Abstract
Granular cell tumors (GCTs) in esophagus are rare tumors lacking of systemic large group reports. In this study, we summarized the clinical characteristics, histological features and therapeutic approaches of 31 cases. GCTs generally located at middle and distal of the esophagus in middle aged and elderly patients with no incidence of gender differences. Histologically, tumor cells were mainly plump and polygonal with abundant, granular, amphophilic or eosinophilic cytoplasm. The growth pattern was solid or nested, usually with minimal infiltration and inflammatory infiltrates and lymphoid aggregation. All GCTs in the present study were benign according to Nasser criteria. Nestin, NSE, CD68 and S100 protein were moderate to strong positive. Moreover, a developmental morphology of a GCT was found, which included areas of relatively normal Schwann cells, transitional cells and typical cells of GCTs. All patients received endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Twenty-six patients were followed up and remained clinically well. In conclusion, GCTs of esophagus are neurogenic origin tumors with favourable prognosis. Definite diagnosis of GCTs relies upon pathological examination. The Nasser criteria for stratification are practical in guiding treatment strategy. ESD is a recommended therapeutic strategy, and the range of application is expanding.
Keywords: Granular cell tumor, esophagus, diagnosis, treatment
Introduction
Granular cell tumors (GCTs) were firstly described by Abrikossoff in the tongue in 1926 [1]. Then, they have been reported in different parts throughout the body, most commonly in oral cavity, skin, and subcutaneous tissue, and less common in the breast, thyroid, respiratory tract, biliary tree, female genital tract, nervous system, and all segments of the gastrointestinal tract [2-6]. Approximately 8% of GCTs develop in the gastrointestinal tract, and the most common site being the esophagus, which is involved in one-third to two-thirds cases [7,8]. In the esophagus, GCTs are most commonly found incidentally during endoscopy. They usually present as a small nodule or plaque with grayish-white to yellow color endoscopically [7-10]. They are generally restricted to the submucosal layer of the esophagus. Endoscopic ultrasound (EUS) is valuable in characterizing these lesions. The most widely accepted theory is that they are neurogenic origin, arising from the Schwann cells, which form part of the submucosal neuronal plexus in the esophagus [8]. Although the majority of GCTs are benign, malignant potential has been described, particularly for larger lesions [7]. The histological criteria of malignancy proposed by Fanburg-Smith are still debatable among pathologists, with metastasis being the sole criterion of malignancy with unanimous agreement [11].
Most GCTs of the esophagus have been reported as small series or single case report, and diagnosis and therapeutic methods varied. In the present study, we summarized clinicopathological characteristics of 31 cases and combined the up-to-date progress trying to propose some accessible approaches in diagnosis and treatment.
Methods and materials
The archives of the department of pathology at Drum Tower Hospital and Veterans Affairs Boston Healthcare System were searched over the period 2003 to 2013 for cases of granular cell tumors occurring in esophagus. Thirty-one cases were identified and reassessed by three experienced pathologists, including 29 cases in Drum Tower Hospital and 2 cases in Veterans Affairs Boston Healthcare System. The medical records of all patients with GCT were reviewed for demographic data, size and site of the tumors, endoscopic ultrasound information, and treatments. For the prognostic evaluation, clinical information was obtained from the follow-up data. The median follow-up time was 18 months (2-54).
The tumor tissues were fixed by 10% neutral buffered formalin and embedded by paraffin, sections of 4 μm thickness were stained with hematoxylin and eosin (HE), immunohistochemistry (IHC) and histochemistry. The IHC stain was performed following EnVision method. The antibodies being used in this study were showed in Table 1. The histochemistry stain was performed with periodic acid–Schiff (PAS). HE slides were reviewed for the following detailed pathologic information: necrosis, atypia, hyperplasia index and mitoses (count per 10HPF).
Table 1.
Antibodies used for immunohistochemistry
| Marker | Supplier | Dilution |
|---|---|---|
| S100 | DakoCytomation (Z0311) | 1:300 |
| CD34 | DakoCytomation (QBEnd/10) | 1:250 |
| CD68 | DakoCytomation (PG-M1) | 1:300 |
| SMA | DakoCytomation (1A4) | 1:300 |
| Desmin | DakoCytomation (D33) | 1:150 |
| CD117 | DakoCytomation (A4502.R) | 1:500 |
| CD8 | DakoCytomation (C8/144B) | 1:400 |
| NSE | DakoCytomation (BBS/NC/V1-H14) | 1:400 |
| Ki67 | DakoCytomation (M1B-1) | 1:400 |
| Nestin | ZSGB-Bio (10C2) | Undiluted |
We evaluated all slides using an Olympus BX 41 microscope by three observers. When discrepancies arose, the cases were reviewed using a multiheaded microscope to achieve a consensus. The Nasser criteria [12] were adopted to evaluate malignancy of these tumors.
Results
The median age of identified patients was 49 years (range, 24 to 71 y) at time of diagnosis. It occurred with equal frequency in male and female (male : female = 16:15). Clinical data showed that most lesions located in middle and distal of the esophagus. Nearly all cases had single lesion, with maximal diameters ranging from 2 mm to 28 mm. Only one patient had two small lesions. Excluding 2 cases with obscure therapeutic information, 12 patients received endoscopic mucosal resection (EMR), and the other 17 patients received endoscopic submucosal dissection (ESD). Bleeding was the only complication that we met, presenting once respectively in patients received EMR and those received ESD. The bleeding was not severe and could be controlled by endoscopic hemostasis. There was no delayed bleeding. Twenty-six patients were followed up, they remained clinically well, without evidences of recurrence or metastasis. Most GCTs located in submucosal of the esophageal wall by endoscopic observation. Clinically, tumor locations were uncertain in 5 cases, therefore, EUS was performed for assistance in diagnosis. As a result, three of the tumors located in submucosal and the other two were muscular propria connected. They were both hypoechoic, homogeneous on EUS. Endoscopic characteristics were showed in Figure 1 and Table 2.
Figure 1.
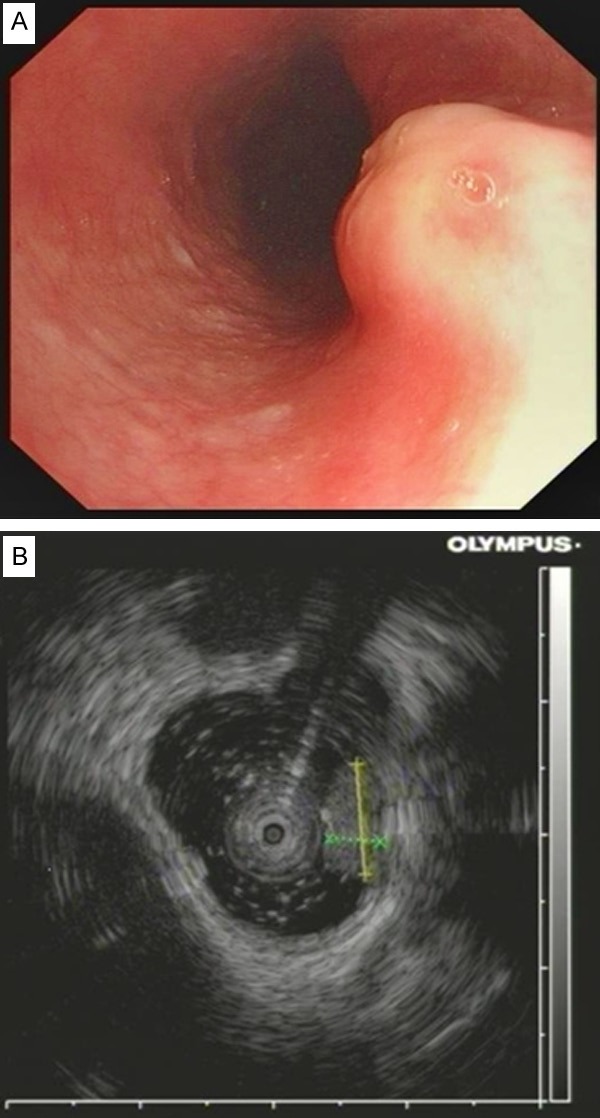
A. The GCT of esophagus presented as a small nodule with grayish-white color endoscopically. B. Endoscopic ultrasound (EUS) showed the GCT was restricted to the submucosal layer of the esophagus.
Table 2.
Results of endoscopy and EUS in 31 patients with GCT
| Endoscopic characteristics | n = 31 | (%) |
|---|---|---|
| Location | ||
| Upper | 4 | 12.9 |
| Mid | 11 | 35.5 |
| Distal | 13 | 41.9 |
| More than one lesion | 1 | 3.2 |
| Not available | 2 | 6.5 |
| Maximum diameter in single patient | ||
| < 10 mm | 19 | 61.3 |
| 10-20 mm | 9 | 29.0 |
| > 20 mm | 1 | 3.2 |
| Not available | 2 | 6.5 |
| Wall layers estimated by EUS* | ||
| Submucosa based | 3 | 60 |
| Muscular propria connected | 2 | 40 |
| Therapeutic management | ||
| EMR | 12 | 38.7 |
| ESD | 17 | 54.8 |
| Surgical resection | 0 | 0 |
| Not available | 2 | 6.5 |
For the 5 cases in which endoscopic ultrasonography (EUS) images were available for review.
Distribution of GCTs in esophagus Layers under microscopy was generally consistent to the result of clinical evaluation. Histologically, all GCTs had normal overlying mucosa (Figure 2A); tumor cells were mainly plump and polygonal with mild or moderate cellularity (Figure 2B); spindling and/or vesicular nuclei with prominent nucleoli could be found in 5 cases (Figure 2D, 2E). The cytoplasm of tumor cells was abundant, granular, amphophilic or eosinophilic (Figure 2B, 2C). The growth pattern was solid or nested, usually with minimal infiltration into adjacent tissues (Figure 2F). Some GCTs had prominent inflammatory infiltrates and lymphoid aggregation (Figure 2F). Mitosis was scarce, necrosis was absent in all cases, thus, they were benign according to Nasser criteria. Interestingly, a developmental morphology of GCT was showed in one case, which included areas of relatively normal Schwann cells (Figure 3A), transitional cells (Figure 3B) and typical cells of GCTs (Figure 3C). The transitional cells generally kept the arrangement pattern of normal Schwann cells, however, the nuclei turned oval and round, and the cytoplasm turned to be granular. Despite of these changes, these transitional cells were general showed a uniform and benign appearance.
Figure 2.
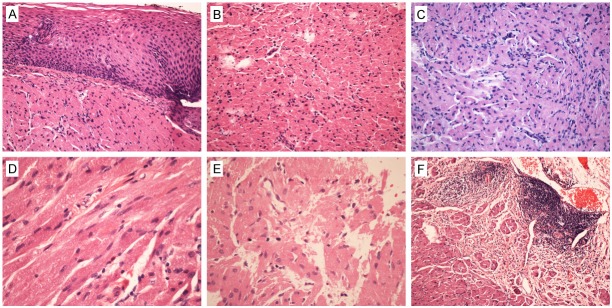
HE stained histological characters of GCTs of esophagus. A. GCTs of esophagus usually had normal overlying mucosa; B. Tumor cells were mainly plump and polygonal with mild or moderate cellularity; D, E. Spindling and/or vesicular nuclei with prominent nucleoli could be found casually. B, C. The cytoplasm of tumor cells were abundant, granular, amphophilic or eosinophilic. F. The growth pattern was solid or nested, usually with minimal infiltration into adjacent tissues and prominent inflammatory infiltrates and lymphoid aggregation.
Figure 3.
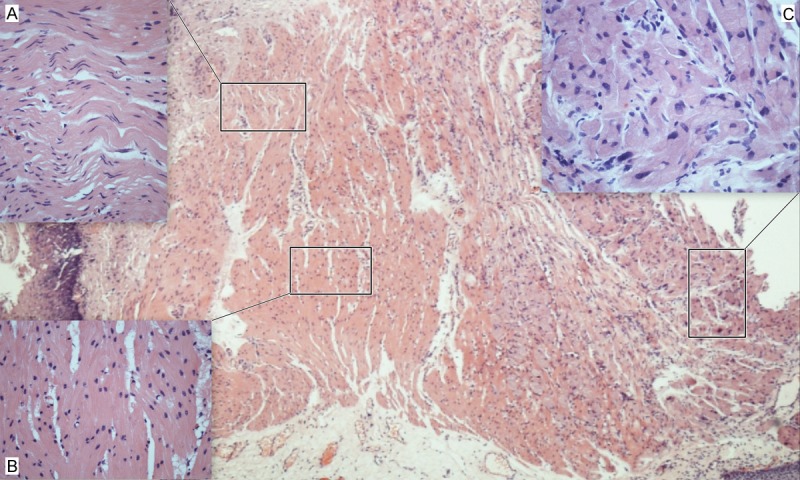
A developmental morphology of a GCT of esophagus. It included areas of (A) relatively normal Schwann cells, (B) transitional cells and (C) typical cells of GCTs. The transitional cells generally kept the arrangement pattern of normal Schwann cells, however, the nuclei turned oval and round, and the cytoplasm turned to be granular.
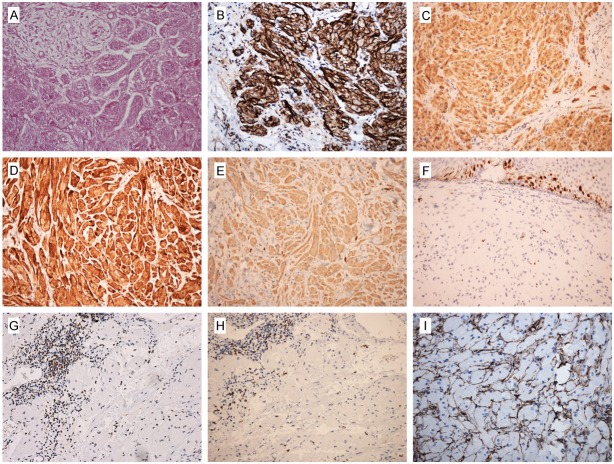
PAS staining was positive and diastase resistant in available 10 cases (Figure 4A). Immunohistochemical results demonstrated that tumor cells were moderate to strong positive staining of Nestin, NSE and S100 protein (Figure 4B-D), and were negative staining of SMA, Desmin, CD117 and CD34. CD68 was typically moderate positive (Figure 4E). Ki67 had a low labelling index (Figure 4F), usually less than 2%. The scattered lymphocytes in tumor stroma or the hyperplastic lymphoid tissues around the tumor were showed positivity of CD45RO (Figure 4G) and CD8 (Figure 4H). Of note, the stroma around the nest of tumor cells were positive for CD34 (Figure 4I).
Figure 4.
Immunohistochemistry (IHC) and histochemistry staining results of GCTs of esophagus. (A) PAS staining was positive and diastase resistant. Tumor cells were moderate to strong positive staining of (B) Nestin, (C) NSE and (D) S100 protein. (E) CD68 was moderate positive. (F) Ki67 had a low positivity. The scattered lymphocytes in tumor stroma or the hyperplastic lymphoid tissues around the tumor were showed positivity of (G) CD45RO and (H) CD8. The stroma around the tumor cells were positive for (I) CD34.
Discussion
The clinical data showed that GCTs occur most commonly in the fourth to sixth decades of life, and generally locate in middle and distal of the esophagus. No gender tendency was observed in this cohort. In a few cases, primary GCTs of the esophagus may occur in multiple sites at the time of initial presentation [13]. In this series, only one case had two adjacent small lesions. If GCTs underlies mucosa, pseudoepitheliomatous hyperplasia may occur, imitating squamous cell carcinoma [14,15], while all our cases had normal overlying mucosa including large lesions. GCTs of the esophagus usually appear as grayish-white to yellow color, sessile mass on endoscopy. They were mainly located in the submucosal layers of the esophagus wall which were hypoechoic, homogeneous and margined on EUS. These findings highly suggest GCTs. Differential diagnosis includes other submucosal tumors, such as leiomyomas arising from the muscularis mucosa, gastrointestinal stromal cell tumors, and metastasis [8,16]. Leiomyomas should especially be considered in differential diagnosis since they are both hypoechoic and margined masses, however, GCTs are usually slightly more echogenic than leiomyomas [13] and display posterior shadowing with a coarse internal echo and high boundary echo [17].
Despite that clinical characters are of great help, the definite diagnosis of GCTs relies upon pathological examination. The histological characteristics of these tumors are distinctive. They are composed by sheet or nested of pump, round or polygonal cells with granular amphophilic or eosinophilic cytoplasm and uniformly small round and pyknotic nuclei. Mitoses and binucleate cells are rarely seen in benign GCTs. PAS staining is positive and diastase resistant. Immunohistochemical staining generally show that GCTs are strongly positive for Nestin, S100, NSE, and various myelin proteins, and negative for SMA, Desmin, CD117 and CD34. It is important to note that the stroma around the tumor cells were positive for CD34. It was reported that GCTs of the gastrointestinal tract express of nestin, a class VI intermediate filament protein present normally in neuroectodermal stem cells and early skeletal muscle cells [18]. This finding suggested that GCTs may arise from a common multipotential stem cell in the digestive tract that has the capability to differentiate between both interstitial cells of Cajal and peripheral nerve pathways. Murakata and Ishak [19] reported that expression of inhibin-α in 100% of 17 gallbladder and extrahepatic biliary GCTs, which was probably useful in differential diagnosis.
The immunohistochemical results imply the neurogenic origin of GCTs. Combining the histological features and immunohistochemical results, it is not difficult to give a correct diagnosis. Interestingly, a developmental morphology recorded the formation process of a GCT, which gave a potent evidence of neurogenic origin.
Histogenesis of malignant granular cell tumor is still vaguely understood. So far, no specific karyotype characterizes these tumors [11,20]. Fanburg-Smith et al proposed six histological criteria for separating of atypical or malignant GCTs [21]. Their criteria are as follows: increased nuclear-to-cytoplasmic ratio; nuclear pleomorphism; necrosis; spindling of tumor cells; vesicular nuclei with prominent nucleoli; and a mitotic count of more than two in 10 high-power fields. They adopted a three-tier classification dividing GCTs into benign (none of the above criteria or focal pleomorphism), atypical (1-2 criteria), and malignant (3-6 criteria). However, some of the proposed criteria, such as pleomorphism and increased nuclear to-cytoplasmic ratio, are subject to interobserver variation and show weak reproducibility among different pathologists complicating the diagnostic spectrum and rendering the separating limits between the different diagnostic subgroups hazy. Nasser et al [12] proposed a simpler and more practical and clear diagnostic criteria. Their diagnostic criteria based on the presence of necrosis (whether single cell or zonal) and/or mitoses (respecting Fanburg-Smith criteria), and designated that tumors without any of the two features as benign granular cell tumors, and tumors demonstrating at least one feature as granular cell tumors with uncertain malignant potential. They considered metastases as the only denominator of malignant cases. Classification of malignant cases did not based on histology alone. All our cases were benign according to Nasser criteria, and 5 cases presenting spindling of tumor cells or vesicular nuclei with prominent nucleoli were classified as atypical GCT following to Fanburg-Smith criteria. We adopt the Nasser criteria in this study because it has clear repercussions on the patient’s treatment strategy. In addition, tumor cells or individual cells usually infiltrate the surroundingt tissues, presenting infiltrative growth pattern. However, we agree with the opinion that the infiltrative feature of the esophageal GCTs, by itself, cannot be considered as a malignant feature [22].
Scattered hyperchromatic degenerate appearing nuclei, inflammatory infiltrates and lymphoid aggregates were often present in GCTs, reminiscent of so-called ‘ancient change’ seen in schwannomas [18]. It was reported that macrophage inflammatory protein-1a (MIP-1a) which belongs to the C-C family of chemokines, is thought to be involved in the recruitment of inflammatory cells and might play a role in the degenerative change of Schwann cell tumors [23]. MIP-1a is a production of pharmacologically stimulated CD4+CD45RA+, CD4+CD45RO+, and CD8+CD45RA+ cells, and the largest amounts of MIP-1a are secreted by CD8+CD45RO+ lymphocytes [24]. We labeled the lymphocytes that in and around esophageal GCTs, as a result, close to half of these cells presented positivity of CD45RO and CD8. This finding suggests the degenerated change of GCTs probably related to these CD8+CD45RO+ lymphocytes.
In contrast to GCTs of the tongue and skin, malignant potential of esophageal GCTs is low [16]. The benign nature of this tumor in esophagus raises questions regarding the need to remove asymptomatic, incidentally detected GCTs. Zhong et al [16] proposed an algorithm of the management of GCTs of esophagus. However, totally endoscopic resection is recommended approach in some Asian countries, and demonstrated to be safe and efficient [25,26]. Twenty-six patients receiving EMR or ESD were followed up in this study, and they remained clinically well, without evidences of recurrence or metastasis. We preferred ESD for its more accurate resection than conventional EMR. ESD is safe and less invasive therapy for smaller tumors, however, we successfully remove an esophageal GCT with maximal diameter of 28 mm, which suggests the feasible application of ESD concerning esophageal GCTs exceeding 20 mm.
In conclusion, GCTs of esophagus are rare tumors. They are neurogenic origin, arising from the Schwann cells, and located in the submucosal layer of the esophagus. Definite diagnosis of GCTs relies upon pathological examination. However, criteria for stratification of them remain disputed. We recommended the Nasser criteria because of its guiding role in treatment strategy. CD8+CD45RO+ lymphocytes may related to the degenerate appearing of GCTs. ESD is a recommended therapeutic strategy for small benign GCTs of esophagus, and a triable approach for large lesions that exceeding 20 mm.
Acknowledgements
We acknowledge Fengan Ding and Ming Chen for the help in data collection. This project was financed by the grants from the National Natural Science Foundation of China (No. 81101815, No. 81201909) and Young Talents Training Project of Health in Nanjing.
Disclosure of conflict of interest
None.
References
- 1.Abrikossoff AI. Uber Myome, augehend von der quergestreiften willkurlichen Muskulatur. Virchows Arch Pathol Anat. 1926;260:214–233. [Google Scholar]
- 2.Lack EE, Worsham R, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Chun B. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 3.Morrison J, Gray G Jr, Dao A, Adkins R Jr. Granular cell tumors. Am Surg. 1987;53:156–160. [PubMed] [Google Scholar]
- 4.Eisen RN, Kirby WM, O’Quinn JL. Granular cell tumor of the biliary tree. A report of two cases and a review of the literature. Am J Surg Pathol. 1991;15:460–465. doi: 10.1097/00000478-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez NG, Mackay B. Granular cell tumor: a review of the pathology and histogenesis. Ultrastruct Pathol. 1999;23:207–222. doi: 10.1080/019131299281545. [DOI] [PubMed] [Google Scholar]
- 6.Johnston MJ, Helwig EB. Granular cell tumors of the gastrointestinal tract and perianal region: a study of 74 cases. Dig Dis Sci. 1981;26:807–816. doi: 10.1007/BF01309613. [DOI] [PubMed] [Google Scholar]
- 7.Radaelli F, Minoli G. Granular Cell Tumors of the Gastrointestinal Tract: Questions and Answers. Gastroenterol Hepatol. 2009;5:798–800. [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima M, Kato H, Muroi H, Sugawara A, Tsumuraya M, Otsuka K, Domeki Y, Onodera S, Sasaki K, Tsubaki M. Esophageal granular cell tumor successfully resected by endoscopic submucosal dissection. Esophagus. 2011;8:203–207. doi: 10.1007/s10388-011-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850–853. doi: 10.1055/s-2007-1004320. [DOI] [PubMed] [Google Scholar]
- 10.Kim DU, Kim GH, Ryu DY, Lee DG, Cheong JH, Lee BE, Song GA, Park DY, Shin NR, Kida M. Endosonographic features of esophageal granular cell tumors using a high-frequency catheter probe. Scand J Gastroenterol. 2011;46:142–147. doi: 10.3109/00365521.2010.525661. [DOI] [PubMed] [Google Scholar]
- 11.Nasser H, Danforth RD, Sunbuli M, Dimitrijevic O. Malignant granular cell tumor: case report with a novel karyotype and review of the literature. Ann Diagn Pathol. 2010;14:273–278. doi: 10.1016/j.anndiagpath.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Nasser H, Ahmed Y, Szpunar SM, Kowalski PJ. Malignant granular cell tumor: a look into the diagnostic criteria. Pathol Res Pract. 2011;207:164–168. doi: 10.1016/j.prp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Lowe DL, Chaudhary AJ, Lee JR, Chamberlain SM, Schade RR, Cuartas-Hoyos U. Four cases of patients with gastrointestinal granular cell tumors. South Med J. 2007;100:298–300. doi: 10.1097/SMJ.0b013e318030eeff. [DOI] [PubMed] [Google Scholar]
- 14.Szumilo J, Dabrowski A, Skomra D, Chibowski D. Coexistence of esophageal granular cell tumor and squamous cell carcinoma: a case report. Dis Esophagus. 2002;15:88–92. doi: 10.1046/j.1442-2050.2002.00232.x. [DOI] [PubMed] [Google Scholar]
- 15.Saito K, Kato H, Fukai Y, Kimura H, Miyazaki T, Kashiwabara K, Nakajima T, Kuwano H. Esophageal granular cell tumor covered by intramucosal squamous cell carcinoma: report of a case. Surg Today. 2008;38:651–655. doi: 10.1007/s00595-007-3694-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhong N, Katzka D, Smyrk T, Wang K, Topazian M. Endoscopic diagnosis and resection of esophageal granular cell tumors. Dis Esophagus. 2011;24:538–543. doi: 10.1111/j.1442-2050.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama K, Kamio T, Hirano A, Seshimo A, Kameoka S. Granular cell tumors: a report of six cases. World J Surg Oncol. 2012;10:204. doi: 10.1186/1477-7819-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfitt J, McLean C, Joseph M, Streutker C, Al-Haddad S, Driman D. Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology. 2006;48:424–430. doi: 10.1111/j.1365-2559.2006.02352.x. [DOI] [PubMed] [Google Scholar]
- 19.Murakata LA, Ishak KG. Expression of inhibin-a by granular cell tumors of the gallbladder and extrahepatic bile ducts. Am J Surg Pathol. 2001;25:1200–1203. doi: 10.1097/00000478-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Rickert CH, Paulus W. Genetic characterization of granular cell tumours. Acta Neuropathol. 2002;103:309–312. doi: 10.1007/s00401-002-0516-x. [DOI] [PubMed] [Google Scholar]
- 21.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Chatelain D, Terris B, Molas G, Belghiti J, Degott C, Flejou J. Infiltrating granular cell tumor of the esophagus: a description of two cases. Ann Pathol. 2000;20:158–162. [PubMed] [Google Scholar]
- 23.Mori K, Chano T, Yamamoto K, Matsusue Y, Okabe H. Expression of macrophage inflammatory protein - 1α in Schwann cell tumors. Neuropathology. 2004;24:131–135. doi: 10.1111/j.1440-1789.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 24.Conlon K, Lloyd A, Chattopadhyay U, Lukacs N, Kunkel S, Schall T, Taub D, Morimoto C, Osborne J, Oppenheim J. CD8+ and CD45RA+ human peripheral blood lymphocytes are potent sources of macrophage inflammatory protein 1α, interleukin - 8 and RANTES. Eur J Immunol. 1995;25:751–756. doi: 10.1002/eji.1830250319. [DOI] [PubMed] [Google Scholar]
- 25.Xu GQ, Chen HT, Xu CF, Teng XD. Esophageal granular cell tumors: Report of 9 cases and a literature review. World J Gastroenterol. 2012;18:7118. doi: 10.3748/wjg.v18.i47.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komori K, Akahoshi K, Tanaka Y, Motomura Y, Kubokawa M, Itaba S, Hisano T, Osoegawa T, Nakama N, Iwao R. Endoscopic submucosal dissection for esophageal granular cell tumor using the clutch cutter. World J Gastrointest Endosc. 2012;4:17. doi: 10.4253/wjge.v4.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]