NF-κB gene play an important role by regulation expression of Matrix metalloproteinases (MMPs) [12,13]. Elevated expression of MMP-2 and MMP-9 promote OS cells metastasis by degrading components of the basement membrane and epimatrix [14]. In this study, the activity of NF-κB was measured by evaluating the effect of decreasing phosphorylation of Akt by silencing Aurora-B on NF-κB (p65) protein expression in OS cells. The results revealed that the expression of NF-κB (p65) protein was significantly inhibited by decreasing Akt phosphorylation mediated by knockdown of Aurora-B.
Taking together, our findings indicate that inhibition of Aurora-B may suppress OS cells invasion and migration via modulation of the PI3K/Akt/NF-κB pathway in vitro. However, further experiments in vivo are necessary to be performed to make it clear whether the Aurora-B blockers could represent a new molecular strategy in the management of OS with metastases.
Acknowledgements
The present study was supported by a grant from Jiangxi Province Education Department of Science and Technology (No. GJJ12097) and the National Natural Science Foundation of China (No. 81260400) and the National Natural Science Foundation of Jiangxi Province (No. 20142BAB205056).
Disclosure of conflict of interest
None.
References
- 1.Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561–2567. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 3.Jawad MU, Cheung MC, Clarke J, Koniaris LG, Scully SP. Osteosarcoma: improvement in survival limited to high-grade patients only. J Cancer Res Clin Oncol. 2011;137:597–607. doi: 10.1007/s00432-010-0923-7. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles AS, Hartmann O. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104:1100–1109. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- 6.Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss J, Szendroi M, Csoka M, Kovacs G. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57:415–422. doi: 10.1002/pbc.23172. [DOI] [PubMed] [Google Scholar]
- 7.Stokkel MP, Linthorst MF, Borm JJ, Taminiau AH, Pauwels EK. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J Cancer Res Clin Oncol. 2002;128:393–399. doi: 10.1007/s00432-002-0350-5. [DOI] [PubMed] [Google Scholar]
- 8.Guise TA, O’Keefe R, Randall RL, Terek RM. Molecular biology and therapeutics in musculoskeletal oncology. J Bone Joint Surg Am. 2009;91:724–732. doi: 10.2106/JBJS.I.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonet C, Giuliano S, Ohanna M, Bille K, Allegra M, Lacour JP, Bahadoran P, Rocchi S, Ballotti R, Bertolotto C. Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells. J Biol Chem. 2012;287:29887–29898. doi: 10.1074/jbc.M112.371682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuncel H, Shimamoto F, Kaneko Guangying Qi H, Aoki E, Jikihara H, Nakai S, Takata T, Tatsuka M. Nuclear Aurora B and cytoplasmic Survivin expression is involved in lymph node metastasis of colorectal cancer. Oncol Lett. 2012;3:1109–1114. doi: 10.3892/ol.2012.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long ZJ, Xu J, Yan M, Zhang JG, Guan Z, Xu DZ, Wang XR, Yao J, Zheng FM, Chu GL, Cao JX, Zeng YX, Liu Q. ZM 447439 inhibition of aurora kinase induces Hep2 cancer cell apoptosis in three-dimensional culture. Cell Cycle. 2008;7:1473–1479. doi: 10.4161/cc.7.10.5949. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang L, Huang W, Huang L, Wang Q. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. 2014;65:24–32. doi: 10.1016/j.cyto.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Lee YD, Cui MN, Yoon HH, Kim HY, Oh IH, Lee JH. Down-modulation of Bis reduces the invasive ability of glioma cells induced by TPA through NF-kappaB mediated activation of MMP-9. BMB Rep. 2014;47:262–7. doi: 10.5483/BMBRep.2014.47.5.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao CL, Lin JH, Lien JC, Hsu SC, Chueh FS, Yu CC, Wu PP, Huang YP, Lin JG, Chung JG. The crude extract of Corni Fructus inhibits the migration and invasion of U-2 OS human osteosarcoma cells through the inhibition of matrix metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol. 2013 doi: 10.1002/tox.21894. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497:118–121. doi: 10.1038/nature12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolanos-Garcia VM. Aurora kinases. Int J Biochem Cell Biol. 2005;37:1572–1577. doi: 10.1016/j.biocel.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Lee EC, Frolov A, Li R, Ayala G, Greenberg NM. Targeting Aurora kinases for the treatment of prostate cancer. Cancer Res. 2006;66:4996–5002. doi: 10.1158/0008-5472.CAN-05-2796. [DOI] [PubMed] [Google Scholar]
- 18.Chen BB, Glasser JR, Coon TA, Mallampalli RK. Skp-cullin-F box E3 ligase component FBXL2 ubiquitinates Aurora B to inhibit tumorigenesis. Cell Death Dis. 2013;4:e759. doi: 10.1038/cddis.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Hu H, Lang Q, Zhang H, Huang Q, Wu Y, Yu L. A thienopyrimidine derivative induces growth inhibition and apoptosis in human cancer cell lines via inhibiting Aurora B kinase activity. Eur J Med Chem. 2013;65:151–157. doi: 10.1016/j.ejmech.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Fields JZ, Opdenaker L, Otevrel T, Masuda E, Palazzo JP, Isenberg GA, Goldstein SD, Brand M, Boman BM. Survivin-induced Aurora-B kinase activation: A mechanism by which APC mutations contribute to increased mitoses during colon cancer development. Am J Pathol. 2010;177:2816–2826. doi: 10.2353/ajpath.2010.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta KB, Tibolla MM, Tiscornia MM, Lorenzati MA, Zapata PD. Recent patents related to phosphorylation signaling pathway on cancer. Recent Pat DNA Gene Seq. 2011;5:175–184. doi: 10.2174/187221511797636257. [DOI] [PubMed] [Google Scholar]
- 22.Yao JE, Yan M, Guan Z, Pan CB, Xia LP, Li CX, Wang LH, Long ZJ, Zhao Y, Li MW, Zheng FM, Xu J, Lin DJ, Liu Q. Aurora-A down-regulates IkappaBalpha via Akt activation and interacts with insulin-like growth factor-1 induced phosphatidylinositol 3-kinase pathway for cancer cell survival. Mol Cancer. 2009;8:95. doi: 10.1186/1476-4598-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]


