Abstract
Purpose: The aim of this study was to make a comparative analysis of the possible different expression of Th22 cells in two subtypes of autoimmune thyroid diseases (AITDs), i.e., Graves’ disease (GD) and Hashimoto’s thyroiditis (HT). Methods: We recruited 61 AITDs patients (31 GD and 30 HT) and 22 controls. Serum level of IL-22 was measured by enzyme linked immunosorbent assay (ELISA). The proportion of Th22 cells in peripheral blood mononuclear cells (PBMCs) was analyzed by flow cytometry. The messenger RNA (mRNA) expressions of IL-22, its receptors (IL10R2, IL22R1) and key transcription factor (aryl hydrocarbon receptor, AHR) in PBMCs were assayed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Several cytokines of the cultured PBMCs were also measured under IL-22 stimulation. Results: The proportion of Th22 cells, serum IL22 level and IL-22 mRNA expression were significantly higher in patients with GD than in healthy controls. Additionally, AHR increased in GD patients compared to healthy controls. However, the elevation of Th22 cells and their relative cytokines was not found in patients with HT. Consistent with specific mRNAs expression of cultured PBMCs, IL-4 increment in supernatant was much higher in GD group than in control group, while IFN-γ levels were decreased under IL-22 stimulation. Conclusion: Th22 cells may participate in the pathogenesis of AITDs as a proinflammatory factor, especially in GD, through expressing and secreting IL-22.
Keywords: Autoimmune thyroid diseases (AITDs), Graves’ disease (GD), Hashimoto’s thyroiditis (HT), T helper 22 (Th22) cell, interleukin 22 (IL-22)
Introduction
Autoimmune thyroid diseases (AITDs), which mainly include Graves’ disease (GD) and Hashimoto’s thyroiditis (HT), are a group of immune-mediated thyroid disorders and characterized by autoantibodies against thyroid antigens, such as TSH receptor (TSHR), thyroglobulin (Tg) and thyroid peroxidase (TPO). Although our studies and others found these two main subtypes of AITDs share some common susceptible genes and may aggregate in the same family [1,2], both of them have big differences not only in clinical manifestation but also in pathophysiology. For instance, GD is characterized by anti-TSHR antibody (TRAb) mediated thyrocyte hyperplasia and hyperthyroidism, while HT is predominated by tissue destruction and the sequent hypothyroidism. Etiologically, GD is thought to be predominated by Th2 lymphocyte [3]; in contrast, HT has a bias towards Th1 profile [4]. The differential T lymphocyte hyperproliferation underlying distinct categories of AITDs may have implication in developing new therapeutic approaches. For example, enhanced IL-10 expression, one typical Th2 producing cytokine, by gene therapy has already shown disease-ameliorating effect on HT animal model [5]. That is why the variants between GD and HT draw great attention and become a hot issue in endocrinology area. In fact, we have demonstrated that the newly defined functioning T help cell, i.e., Th17, varies in GD and HT [4].
T helper 22 cells (Th22), which have a stable effective factor system and specific biological function, is another newly identified T cell subtype. IL-22, as the dominant functional cytokine of Th22 cells, which belongs to the IL-10 cytokine superfamily, was once named as IL-10 related T cell-derived inducible factor (IL-TIF). The functional IL-22 receptor consists of two receptor subunits, IL22R1 and IL10R2. Aryl hydrocarbon receptor (AHR) is the key transcription factor of IL-22. Of note, many researches have found that Th22 cell and its effective factor IL-22 not only play vital roles in host defense against exogenous microorganisms [6,7], but also are implicated in the pathology of many autoimmune inflammatory diseases like rheumatoid arthritis [8], multiple sclerosis [9], immune thrombocytopenia [10] and ankylosing spondylitis [11]. Meanwhile, it has been found that the role of the novel cytokine IL-22 in different autoimmune diseases can be beneficial or detrimental [8,12].
Two recent studies preliminarily reported that the frequency of circulating Th22 cells is higher in newly-onset patients with GD [13] and HT [14]. However, in the present study, we found GD has a different Th22 pattern from that of HT. Additionally, we also primitively explored the possible role of Th22 in the development of AITDs.
Subjects and methods
Subjects
A total of 31 patients (8 males and 23 females) with newly diagnosed GD and 30 HT patients (3 males and 27 females) were enrolled from the Outpatient Department of Jinshan Hospital, Fudan University (Shanghai, China). Another 22 age- and sex-matched healthy controls (5 males and 17 females) were collected from the Health Check-Up Center of the same hospital. All the subjects including AITDs patients (AITDs group) and normal controls (NC group) were of Chinese Han population and unrelated to each other.
GD patients were diagnosed by clinical manifestations and biochemical assessments of hyperthyroidism and the positive circulating TSH receptor antibody (TRAb), with or without positive antibody against thyroid peroxidase (TPOAb) or antibody against thyroglobulin (TgAb) or diffuse goiter of the thyroid. HT was defined based on the high level of either TPOAb or TgAb, with or without clinical and biochemical hypothyroidism, the presence of an enlarged thyroid. HT in a minority of patients was also confirmed by fine needle aspiration biopsies. Only the ones with negative thyroid antibodies were recruited in NC group, and others with acute or chronic diseases and medication history during the past three months were excluded in this group.
Written informed consent was obtained from all the subjects, and the research protocol was approved by the Ethics Committee of Jinshan Hospital.
Sample preparation
Peripheral blood samples were collected in coagulant-containing tubes and heparin sodium-containing tubes, respectively, at room temperature. Then serum samples were separated through centrifugation, while peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation with lymphocyte separation medium (Tianjin Haoyang Company, China). Serum supernatant was stored at -80°C until use. The harvested PBMCs were stored in liquid nitrogen before use. After they were cultured for 48 h, PBMCs and the supernatant were collected for the next arrays.
ELISA
IL-22 concentration in the serum samples was validated using the ELISA kit (R&D Systems, USA). 100 μl serum samples of each patient and each control were used according to the manufacturer’s protocol. Samples were coded until the end of the experiment so that the experimenters were unaware of the group designation during the procedure. A standard curve was prepared by serial dilution of purified recombinant protein supplied with the kit. 100 μl standards or serum samples (in duplicate) were added to the appropriate well in a 96-well plate containing immobilized antibodies specific to IL-22. Samples were incubated for 2 h at room temperature, with gentle shaking. After adding 200 μl of IL22 conjugate to each well, they were mixed and incubated for 2 h. Following extensive washing, bound IL-22 was detected using a horseradish peroxidase (HRP)-conjugated monoclonal antibody against the target protein. The detection antibody was incubated for 30 min at room temperature and detected using hydrogen peroxide and chromogen (tetramethylbenzidine). The reaction was terminated by addition of sulfuric acid provided in the kit. The resultant optical density was detected with an enzymes labeling reader at 450 nm with a wavelength correction reading at 540 nm. The concentrations of the unknowns were calculated from the standard curve.
Flow cytometric analysis
Fifty thousand to one million PBMCs with 1 ml of Roswell Park Memorial Institute (PRMI)-1640 medium was incubated for 6 h at 37°C in 5% CO2 in the presence of 50 ng/ml of phorbol myristate acetate (PMA), 1 μg/ml of ionomycin and 0.7 μl/ml of monensin (all from BD Biosicence Pharmingen, San Diego, CA).
PMA and ionomycin were pharmacologic T cell-activating agents that mimic signals generated by the T cell receptor (TCR) complex and help to stimulate T cells of any antigen specificity. Monensin was used to block the intracellular transport mechanisms, thereby leading to an accumulation of cytokines in the cells.
After incubation, the cells were stained with PE-Cy7-conjugated anti-CD4 monoclonal antibodies at 4°C in the dark for 20 min. The cells were then stained with FITC-conjugated anti- IFN-γ monoclonal antibodies, PE-conjugated anti-IL-17A monoclonal antibodies, APC-Cy7-conjugated anti-IL-4 monoclonal antibodies, and APC-conjugated anti-IL-22 monoclonal antibodies for 30 min after fixation and permeabilization (20 min). Isotype controls were used to ensure correct compensation and to identify antibody specificity. Stained cells were measured by flow cytometric analysis using an FACS Calibur cytometer equipped with CellQuest software (BD Bioscience Pharmingen, San Diego, CA). All the above antibodies were obtained commercially from BD (BD Biosicence Pharmingen, San Diego, CA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from PBMCs using Trizol (Invitrogen, USA) according to the introduction. The RNA was quantified and 1 μg was used for synthesizing cDNA through the reverse-transcription kit (TaKaRa, Japan). Reverse transcription reaction was accomplished at 37°C for 15 min, 85°C for 5 s. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in 96 well plate format using SYBR Green (TaKaRa, Japan) with each 10 μl reaction containing approximately 50 ng cDNA, 0.2 μM of forward and reverse primers, and 1× SYBR Premix Ex Taq II. The plate was sealed and cycled under the following conditions: 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 31 s. Each reaction was performed in triplicates. In order to normalize PCR, mRNA levels of the house-keeping gene β-actin (also known as ACTB) were used. PCR efficiencies were determined from a slope of a standard curve generated by ten-fold dilution series of the DNA templates. Amplification efficiency of all the genes was almost the same through evaluating the standard curve, so fold induction was determined from Ct values using 2-ΔΔCt method. The primers for human IL-22, IL22R1, IL10R2, AHR, IFN-γ, IL-4 and β-actin were synthesized by Shanghai Jikang Biology Company (Table 1).
Table 1.
Primer sequences of qRT-PCR
| Gene | Forward Primer (5’-3’) | Reverse Primer (5’-3’) | Gene Length (bp) |
|---|---|---|---|
| IL-22 | TGCTGTTCCCTCAATCTG | TGTGCTTAGCCTGTTGCTG | 86 |
| IL22R1 | TGACGGTGGAGACGGGC | GGTGGCTTGAGGGTAGTGTG | 130 |
| IL10R2 | CGCCTTGCTGTGGTGC | AACTCTTTCAGGTGCTGTGGA | 81 |
| AHR | TTACAGGCTCTGAATGGCTT | TTTTCTGGAGGAATCTGGTCT | 281 |
| IFN-γ | TTTTGAAGAATTGGAAAGAGGA | CACTTGGATGAGTTCATGTATTG | 254 |
| IL-4 | GAAGAGAGGTGCTGATTG | GGAAGAACAGAGGGGGAAG | 234 |
| β-actin | CATTGCCGACAGGATGCAG | CTCGTCATACTCCTGCTTGCTG | 169 |
Cell stimulation
A total of one million isolated PBMCs were incubated in RPMI1640 supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin at 37% in an incubator containing 5% CO2. Simultaneously, 40 ng (20 μl, 40 ng/μl) recombinant human IL-22 and 40 ng (10 μl, 40 ng/μl) recombinant human IL-2 were added. Adding only 40 ng (10 μl, 40 ng/μl) recombinant human IL-2 served as the negative control. After 48 h of stimulation, cells and supernatant were harvested, frozen in liquid nitrogen and kept at -80°C until further analysis. Cytokines content in the supernatant, like IFN-γ and IL-4, was measured. The mRNA expression of cytokines in stimulated PBMCs was detected using qRT-PCR.
Cytokine assays
Collected supernatants were centrifuged at 4000 rpm for 15 min at 4°C and then detected by Bio-Plex ProTM Human Cytokine Assays (Bio-Rad, USA), which are magnetic bead-based multiplex immunoasssays consisting of a comprehensive blend of soluble biomarkers, IFN-γ and IL-4. First, we washed the plate with Assay Buffer for one time added 50 μl assay buffer containing coupled beads (antibody to IFN-γ and IL-4) to each well, and then assigned 50 μl standards and the supernatant samples for incubating for 1 h. We next washed the plate three times using assay buffer, added 30 μl antibody diluent and incubated for 30 min. We assigned 50 μl assay buffer containing streptavidin-PE and incubated for 10 min. Finally, 125 μl assay buffer was added to all the wells, vortexed for 3 min, and Bio-Plex ManagerTM software was recommended for all the data acquisition and analysis.
Statistical analysis
Results are presented as mean ± SD. The mean values were analyzed between the two groups (AITDs group and NC group) using two-independent-sample T test or nonparametric test (two-independent-samples test). SPSS 17.0 was used for statistical analysis. P value less than 0.05 was considered to be statistically significant.
Results
Serum levels of IL-22
Serum IL-22 level was measured in both AITDs patients and controls. It was found below the lowest detection limit of the ELISA kit (2.7 pg/ml) in most AITDs patients and controls. Serum IL-22 level was detected in some AITDs patients (36.07%) [GD patients (35.48%) and HT patients (36.67%), respectively] and in some control subjects (52.63%). AITDs patients were found to have an increased serum IL-22 level (10.28 pg/ml) compared with controls (4.14 pg/ml) (P = 0.025) among these detectable individuals. When further analyzed, serum IL-22 level was significantly higher in GD patients (11.35 pg/ml) than in the control group (P = 0.04). HT patients showed an increased IL-22 serum level (9.22 pg/ml) than that of controls, with the P value was nearly significant (P = 0.066). But compared to GD group, serum IL-22 level was decreased in HT group showing no statistically significance (P = 0.397). All data are shown in Figure 1.
Figure 1.
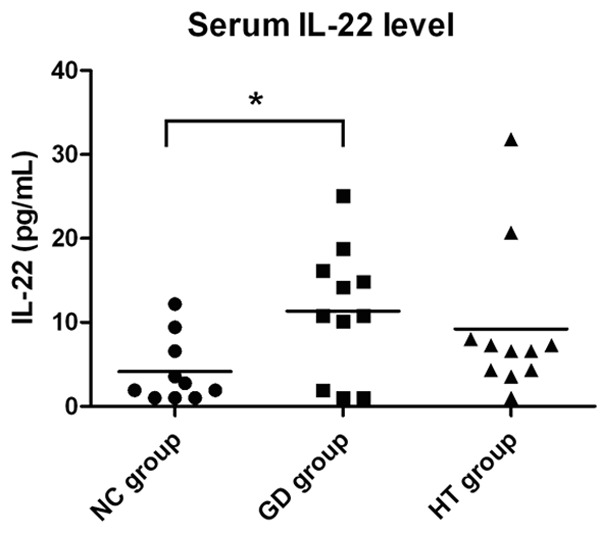
Serum IL-22 levels of AITDs patients and healthy controls. The concentrations of serum IL-22 in AITDs patients (including GD, HT patients) and healthy controls were measured by ELISA. Data was expressed as the mean values of cases and controls. *P<0.05.
Frequency of Th22 cells in PBMCs
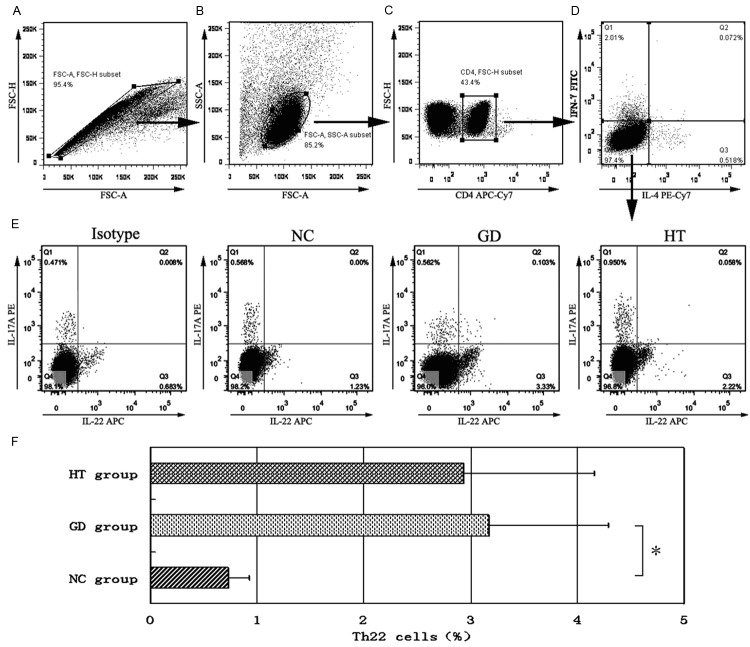
As shown in Figure 2, we used CD4+IL22+IFNγ-IL4-IL17A- as the phenotype of Th22 cells. Flow cytometry showed that compared with normal controls, there was an elevated tendency in both GD and HT patients, especially the GD group with a significant difference (P = 0.043). Th22 cells ratio of HT patients was higher than that of normal controls (P = 0.051, nearly approaching statistically significant), but lower than GD patients (P = 0.732).
Figure 2.
Flow cytometry analysis detected Th22 cells. PBMCs were isolated from participants with PMA/ionomycin stimulation and harvested for staining with PE-Cy7-conjugated anti-CD4 antibody, fixed and permeabilized, followed by intracellular staining with FITC-conjugated anti-IFN-γ monoclonal antibody, PE-conjugated anti-IL-17A monoclonal antibody, APC-Cy7-conjugated anti-IL-4 monoclonal antibody, and APC-conjugated anti-IL-22 monoclonal antibody, and finally tested by flow cytometry. Subsequently, the cells were gated first on CD4+ cells for analysis of the frequency of CD4+IFNγ-IL4- cells, which were further analyzed for CD4+IFNγ-IL4- cells. The CD4+IFNγ-IL4- cells were then analyzed for CD4+IL22+IFNγ-IL4-IL17A- cells, followed by quantitative analyses. Data were represented as the mean values of individual subjects. A-E: representative charts of flow cytometry analysis: A: PBMCs subtype; B: T cells subtype; C: CD3+ cells subtype; D: CD4+IL4- IFN-γ- cells subtype; E: Th22 cells (CD4+IL22+IFNγ-IL4-IL17A-) subtype. F: frequencies of Th22 cells in AITDs and NC groups. *P<0.05.
mRNA expression of IL-22, AHR, IL10R2 and IL22R1 in PBMCs
IL-22 mRNA expression in PBMCs of GD patients was obviously higher than that of normal controls and HT patients (P = 0.044 and 0.047, respectively). Compared with control group, IL-22 mRNA expression in HT group only showed an increased trend, but without significant difference (P = 0.703). We found no expression of IL22R1 mRNA in PBMCs. There was an increased trend of IL10R2 in GD and HT patients compared with healthy controls, though P value were both larger than 0.05. A higher AHR mRNA expression was observed in PBMCs of GD group (P = 0.041), not in HT group (P = 0.093). The data comparisons are displayed in Figure 3.
Figure 3.
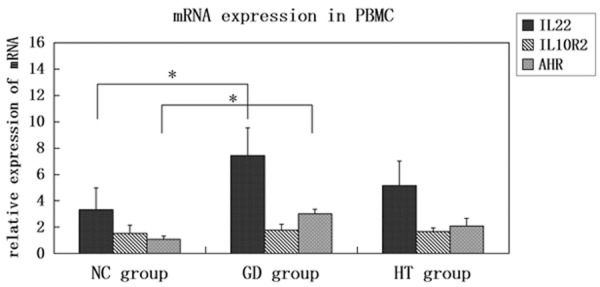
mRNA expressions of IL-22, IL10R2, and AHR in PBMCs. The mRNA expressions of IL-22, IL10R2, and AHR were measured in AITDs and control samples by qRT-PCR, with β-action as an endogenous control. The results of individual participants were determined from Ct values using 2-ΔΔCt method. The fold changes were shown relative to control samples. The data were displayed as bar chart with error bars. *P<0.05.
Cytokines expressions in cultured PBMCs after IL-22 stimulation
After 48-hour stimulation with IL-2 and IL-22, IL-4 level of the supernatant in control and GD groups was greatly increased, especially in GD group (P = 0.045), while IFN-γ level was decreased (P = 0.047). Moreover, IL-4 mRNA expressed in PBMCs was also elevated (P = 0.043), IFN-γ mRNA expression declined in both of the two groups (P = 0.141). However, in HT group, IFN-γ level in the supernatant was greatly increased, while IL-4 level was decreased. Data are shown in Figure 4.
Figure 4.
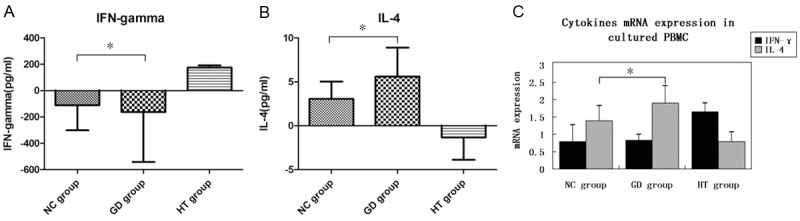
Cytokines expressions in the cultured PBMCs after IL-22 stimulation. After 48-hour stimulation with IL-2 and IL-22, IL-4 and IFN-γ level of the supernatant in AITDs and control groups was examined by cytokine assays. Then, the results were then compared by mean values. In addition, IL-4 and IFN-γ mRNA expressions in PBMCs of all the groups were also measured by qRT-PCR and the results were determined from Ct values using 2-ΔΔCt method. A: Changes of IFN-γ expression in the supernatant of cultured PBMCs; B: Changes of IL-4 expression in the supernatant of cultured PBMCs; C: Changes of cytokines mRNA expressions in the cultured PBMCs. *P<0.05.
Discussion
As two main subclasses of AITDs, GD and HT are thought to have different clinical presentations caused by different overwhelming lymphocytes on specific conditions. For decades, the traditional Th1/Th2 imbalance has been used to explain the two variants. We all know that Th1 may initiate apoptosis and other destructive processes in HT through secreting proinflammatory cytokines such as INF-γ [4]. And Th2 cells with other cytokines like IL-4 secretion may aid plasma cells and cause antibody dependent pathological process such as TRAb in GD [3]. However, they cannot explain the whole pathogenesis of AITDs.
Initially, IL-22 was thought to be a kind of cytokine of Th1 cells. In the pathological studies on psoriasis and allergic dermatitis, abundant expression of IL-22 was detected in circulation and skin lesions of the patients, while other cytokines like IL-17 and IL-23 were undetectable [15]. Then this specific T lymphocyte, which independently secretes IL-22, was named as Th22 cell [6].
Nowadays, the role of Th22 cell in autoimmune diseases has become a hot issue in immunology community and draws intensive attention. A significant amount of IL-22 and IL22R1 expressions were found in the colonic epithelial mucosa of patients with ulcerative colitis [16]. Elevated serum IL-22 level is positively correlated to the disease activity of Crohn’s disease [17]. One study showed that Th22 cells level in circulation was higher in psoriatic patients [18]. Lo et al. found that IL-22 level in the serum of patients with psoriasis was greatly elevated and was related to the severity extent of the disease [19]. Th22 cell was also at a higher level in other diseases like rheumatoid arthritis (RA) [11].
Interestingly, our present study found that the serum level of IL-22, the expressions of its mRNA, its receptor and even Th22 were all increased in patients with GD. This is in accordance with the overexpression of key transcription factor of IL-22, i.e., AHR in PBMCs of GD. For HT, IL-22 expression did not differ from that in normal individuals neither at protein nor at mRNA level, although there was an obvious trend towards increment. Our results are consistent with those found in GD [13], but to some extent different from those found in HT [14], which also showed an increased Th22 expression. Although the specific samples and the confined sensitivity of assays might contribute to the difference, we did discover a distinctive Th22 expression mode in GD and HT. In addition, we believe the experiments performed under the same condition may help us disclose the entities of different AITDs more clearly.
The augmentation of Th22 cell and its pathway-molecules in AITDs do not definitely mean that they are detrimental to the patients. For example, IL-22 is deemed as a proinflammatory factor in many autoimmune inflammatory diseases such as RA and SSc; it seems to play a protective role in patients with SLE [12,20]. Because the importance of Th1/Th2 imbalance in AITDs is well documented, we then conducted the lymphocyte stimulation experiment for further investigation. We found that the PBMCs from GD patients with additional stimulation of IL-22 elicited more production of IL-4, while PBMCs from HT patients synthesized more INF-γ (although with no statistical significance). It is well known that both IL-4 and INF-γ take dominant proinflammatory parts in GD and HT, respectively. Taking together our results, we postulate that Th22 cell and its effector cytokine IL-22 may initiate or perpetuate the pathophysiological progression of AITDs, especially in GD, via strengthening the other T lymphocytes and inducing other immune mediators.
In summary, Th22 cells may have a cooperative or synergetic function with Th2 cells in the immunopathogenesis of GD. Therefore, reducing the abnormally increased Th22 relevant cytokine may lead to a novel therapeutic strategy for GD.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81270871) and Technology Project of Xi’an Municipal Health Bureau (No. 2013045).
Disclosure of conflict of interest
None.
References
- 1.Muhali FS, Song RH, Wang X, Shi XH, Jiang WJ, Xiao L, Li DF, He ST, Xu J, Zhang JA. Genetic variants of BANK1 gene in autoimmune thyroid diseases: a case-control association study. Exp Clin Endocrinol Diabetes. 2013;121:556–560. doi: 10.1055/s-0033-1348220. [DOI] [PubMed] [Google Scholar]
- 2.Song RH, Yu ZY, Wang Q, Muhali FS, Jiang WJ, Xiao L, Shi XH, He ST, Xu J, Zhang JA. Polymorphisms of the TNFAIP3 region and Graves’ disease. Autoimmunity. 2014 doi: 10.3109/08916934.2014.914504. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Marique L, Van Regemorter V, Gerard AC, Craps J, Senou M, Marbaix E, Rahier J, Daumerie C, Mourad M, Lengele B, Colin IM, Many MC. The Expression of Dual Oxidase, Thyroid Peroxidase, and Caveolin-1 Differs According to the Type of Immune Response (TH1/TH2) Involved in Thyroid Autoimmune Disorders. J Clin Endocrinol Metab. 2014;99:1722–1732. doi: 10.1210/jc.2013-3469. [DOI] [PubMed] [Google Scholar]
- 4.Qin Q, Liu P, Liu L, Wang R, Yan N, Yang J, Wang X, Pandey M, Zhang JA. The increased but non-predominant expression of Th17-and Th1-specific cytokines in Hashimoto’s thyroiditis but not in Graves’ disease. Braz J Med Biol Res. 2012;45:1202–1208. doi: 10.1590/S0100-879X2012007500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZL, Lin B, Yu LY, Shen SX, Zhu LH, Wang WP, Guo LH. Gene therapy of experimental autoimmune thyroiditis mice by in vivo administration of plasmid DNA coding for human interleukin-10. Acta Pharmacol Sin. 2003;24:885–890. [PubMed] [Google Scholar]
- 6.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 7.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Li JM, Liu XG, Ma DX, Hu NW, Li YG, Li W, Hu Y, Yu S, Qu X, Yang MX, Feng AL, Wang GH. Elevated Th22 Cells Correlated with Th17 Cells in Patients with Rheumatoid Arthritis. J Clin Immunol. 2011;31:606–614. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 9.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Li H, Zhang L, Shan B, Xu X, Li H, Liu X, Xu S, Yu S, Ma D, Peng J, Hou M. Elevated profiles of Th22 cells and correlations with Th17 cells in patients with immune thrombocytopenia. Hum Immunol. 2012;73:629–635. doi: 10.1016/j.humimm.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, Hu NW, Ma DX, Li ZF, Yang Q. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. doi: 10.1371/journal.pone.0031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan HF, Zhao XF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Tang XW, Li WX, Li LH, Feng JB, Hu CS, Ye DQ. Decreased serum IL-22 levels in patients with systemic lupus erythematosus. Clin Chim Acta. 2009;401:179–180. doi: 10.1016/j.cca.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Peng D, Xu B, Wang Y, Guo H, Jiang Y. A High Frequency of circulating Th22 and Th17 Cells in patients with new onset graves’ disease. PLoS One. 2013;8:e68446. doi: 10.1371/journal.pone.0068446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Peng D, Yang XG, Wang Y, Xu BC, Ni JS, Meng W, Jiang YF. A higher frequency of circulating IL-22(+)CD4(+) T cells in Chinese patients with newly diagnosed Hashimoto’s thyroiditis. PLoS One. 2014;9:e84545. doi: 10.1371/journal.pone.0084545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. e1242. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T. Involvement of the IL-22/REG Iα axis in ulcerative colitis. Lab Invest. 2010;90:496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 17.Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, Lohse P, Goke B, Brand S. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 18.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo YH, Torii K, Saito C, Furuhashi T, Maeda A, Morita A. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J Dermatol Sci. 2010;58:225–227. doi: 10.1016/j.jdermsci.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604–606. doi: 10.1136/ard.2008.097089. [DOI] [PubMed] [Google Scholar]