Abstract
Neutrophil-to-lymphocyte ratio (NLR) has been reported to predict cardiovascular risks and mortality in coronary artery diseases. We aimed to evaluate the capacity of NLR to predict long-term mortality in Chinese patients presenting with ST-segment elevation myocardial infarction (STEMI). We recorded NLR at admission, 24 or 72 hours after admission, and at discharge (14±2 days) of 692 patients presenting with STEMI at Xuanwu hospital, Beijing between 2002 and 2005, and assessed the capacity of NLR to predict mortality during follow up (median 9.43, interquartile range (IQR) 8.65-10.28 years). Backward stepwise multivariate Cox regression revealed that average inpatient NLR (NLRaverage) predicted all-cause mortality (Hazard ratio 1.481) more accurately than absolute leukocyte and neutrophil counts (P<0.001). When patients were stratified into tertiles by NLRaverage (T1 NLR<3.16, T3 NLR>4.75), patients in T3 exhibited a 4.621-fold higher risk of mortality than patients in T1 (P=0.002). Patients in T3 had a significantly higher incidence of all-cause mortality (10.00%) than T1 (2.17%) and T2 (4.31%), cardiac-mortality (8.70%) than T1 (2.17%) and T2 (4.31%), hypotension (20.00%) than T1 (5.65%) and T2 (12.93%), arrhythmia (43.91%) than T1 (24.14%) and T2 (24.35%), and defibrillation (7.83%) than T1 (1.74%) and T2 (5.17%) in hospital; and suffered from higher mortality (46.09%) than T1 (9.13%) and T2 (29.74%), cardiac mortality (27.83%) than T1 (5.22%) and T2 (15.52%) and MACE events (36.52%) than T1 (13.04%) and T2 (31.9%) during long-term follow-up. Average NLR was a useful and powerful predictor of mortality and adverse-outcomes in Chinese patients presenting with STEMI.
Keywords: Myocardial infarction, inflammation, biological markers, leukocyte count, prognosis
Introduction
Leukocytes play a major role in both initiation and progression of atherosclerosis, and have been implicated in acute rupture of atherosclerotic plaques with superimposed thrombus formation [1-6]. During acute myocardial infarction (AMI), activated-neutrophils infiltrate into the infarcted zone [2,3] contributing to fibrotic scar formation, a cause of arrhythmia [7,8]. Furthermore, neutrophils aggregate with platelets to exacerbate vascular plugging in the microcirculation [4,5,9], and induce no-reflow phenomenon [10-14]. Neutrophils also prompt the secretion of inflammatory mediators [15], aggravating myocardial ischemia [16,17] and extending the infarct area [3,18,19]. Depressed levels of circulating lymphocytes are often observed to follow AMI in response to elevated cortisol secretion and apoptotic stress reactions [3,20,21]. Lower lymphocyte levels were associated with advanced heart failure [2,19] and mortality [5] in STEMI patients.
Recently, neutrophil-to-lymphocyte ratio (NLR) has emerged as a potent composite inflammatory marker [10,15,20]. NLR, obtained on admission [4-7,9-13,15-20,22-26], 24-hour after percutaneous coronary intervention (PCI) [14], or calculated as an average [21] or maximum value [27], has been found to be an independent predictor of mortality [1,4-9,11-27] and adverse-outcomes including major adverse cardiovascular events (MACE) [5,13,16,18,25], advanced heart failure [3,19,23], re-infarction [12], and arrhythmia [8,21,27] in patients with ST-segment elevation myocardial infarction (STEMI) [4,5,7,10-15,17-20,24-27], non-STEMI [21,23] or stable chronic coronary artery disease [6,9,16,22]. NLR measured at admission was also an independent predictor of poor myocardial perfusion [9,16] and worse angiographic outcomes pre- [7,10,15,26] or post- [13,17] PCI.
However, catecholamine release [4,21], dehydration [4,21] or reperfusion therapy [24] may artificially increase the levels of circulating neutrophils and lymphocytes, and blur their truthful prognostic value. Additionally, NLR is not static, and varies with progression of critical illness [27]. It is unclear when NLR values should be calculated to offer the best prognostic value for Chinese STEMI patients. Therefore, we conducted this study to identify the predictive value of NLR calculated at different time points in Chinese patients presenting with STEMI.
Materials and methods
Patients
We enrolled 826 STEMI patients hospitalized at Xuanwu hospital, Beijing, China between January 2, 2002 and January 16, 2005 within the first 12 hours of symptom onset. The study was approved by the ethics committee of Xuanwu hospital and was carried out according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all included patients (or their relatives). STEMI was defined according to the criteria defined by the American College of Cardiology and the European Society of Cardiology [28].
Patients with clinical evidence of active cancer, severe renal failure (creatinine exceeding the upper limit of the normal value on admission by 150%), hematological proliferative disorders, active hepatobiliary diseases, active infection, chronic inflammatory disease, or receiving steroid therapy for autoimmune disease were excluded from this study. A total of 692 patients were included in the analysis. We recorded age, gender, personal and family history, systolic/diastolic blood pressure, heart rate, killip class, and complications from complete medical history records.
Hematology
Routine blood tests were carried out at admission, 24 and 72 hours later, and at discharge. Leukocyte, neutrophil, lymphocyte, monocyte counts were determined using an automated blood cell counter (MEK-7222K, NIHON KOHEN, JAPAN). NLR was calculated as the ratio of neutrophils to lymphocytes, and both obtained from the same blood sample. And average, maximum and minimum NLR values were recorded.
Biochemical parameters (Table 1) were determined using commercially available methods and kits (7170, HITACHI, JAPAN), as determined by the attending cardiologist.
Table 1.
Baseline characteristics of patients according to average NLR tertiles
| Tertile1 (n=230) | Tertile2 (n=232) | Tettile3 (n=230) | P values | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| (NLR<3.16) | (3.16-4.75) | (NLR>4.75) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | Overall | |
| NLRaverage | 2.49±0.52 | 3.89±0.47 | 6.87±2.42 | <0.001* | <0.001* | <0.001* | <0.001* |
| Age | 57.09±11.57 | 60.03±11.04 | 63.69±11.31 | 0.004* | <0.001* | 0.001* | <0.001* |
| Body mass index (kg/m2) | 25.45±6.13 | 25.15±5.20 | 25.14±3.15 | 0.648 | 0.660 | 0.980 | 0.874 |
| Men/Women | 184/46 | 170/62 | 192/38 | 0.076 | 0.359 | 0.007* | 0.024* |
| History | |||||||
| Hypertension | 53.91% (124) | 64.66% (150) | 57.39% (132) | 0.019* | 0.448 | 0.113 | 0.057 |
| Dyslipidemia | 27.83% (64) | 30.60% (71) | 14.78% (34) | 0.483 | 0.001* | <0.001* | <0.001* |
| Diabetes mellitus | 25.65% (59) | 27.59% (64) | 23.91% (55) | 0.635 | 0.670 | 0.368 | 0.666 |
| Cerebral Stroke | 15.22% (35) | 15.52% (36) | 14.78% (34) | 0.929 | 0.897 | 0.826 | 0.976 |
| Smoking | 68.70% (158) | 65.95% (153) | 69.57% (160) | 0.527 | 0.842 | 0.405 | 0.685 |
| Previous myocardial infarction | 6.09% (14) | 6.03% (14) | 6.96% (16) | 0.982 | 0.703 | 0.685 | 0.902 |
| Previous PTCA | 1.74% (4) | 0.86% (2) | 1.74% (4) | 0.431 | 1.000 | 0.431 | 0.660 |
| History of heart pass | 0.43% (1) | 0.86% (2) | 0.00% (0) | 0.485 | 0.478 | 0.159 | 0.371 |
| Admission condition | |||||||
| Systolic blood pressure (mmHg) | 126.20±21.46 | 128.69±21.25 | 126.59±23.63 | 0.692 | 0.991 | 0.764 | 0.678 |
| Diastolic blood pressure (mmHg) | 77.88±13.22 | 78.92±13.51 | 76.77±13.37 | 0.569 | 0.544 | 0.237 | 0.497 |
| Heart rates (beats/min) | 72.33±11.14 | 76.25±15.81 | 81.62±17.36 | 0.013* | <0.001* | <0.001* | <0.001* |
| Killip class (≥2) | 30.00% (69) | 31.90% (74) | 50.43% (116) | 0.669 | <0.001* | <0.001* | <0.001* |
| Biochemical measurements | |||||||
| Peak of CKMB (ng/ml) | 384.97±468.69 | 327.98±577.39 | 352.82±317.04 | 0.221 | 0.238 | 0.944 | 0.389 |
| Peak of creatine kinase (ng/ml) | 2278.15±1862.42 | 2076.35±1710.98 | 2863.90±2186.00 | 0.505 | 0.049* | 0.007* | 0.019* |
| Creatinine (mg/dl) | 1.09±0.96 | 1.11±0.97 | 1.12±0.31 | 0.709 | 0.669 | 0.956 | 0.898 |
| Cholesterol (mg/dl) | 193.76±58.28 | 188.45±43.26 | 180.73±36.40 | 0.235 | 0.011* | 0.136 | 0.038* |
| Triglyceride (mg/dl) | 205.85±137.12 | 150.62±101.77 | 136.44±80.81 | 0.001* | <0.001* | 0.388 | <0.001* |
| HDL (mg/dl) | 43.35±14.72 | 42.36±11.20 | 40.79±9.67 | 0.547 | 0.130 | 0.335 | 0.308 |
| LDL (mg/dl) | 119.07±40.61 | 116.82±35.18 | 112.33±33.30 | 0.654 | 0.189 | 0.363 | 0.404 |
| Glucose (mg/dl) | 156.79±64.59 | 163.11±79.34 | 177.79±79.97 | 0.376 | 0.004* | 0.039* | 0.012* |
| Hematological measurements on admission | |||||||
| Leukocyte counts (109/L) | 9.04±2.55 | 10.41±3.34 | 11.17±2.87 | 0.001* | <0.001* | 0.045* | <0.001* |
| Neutrophils (109/L) | 6.11±2.28 | 8.25±2.66 | 9.77±2.75 | <0.001* | <0.001* | <0.001* | <0.001* |
| lymphocytes (109/L)) | 2.56±1.03 | 1.80±0.66 | 1.14±0.37 | <0.001* | <0.001* | <0.001* | <0.001* |
| Monocytes (109/L) | 0.33±0.18 | 0.30±0.21 | 0.22±0.16 | 0. 389 | <0.001* | <0.001* | <0.001* |
| Platelets (109/L) | 212.19±56.14 | 210.20±61.33 | 199.61±56.38 | 0.827 | 0.173 | 0.245 | 0.341 |
| Red blood cell counts (1012/L) | 4.57±0.52 | 4.54±0.59 | 4.53±0.57 | 0.660 | 0.553 | 0.866 | 0.829 |
| Hemoglobin (g/L) | 140.08±21.05 | 138.09±16.46 | 140.29±17.9 | 0.453 | 0.940 | 0.396 | 0.644 |
T1 NLRaverage<3.16, T2 3.16≤NLRaverage≤4.75, T3 NLRaverage>4.75. Data shown are % (n), median (IQR) or mean ± SD.
P<0.05.
CK-MB: MB isoenzyme of creatine kinase; HDL: High-density lipoprotein cholesterol; LDL: Low-density lipoprotein cholesterol; NLR: neutrophil-to-lymphocyte ratio; PCTA: percutaneous transluminal coronary angioplasty.
Electrocardiograph (ECG) and echocardiograph examinations
Each patient received an 18-lead ECG immediately after admission and repeated per 2-4 hours. The number of leads with ST-segment elevation, sites of myocardial infarction, episodes of sustained arrhythmia (ventricular tachycardia, ventricular fibrillation, atrial fibrillation, atrio-ventricular block and/or new bundle branch block) were recorded. All patients underwent a complete two-dimensional echocardiography evaluation within 48 hours of admission, and left ventricular ejection fraction (LVEF) was calculated by Simpson’s method [29].
Interventions and pharmacotherapy
All patients received primary PCI (n=381) or PCI within 90 minutes of thrombolysis (n=311), and the reperfusion strategy was chosen by the attending cardiologists. Concomitant medical treatments during hospitalization and after discharge were prescribed according to American College of Cardiology/American Heart Association guidelines [28] and the judgment of attending cardiologists.
Clinical follow-up and study end points
Patients were followed for a median of 9.43 years (Interquartile range (IQR) 8.65-10.28 years, maximum 11.42 years) by regular clinical examinations, telephone contact with patients or their family, and/or by consulting the Chinese population register.
All-cause mortality, cardiac mortality and MACE events were recorded. MACE events were defined as non-fatal re-infarction, target vessel revascularization, ischemia stroke and cardiac mortality.
Statistical analysis
All data was analyzed using SPSS software (Version 17.0, SPSS Chicago, USA). One-sample Kolmogorov-Smirnov and Levene’s tests were employed to determine the distribution characteristics of variables and variance homogeneity. Continuous normally distributed variables were given as mean±standard deviation (SD). Skewed distributed variables were given as median IQR.
The study population was subdivided into tertiles by average NLR (T1<3.16, T3>4.75). One-way analysis of variance (ANOVA) was employed to compare clinical characteristics of the tertiles. Categorical variables were given as percentages and compared with the Chi-square test.
The predictive capacity of leukocyte parameters was investigated in 2 ways. Firstly, the mean of leukocyte differential and NLR at given time points was compared between survivor and mortality groups using receiver-operating characteristics (ROC) curve analysis, and Z test was used to disclose the significant difference of area under the curve (AUC) values. Secondly, the influence of NLR and leukocyte differential on long-term mortality were assessed by Cox regression analysis, and crude and adjusted hazard ratios (HR) were presented with their respective 95% confidence intervals (95% CI). Candidate covariates for multivariable analysis were chosen based on previous medical knowledge and independent of their p value. From this initial model, a parsimonious, highly predictive model was derived using backward step down selection. The discriminative ability of all multivariable models was assessed by Harrell’s C statistics.
The Kaplan-Meier method was used in order to demonstrate the timing of events during long-term follow-up in relation to average NLR, and statistical evaluation was carried out using the Log-rank test. A P value <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Of the 826 STEMI patients hospitalized at Xuanwu hospital, Beijing, China between January 2, 2002 and January 16, 2005 within the first 12 hours of symptom onset, 692 patients were available for the final analysis, of which 196 died during the clinical follow-up period (median 9.43 years). Blood samples were taken following admission, a median of 6.86 hours (IQR 6.12-7.60 hours) after symptom onset (n=692), 24 and 72 hours later (n=692, n=619, respectively), and before discharge (about 14±2 days after symptom onset, n=632).
Patients that survived during the follow up period (n=496) had lower leukocyte and neutrophil counts, lower NLR values, and higher lymphocyte counts throughout hospitalization than patients that did not survive (Figure 1).
Figure 1.
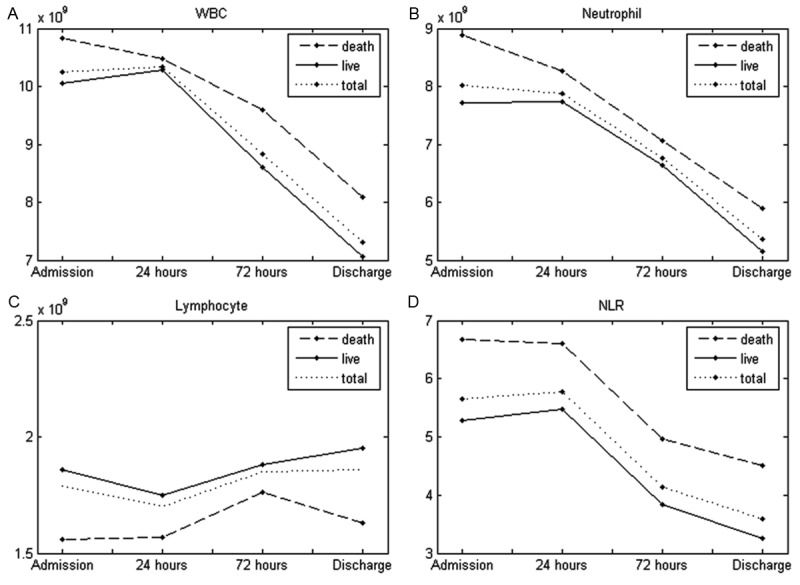
Leukocyte counts and NLR in patients presenting with ST-segment elevation acute myocardial infarction that died or survived during follow up. The leukocyte counts were assessed in patient blood samples at admission (6.12-7.60 hours after symptom onset, n=692); 24 hours after admission (n=692); 72 hours after admission (n=614); Before discharge (14±2 days after symptom onset, n=632). Patients that died during follow up had higher total leucocyte or neutrophil counts and higher NLRs, and lower lymphocyte counts in comparison with patients that did not die during the follow up period.
Neutrophil-to-lymphocyte ratio
The average NLR value detected during hospitalization was calculated for each patient, and patients were divided into tertiles according to average NLR: T1 NLRaverage<3.16, T2 3.16≤NLRaverage≤4.75, T3 NLRaverage>4.75. Patients with NLRaverage>4.75 tended to be older and were less likely to have a history of dyslipedimia. In addition, faster heart rates, advanced Killip class were more prevalent in T3, and patients in T3 had higher creatine-kinase and glucose levels, and lower triglyceride and cholesterol levels on admission. Furthermore, higher NLR was positively associated with higher leukocyte and neutrophil counts, and negatively related with lower lymphocyte and monocyte counts (Table 1).
Neither the reperfusion time, electrocardiography at admission, nor intervention methods employed differed significantly between T1, T2 or T3. However, the fraction of patients with thrombolysis in myocardial infarction (TIMI) flow classification under three either pre- or post-surgery was significantly higher in T3 than T1 (P<0.05). The number of coronary arteries narrowed was significantly higher in T3 than T1 and T2 (P<0.05) (Table 2). In addition, the average end systolic- and diastolic-volumes of T3, were significantly higher than the other groups, and T3 patients had the lowest LVEF (P<0.05) (Table 2).
Table 2.
Angiographic and ECG characteristics, Echocardiography results according to average NLR tertiles
| Tertile1 (n=230) | Tertile2 (n=232) | Tettile3 (n=230) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | Total P | |
|---|---|---|---|---|---|---|---|
| Pain to reperfusion time (hours) | 6.36±0.66 | 6.62±0.41 | 6.14±0.33 | 0.698 | 0.865 | 0.478 | 0.833 |
| Admission Electrocardiography | |||||||
| Number of leads with ST segment elevation | 3.01±5.57 | 4.23±6.81 | 4.49±7.85 | 0.145 | 0.076 | 0.745 | 0.165 |
| The elevation degree of ST segment (mm) | 4.74±1.95 | 4.80±1.91 | 5.19±1.91 | 0.800 | 0.057 | 0.098 | 0.118 |
| Intervention methods | |||||||
| Primary PCI | 55.22% (127) | 51.72% (120) | 58.26% (134) | 0.451 | 0.512 | 0.159 | 0.369 |
| PCI after thrombolysis | 44.78% (103) | 48.28% (112) | 41.74% (96) | ||||
| TIMI flow classification | |||||||
| Pre-procedure TIMI flow (<3) | 72.17% (166) | 81.90% (190) | 81.74% (188) | 0.011* | 0.012* | 0.967 | 0.014* |
| Post-procedure TIMI flow (<3) | 3.04% (7) | 5.60% (13) | 7.83% (18) | 0.227 | 0.024* | 0.294 | 0.079 |
| Number of coronary arteries narrowed | |||||||
| Single-vessel disease | 50.87% (117) | 49.13% (114) | 40.87% (94) | 0.727 | 0.004* | 0.001* | 0.002* |
| Two-vessel disease | 27.83% (64) | 34.05% (79) | 30.00% (69) | ||||
| Three-vessel disease | 20.00% (46) | 15.52% (36) | 22.61% (52) | ||||
| With left main coronary artery disease | 1.30% (3) | 1.29% (3) | 6.52% (15) | ||||
| Echocardiography | |||||||
| Ejection fraction (%) | 57.88±9.83 | 58.08±9.22 | 54.90±9.74 | 0.876 | 0.023* | 0.017* | 0.028* |
| End-systolic volume (ml) | 60.01±25.51 | 58.62±21.45 | 69.43±29.40 | 0.690 | 0.007* | 0.003* | 0.004* |
| End-diastolic volume (ml) | 137.49±32.90 | 137.37±27.98 | 149.80±37.11 | 0.978 | 0.006* | 0.007* | 0.008* |
T1 NLRaverage<3.16, T2 3.16≤NLRaverage≤4.75, T3 NLRaverage>4.75. Data shown are % (n), median (IQR) or mean ± SD.
P<0.05.
PCI: percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction.
While there was no significant difference in the length of hospital stay between T1, T2 and T3, β-receptor blockers, calcium channel blockers, nitrates, statins and low molecular heparins were less often employed to treat T3 than T1 (P<0.05). Hypotension was observed significantly less frequently in T1 (5.65% patients) than T2 or T3 (12.93% or 20.00% patients, P<0.001). Intra-aortic balloon pumps (IABP) were also used less often in T1 (1.30%) than T2 or T3 (9.05% or 11.3%, P<0.001), and arrhythmia was less frequently observed in T1 (24.35%) than T2 or T3 (24.14% or 43.91%, P<0.001), as was defibrillation (1.74% vs. 5.17% vs. 7.83%, P=0.010). Within the period of hospitalization cardiac mortality was less frequently observed in T1 (2.17%) than T2 (4.31% or 8.7%, P=0.005), as was all-cause mortality (2.17% vs. 4.31% vs. 10%, P=0.001) (Table 3).
Table 3.
Medical therapies and clinical outcomes of population according to average NLR tertiles
| Tertile1 (n=230) | Tertile2 (n=232) | Tettile3 (n=230) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | Total P | |
|---|---|---|---|---|---|---|---|
| Medical therapies | |||||||
| ACEI/ARB | 84.35% (194) | 87.93% (204) | 78.26% (180) | 0.298 | 0.078 | 0.005* | 0.018* |
| β-receptor blocker | 82.61% (190) | 78.45% (182) | 65.25% (150) | 0.293 | <0.001* | <0.001* | <0.001* |
| Nitrate | 86.09% (198) | 85.34% (198) | 71.30% (164) | 0.837 | <0.001* | <0.001* | <0.001* |
| Calcium channel blocker | 14.78% (34) | 12.07% (28) | 6.96% (16) | 0.355 | 0.008* | 0.082 | 0.026* |
| Statin | 67.83% (156) | 69.83% (162) | 53.91% (124) | 0.652 | 0.002* | <0.001* | 0.001* |
| Low molecular heparin | 79.13% (182) | 79.31% (184) | 67.82% (156) | 0.964 | 0.005* | 0.004* | 0.005* |
| Anti-platelet therapies | |||||||
| Aspirin | 25.65% (59) | 26.29% (61) | 30.00% (69) | 0.660 | 0.253 | 0.474 | 0.514 |
| Clopidogel | 0.87% (2) | 2.16% (5) | 1.30% (3) | ||||
| Aspirin+Clopidogel | 52.17% (120) | 47.41% (110) | 49.57% (114) | ||||
| Aspirin+Ticlid | 19.13% (44) | 21.12% (49) | 17.83% (41) | ||||
| Aspirin+clopidogel+Ticlid | 2.17% (5) | 3.02% (7) | 1.30% (3) | ||||
| In hospital events | |||||||
| Hospital stay (days) | 14.65±9.44 | 14.32±7.08 | 14.24±10.21 | 0.550 | 0.577 | 0.967 | 0.798 |
| Hypotension‡ | 5.65% (13) | 12.93% (30) | 20.00% (46) | 0.018* | <0.001* | 0.022* | <0.001* |
| Usage of IABP | 1.30% (3) | 9.05% (21) | 11.30% (26) | 0.001* | <0.001* | 0.344 | <0.001* |
| Arrhythmia† | 24.35% (56) | 24.14% (56) | 43.91% (101) | 0.960 | <0.001* | <0.001* | <0.001* |
| Defibrillation | 1.74% (4) | 5.17% (12) | 7.83% (18) | 0.087 | 0.003* | 0.185 | 0.010* |
| Cardiac mortality | 2.17% (5) | 4.31% (10) | 8.70% (20) | 0.292 | 0.001* | 0.031* | 0.005* |
| All cause Mortality | 2.17% (5) | 4.31% (10) | 10.00% (23) | 0.310 | <0.001* | 0.007* | 0.001* |
| Long-term events | |||||||
| Survival time (years) | 9.40±1.69 | 8.80±2.46 | 7.16±3.92 | 0.023* | <0.001* | <0.001* | <0.001* |
| Cardiac mortality | 5.22% (12) | 15.52% (36) | 27.83% (64) | 0.002* | <0.001* | <0.001* | <0.001* |
| All-cause mortality | 9.13% (21) | 29.74% (69) | 46.09% (106) | <0.001* | <0.001* | <0.001* | <0.001* |
| MACE events§ | 13.04% (30) | 31.90% (74) | 36.52% (84) | <0.001* | <0.001* | 0.054 | <0.001* |
T1 NLRaverage<3.16, T2 3.16≤NLRaverage≤4.75, T3 NLRaverage>4.75. Data shown are % (n), median (IQR) or mean±SD.
P<0.05.
ACEI/ARB, Angiotensin-converting enzyme inhibitors/Angiotensin II receptor antagonists; IABP: intra-aortic balloon pump.
Arrhythmia was defined as ventricular tachycardia, ventricular fibrillation, atrial fibrillation, atrio-ventricular block and new bundle-branch block during hospitalization.
Hypotension was defined as lower blood pressure which was continuously less than 90/60 mmHg, and need to volume expansion therapy or step-up therapy.
MACE events was defined as non-fatal re-infarction, target vessel revascularization, ischemia stroke, and cardiac mortality at 9.43-year clinical follow-up.
Patients in T3 survived, on average, less long than patients in T2 or T1 (9.40±1.69 vs. 8.80±2.46 vs. 7.16±3.92 years, P<0.001), and had the highest incidence of cardiac mortality (5.22% vs. 15.52% vs. 27.83%, P<0.001), all-cause mortality (9.13% vs. 29.74% vs. 46.09%, P<0.001) and MACE events (13.04% vs. 31.90% vs. 36.52%, P<0.001) within the follow up period (median 9.43 (8.65-10.28) years) (Table 3).
Predictive value of NLR
We sought to determine the relative capacities for leukocyte counts to predict mortality via ROC curve analysis. The area under the curve (AUC) for NLRaverage (0.726, 95% CI 0.683-0.769, P<0.001) was greater than that for neutrophilaverage (AUC=0.655, 95% CI 0.608-0.701, P<0.001) and leukocyteaverage (AUC =0.600, 95% CI 0.551-0.649, P<0.001) (Z test: NLRaverage vs. neutrophilaverage, Z value=2.96>1.96, P<0.05; NLRaverage vs. leukocyteaverage, Z value=3.78>1.96, P<0.05) (Figure 2). The optimal NLRaverage cut-off, 4.22, predicted mortality with a specificity of 68.8%, sensitivity of 69.3%, positive predictive value of 50.37%, and negative predictive value of 83.26%.
Figure 2.
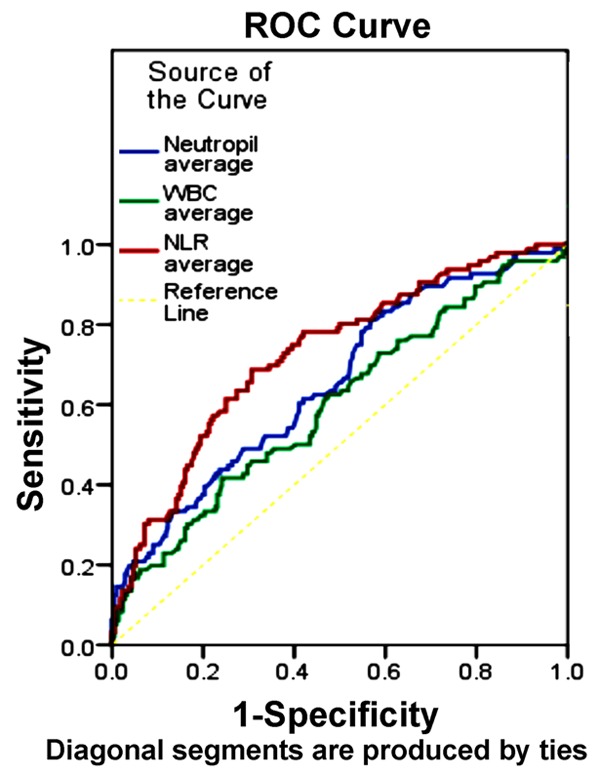
Receiver-operating characteristic curves for NLR and leukocyte counts prediction of mortality. ROC curves were plotted for the average NLR values and leukocyte and neutrophil counts. The NLRaverage curve AUC (0.726) was significantly higher than ther leukocyteaverage (0.600) and Neutrophilaverage (0.655). The optimal threshold maximizing the composite of specificity and sensitivity in the NLRaverage prediction of mortality was calculated to be 4.22. The optimal cut-off, 4.22, predicted mortality with a specificity of 68.8%, sensitivity of 69.3%, positive predictive value of 50.37%, and negative predictive value of 83.26%.
Independent predictors of long-term mortality were determined by a backward stepwise multivariate COX regression analysis (Table 4). In univariable COX regression analysis, the HR for mortality according to NLRaverage was 1.407 (p<0.001); After adjustment for confounding factors (gender, age, smoking, previous myocardial infarction, diabetes mellitus, systolic blood pressure, heart rates, glucose level, invention methods, impaired vessels, ejection fraction, defibrillation, usage of pacemaker, usage of IABP, statin use or ACEI/ARB use) NLRaverage remained a strong predictor of long-term mortality, with an HR of 1.481 (P<0.001); Furthermore, stratified into tertiles, the HR increased by 2.138, 95% CI (0.823-5.557) per tertile (P=0.119) and 4.621, 95% CI (1.776-12.025), (P=0.002) for mortality. Otherwise, when NLRaverage was assessed as binary variables, the HR was 2.989, 95% CI (1.238-7.215), (P=0.015). Higher Harrell’s c statistic values were obtained from the model including NLRaverage Tertiles (0.861) or NLRaverage binary (0.852), in contrast with the lower values derived from the model employing NLR as a continuous variable (0.837) (Table 5).
Table 4.
Hazard ratios for long-term all-cause mortality according to leukocyte and neutrophil counts by univariate Cox regression analysis and stepwise multivariate Cox regression
| Univariate Cox regression analyses | Multivariate COX regression analyses# | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Variables | Non-adjusted HR (95% CI) | P value | Variables | Non-adjusted HR (95% CI) | P value | Variables | Adjusted HR (95% CI) | P value |
| NLRAverage | 1.407 (1.301-1.521) | <0.001* | Gender | 2.288 (1.569-3.336) | <0.001* | NLRAverage | 1.481 (1.279-1.715) | <0.001* |
| Maximum | 1.064 (1.040-1.088) | <0.001* | Age | 1.078 (1.056-1.100) | <0.001* | Maximum | 1.043 (1.003-1.083) | 0.033* |
| Admission | 1.244 (1.172-1.320) | <0.001* | IABP use | 2.000 (1.029-3.884) | 0.041* | Admission | 1.298 (1.183-1.425) | <0.001* |
| 24hours | 1.135 (1.077-1.195) | <0.001* | Smoking | 0.613 (0.419-0.895) | 0.011* | 24hours | 1.166 (1.058-1.285) | 0.025* |
| 72hours | 1.145 (1.095-1.198) | <0.001* | HR | 1.040 (1.030-1.051) | <0.001* | 72hours | 1.160 (1.058-1.271) | 0.002* |
| Last | 1.057 (1.032-1.082) | <0.001* | SBP | 0.993 (0.984-1.001) | 0.099 | Last | 1.046 (1.009-1.085) | 0.015* |
| WBCAverage | 1.261 (1.167-1.362) | <0.001* | Defibrillation | 3.330 (1.964-5.464) | <0.001* | WBCAverage | 1.222 (1.077-1.386) | 0.002* |
| Maximum | 1.128 (1.070-1.189) | <0.001* | Diabetes mellitus | 1.258 (0.838-1.888) | 0.268 | Maximum | 1.130 (1.040-1.228) | 0.004* |
| Admission | 1.169 (1.100-1.243) | <0.001* | OMI | 0.538 (0.198-1.463) | 0.225 | Admission | 1.238 (1.143-1.341) | <0.001* |
| 24hours | 1.087 (1.024-1.154) | <0.001* | Pacemaker use | 1.802 (0.094-3.448) | 0.075 | 24hours | 1.054 (0.965-1.152) | 0.241 |
| 72hours | 1.167 (1.093-1.247) | <0.001* | EF values | 0.035 (0.003-0.398) | 0.007* | 72hours | 1.228 (1.108-1.360) | <0.001* |
| Last | 1.179 (1.114-1.247) | <0.001* | ACEI use | 0.397 (0.262-0.601) | <0.001* | Last | 1.070 (0.969-1.181) | 0.179 |
| NAverage | 1.279 (1.218-1.344) | <0.001* | Statin use | 0.573 (0.399-0.823) | 0.003* | NAverage | 1.475 (1.278-1.702) | <0.001* |
| Maximum | 1.165 (1.106-1.228) | <0.001* | Glucose | 1.004 (1.002-1.006) | <0.001* | Maximum | 1.212 (1.113-1.319) | <0.001* |
| Admission | 1.257 (1.175-1.344) | <0.001* | Creatinine | 1.063 (0.880-1.284) | 0.525 | Admission | 1.369 (1.244-1.507) | <0.001* |
| 24hours | 1.150 (1.081-1.222) | <0.001* | Impaired vessels | 1.734 (1.399-2.148) | <0.001* | 24hours | 1.103 (0.994-1.225) | 0.065 |
| 72hours | 1.209 (1.135-1.288) | <0.001* | P-to-R time | 0.997 (0.968-1.027) | 0.852 | 72hours | 1.267 (1.140-1.408) | <0.001* |
| Last | 1.216 (1.157-1.279) | <0.001* | Invention methods | 0.926 (0.824-1.041) | 0.197 | Last | 1.245 (1.112-1.395) | <0.001* |
| LAverage | 0.556 (0.404-0.766) | <0.001* | LAverage | 0.559 (0.337-0.926) | 0.024* | |||
| Minimum | 0.495 (0.343-0.713) | <0.001* | Minimum | 0.499 (0.258-0.996) | 0.039* | |||
| Admission | 0.625 (0.462-0.847) | 0.002* | Admission | 0.584 (0.388-0.878) | 0.010* | |||
| 24hours | 0.809 (0.626-1.045) | 0.105 | 24hours | 1.050 (0.691-1.597) | 0.818 | |||
| 72hours | 0.796 (0.602-1.054) | 0.111 | 72hours | 1.265 (0.814-1.966) | 0.295 | |||
| Last | 0.561 (0.393-0.801) | 0.001* | Last | 0.506 (0.316-0.808) | 0.004* | |||
| MAverage | 0.195 (0.069-0.550) | 0.002* | MAverage | 0.183 (0.032-1.057) | 0.058 | |||
| Maximum | 0.310 (0.147-0.653) | 0.002* | Maximum | 0.530 (0.191-1.466) | 0.221 | |||
| Admission | 0.266 (0.069-1.022) | 0.054 | Admission | 0.732 (0.211-2.543) | 0.624 | |||
| 24hours | 0.333 (0.124-0.891) | 0.028* | 24hours | 0.734 (0.229-2.347) | 0.602 | |||
| 72hours | 0.331 (0.142-0.768) | 0.010* | 72hours | 0.166 (0.026-1.057) | 0.057 | |||
| Last | 0.170 (0.052-0.559) | 0.004* | Last | 0.034 (0.003-0.3600 | 0.005 | |||
HR: hazard ratio; CI: confidence interval;
P<0.05.
ACEI/ARB: Angiotensin-converting enzyme inhibitors/Angiotensin II receptor antagonists; EF: ejection fraction; SBP: systolic blood pressure; IABP: intra-aortic balloon pump; HR: heart rates; P-to-R time: pain to reperfusion time; NLR: neutrophil-to-lymphocyte ratio; N: neutrophil count; M: monocyte count; L: lymphocyte count; OMI: previous myocardial infarction. Imparied vessels, numbers of coronary arteries narrowed (>70%).
Final Cox model adjusted by: average NLR, gender, age, systolic blood pressure, heart rates, ejection fraction, statin use, ACEI/ARB use, defibrillation, glucose, usage of pacemaker, usage of IABP, previous myocardial infarction, smoking, diabetes mellitus, impaired vessels (numbers of coronary arteries narrowed >70%), invention methods (including: primary PCI or PCI after thrombolysis).
Table 5.
Hazard ratios for all-cause mortality according to NLRaverage in different multivariate Cox regression models
| Multivariate Cox regression models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Model 1 (NLR as continuous variables) | Model 2 (NLR as binary variables)† | Model 3 (NLR as tertile variables)& | |||||||
|
|
|||||||||
| Variable | Adjusted HR (95% CI) | P value | C Statistics | Adjusted HR (95% CI) | P value | C Statistics | Adjusted HR (95% CI) | P value | C Statistics |
| Smoking | 0.559 (0.285-1.099) | 0.092 | 0.837 | - | - | 0.852 | - | - | 0.861 |
| Diabetes mellitus | 3.115 (1.481-6.554) | 0.003* | - | - | - | - | |||
| Gender (women) | 1.997 (0.971-4.111) | 0.060 | 2.036 (1.061-3.905) | 0.032* | 1.957 (1.323-2.894) | 0.001* | |||
| Heart rates | - | - | 1.026 (1.007-1.046) | 0.008* | 1.022 (1.003-1.041) | 0.023* | |||
| Defibrillation | 14.040 (4.439-44.407) | <0.001* | 9.998 (3.993-25.035) | <0.001* | 13.226 (4.957-35.289) | <0.001* | |||
| Impaired-vessels | 1.787 (1.239-2.577) | 0.002* | 1.918 (1.286-2.960) | 0.001* | 1.957 (1.323-2.894) | 0.001* | |||
| ACEI use | - | - | 0.476 (0.220-1.026) | 0.058 | 0.452 (0.206-0.992) | 0.048* | |||
| NLR | 1.481 (1.279-1.715) | <0.001* | - | - | - | - | |||
| NLR>4.22 | - | - | 2.989 (1.238-7.215) | 0.015* | - | - | |||
| NLR tertiles | - | - | - | - | 0.004* | ||||
| Tertile2 | - | - | - | - | 2.138 (0.823-5.557) | 0.119 | |||
| Tertile3 | - | - | - | - | 4.621 (1.776-12.025) | 0.002* | |||
HR: hazard ratio; CI: confidence interval;
P<0.05.
ACEI/ARB: Angiotensin-converting enzyme inhibitors/Angiotensin II receptor antagonists; Impaired vessels: numbers of coronary arteries narrowed (>70%). #Final Cox model adjusted by average NLR, gender, age, systolic blood pressure, heart rates, ejection fraction, statin use, ACEI/ARB use, defibrillation, glucose, usage of pacemaker, usage of IABP, previous myocardial infarction, smoking, diabetes mellitus, impaired vessels (numbers of coronary arteries narrowed >70%), invention methods (including primary PCI or PCI after thrombolysis).}
NLR as a binary variable: average NLR as binary variables categorized by best cut-offs of ROC analysis (4.22).
NLR tertiles: average NLR as categorical variables stratified into tertiles with cut offs of NLR at the 33rd percentile (NLR=3.16) and 67th percentile (NLR=4.75) when arranged in ascending order.
Kaplan-Meier analysis revealed significantly reduced long-term event-free survival in patients in the highest T3 in comparison with those in T1, and all-cause mortality differed significantly between tertiles. (Log rank test, p<0.001) (Figure 3).
Figure 3.
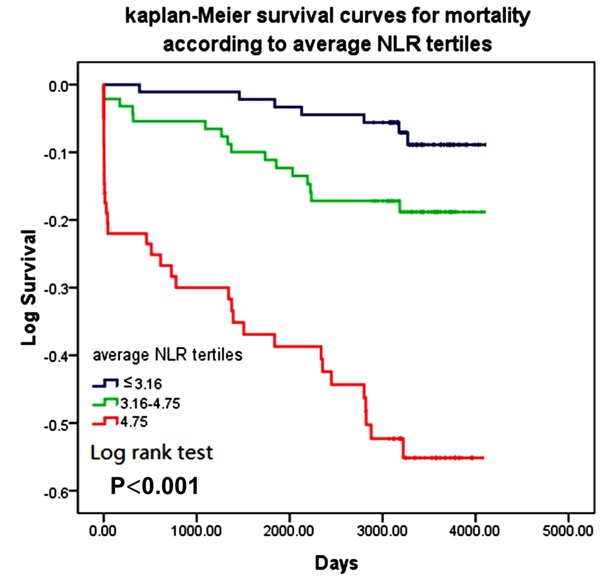
Kaplan-Meier survival curves for 9.43 (8.65-10.28) years mortality according to NLRaverage at tertiles, T1 NLRaverage<3.16, T2 3.16≤NLRaverage≤4.75, T3 NLRaverage>4.75) (Log rank test, P<0.001).
Discussion
Many studies have demonstrated a correlation between NLR and clinical adverse outcomes [3,6-9,12,13,15-17,19,20], but few have compared the prognostic value of NLR and leukocyte subgroups at different time points following myocardial infarction. We conducted this study to determine the predictive value of NLR in Chinese patients presenting with STEMI.
Average NLR was a significant predictor of subsequent all-cause mortality and outperformed the predictive ability of absolute leukocyte, lymphocyte or neutrophil counts. After adjustment for potential confounding factors, average NLR remained a significant predictor of mortality. When patients were divided into tertiles according to NLR, the highest incidence of adverse events, both during hospitalization and followup, was recorded in patients in the third tertile.
Only three studies have been previously published concerning the predictive capacity of NLR in STEMI or NSTEMI [14,21,27] in different time. Park et al. reported that when patients were stratified by NLR measured 24 hours after admission for STEMI, NLR predicted mortality more accurately than when NLR at admission was used [14]. However, Azab et al. employed average NLR, calculated as an average of the NLR during hospitalization, was a better predictor of outcome than other max NLR, or NLR at admission or discharge in patients presenting with NSTEMI [21]. And Núñez et al. reported that the maximum NLR recorded during the first 96 hours after STEMI predicted mortality better than the maximum value of leucocyte [27].
We found that NLR measured at admission, 24- and 72-hour after admission, before discharge, and both max and average NLR during hospitalization all predicted mortality in patients presenting with STEMI [4,13,18]. NLRaverage predicted more accurately than other absolute leucocyte, lymphocyte or neutrophil counts. Confirming the work of Azab et al. [21] we found that NLRaverage was significantly associated with clinical adverse-outcomes (including: lower TIMI grade pre- [15] or post- [13,17] PCI, multi-vessel disease [6,7,16], advanced heart failure [19,23] and arrhythmia [27]), a high incidence of MACE events [13,16,18,25] and mortality [12,20], both during hospitalization or long-term follow-up, consistent with previous studies of NLRadmission [6,7,13,15-17,19,23,27]. Furthermore, we found high NLRaverage predicted distinctive cardiac dilatation, and elevated incidence of hypotension and defibrillation during hospitalization, representing activated-neutrophils, activation of sympathetic nervous, the first report of this finding to our knowledge. After adjustment for recognized risk factors (gender, heart rates, defibrillation and multi-vessel disease and etc.) NLRaverage remained a significant predictor of mortality. Therefore we conclude that NLRaverage represents a useful indictor for risk stratification and prognostics.
One major obstacle to implementing NLR-based predictive prognostic matrices is determining an appropriate cut-off value. The appropriate NLR cut-off is likely to change with the stage of illness, laboratory techniques employed, concomitant infection and patient demographic characteristics [4]. Previous studies have recommended NLR cut-off points between 4.0-5.4 in AMI via ROC analysis [13,14,21,25]. However, In this study, a multivariable models in which patients were stratified into tertliles by NLRaverage achieved a higher c value than a model employing a binary NLRaverage categorized by ROC cutoffs, and the tertile cut-offs were very close to those employed by Azab et al. [21]. Therefore, a large multicenter study employing a clinically significant NLR cutoff rather than NLR interval grouping will be required to develop an appropriate cut-off for NLR [21].
We found that patients’ NLRaverage corelatred negatively with employment of medical therapies, suggesting that either medication promoted reduction of NLR via anti-inflammatory actions [15,21], or that high NLRaverage indicated that currently employed medical strategies were inapropriate. Patients in highest NLRaverage tertile presented with the highest frequency of severe illness (hypotension or arrhythmia) inhibiting the use of medicine, and suggesting treatment intolerance of patients themselves. Therefore, high NLRaverage might represent an index for therapeutic modulation [13].
Consistent with previous reports, we observed that older patients had highest tertile NLRaverage. Older patients more frequently present with a higher inflammatory burden and age-associated diseases [20,23]. Furthermore, we found that the lowest tertile NLRaverage was associated with hyperlipidemia. Low NLR may result from both increased migration of neutrophils from blood vessels to peripheral tissues, and increased lymphocyte counts in the acute phase of STEMI [12-14,21]. We also found the highest NLRaverage tertile to be associated with increased heart rates, glucose and CK, suggesting activation of the sympathetic nervous system and renin-angiotensin system [19]. These observations highlight the hypothesis that NLR may be a pathogenesis factor of atherosclerosis, not only represent a mere systemic inflammatory response to an infarcted myocardium, and these issues require further study [21].
Limitations
The limitations of our study were (1) This was a non-randomized single center study that included a relatively small number of patients was subject to selective bias; (2) NLR might be a useful marker in risk scoring, while the cut-off points and normal ranges should be furthermore determined by randomized multicenter trials. (3) We did not compare the prognostic value of NLR with other inflammatory markers such as: Hs-CRP, pro-BNP and etc.
Conclusions
This study demonstrated that average NLR was a useful and powerful indicator of mortality and adverse-outcomes both during hospitalization and long-term follow-up in Chinese patients presenting with STEMI.
Acknowledgements
This study was supported by 973 Program (No.2012CB517805) and National Department Public Benefit Research Foundation by Ministry of Health P.R China (No.201002011).
Disclosure of conflict of interest
None.
References
- 1.Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 2.Dragu R, Khoury S, Zuckerman R, Suleiman M, Mutlak D, Agmon Y, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H, Aronson D. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. 2008;196:405–412. doi: 10.1016/j.atherosclerosis.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi: 10.1016/j.amjcard.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Lee GK, Lee LC, Chong E, Lee CH, Teo SG, Chia BL, Poh KK. The long-term predictive value of the neutrophil-to-lymphocyte ratio in Type 2 diabetic patients presenting with acute myocardial infarction. Qjm. 2012;105:1075–1082. doi: 10.1093/qjmed/hcs123. [DOI] [PubMed] [Google Scholar]
- 5.Cho KH, Jeong MH, Ahmed K, Hachinohe D, Choi HS, Chang SY, Kim MC, Hwang SH, Park KH, Lee MG, Ko JS, Sim DS, Yoon NS, Yoon HJ, Hong YJ, Kim KH, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107:849–856. doi: 10.1016/j.amjcard.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 6.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Sahin DY, Elbasan Z, Gur M, Yildiz A, Akpinar O, Icen YK, Turkoglu C, Tekin K, Kuloglu O, Cayli M. Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology. 2013;64:423–429. doi: 10.1177/0003319712453305. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Chandra P, Guha G, Kalra V, Chakraborty A, Frankel R. Pre-procedural Elevated White Blood Cell Count and Neutrophil-Lymphocyte (N/L) Ratio are Predictors of Ventricular Arrhythmias During Percutaneous Coronary Intervention. Cardiovasc Hematol Disord Drug Targets. 2011 doi: 10.2174/187152911798346981. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Williams BA, Merhige ME. Association between neutrophil-lymphocyte ratio and impaired myocardial perfusion in patients with known or suspected coronary disease. Heart Lung. 2013;42:436–441. doi: 10.1016/j.hrtlng.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Tanboga IH, Topcu S, Aksakal E, Kalkan K, Sevimli S, Acikel M. Determinants of Angiographic Thrombus Burden in Patients With ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2013 doi: 10.1177/1076029613483169. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Khan HA, Alhomida AS, Sobki SH, Moghairi AA, Koronki HE. Blood cell counts and their correlation with creatine kinase and C-reactive protein in patients with acute myocardial infarction. Int J Clin Exp Med. 2012;5:50–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaya MG, Akpek M, Lam YY, Yarlioglues M, Celik T, Gunebakmaz O, Duran M, Ulucan S, Keser A, Oguzhan A, Gibson MC. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol. 2013;168:1154–1159. doi: 10.1016/j.ijcard.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Sen N, Afsar B, Ozcan F, Buyukkaya E, Isleyen A, Akcay AB, Yuzgecer H, Kurt M, Karakas MF, Basar N, Hajro E, Kanbay M. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228:203–210. doi: 10.1016/j.atherosclerosis.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, Youn TJ, Cho GY, Chae IH, Choi DJ. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Sahin DY, Gur M, Elbasan Z, Yildiz A, Kaya Z, Icen YK, Kivrak A, Turkoglu C, Yilmaz R, Cayli M. Predictors of preinterventional patency of infarct-related artery in patients with ST-segment elevation myocardial infarction: Importance of neutrophil to lymphocyte ratio and uric acid level. Exp Clin Cardiol. 2013;18:e77–81. [PMC free article] [PubMed] [Google Scholar]
- 16.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Turkmen S, Dogdu O, Tekin K, Kucukdurmaz Z, Cagliyan CE, Sarikaya S, Yucel H, Karapinar H, Ozkan B, Uysal OK, Basara A, Sancaktar E, Yilmaz A. The relationship between neutrophil/lymphocyte ratio and the TIMI flow grade in patients with STEMI undergoing primary PCI. Eur Rev Med Pharmacol Sci. 2013;17:2185–2189. [PubMed] [Google Scholar]
- 18.Han YC, Yang TH, Kim DI, Jin HY, Chung SR, Seo JS, Jang JS, Kim DK, Kim DK, Kim KH, Seol SH, Kim DS. Neutrophil to Lymphocyte Ratio Predicts Long-Term Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Korean Circ J. 2013;43:93–99. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, Capodilupo R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–438. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Ergelen M, Uyarel H, Altay S, Kul S, Ayhan E, Isik T, Kemaloglu T, Gul M, Sonmez O, Erdogan E, Turfan M. Predictive Value of Elevated Neutrophil to Lymphocyte Ratio in Patients Undergoing Primary Angioplasty for ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2013 doi: 10.1177/1076029612473516. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D, Lafferty J. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Sbrana F, Cocci F, Papa A, Landi P, Sampietro T, Rossi G, Rovai D. Routine laboratory tests to risk-stratify patients with chronic coronary artery disease. J Cardiol. 2013;61:132–137. doi: 10.1016/j.jjcc.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Gul M, Uyarel H, Ergelen M, Ugur M, Isik T, Ayhan E, Turkkan C, Aksu HU, Akgul O, Uslu N. Predictive Value of Neutrophil to Lymphocyte Ratio in Clinical Outcomes of Non-ST Elevation Myocardial Infarction and Unstable Angina Pectoris: A 3-Year Follow-Up. Clin Appl Thromb Hemost. 2014;20:378–84. doi: 10.1177/1076029612465669. [DOI] [PubMed] [Google Scholar]
- 24.Poludasu S, Cavusoglu E, Khan W, Marmur JD. Neutrophil to lymphocyte ratio as a predictor of long-term mortality in African Americans undergoing percutaneous coronary intervention. Clin Cardiol. 2009;32:E6–E10. doi: 10.1002/clc.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meissner J, Irfan A, Twerenbold R, Mueller S, Reiter M, Haaf P, Reichlin T, Schaub N, Winkler K, Pfister O, Heinisch C, Mueller C. Use of neutrophil count in early diagnosis and risk stratification of AMI. Am J Med. 2011;124:534–542. doi: 10.1016/j.amjmed.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Soylu K, Yuksel S, Gulel O, Erbay AR, Meric M, Zengin H, Museyibov M, Yasar E, Demircan S. The relationship of coronary flow to neutrophil/lymphocyte ratio in patients undergoing primary percutaneous coronary intervention. J Thorac Dis. 2013;5:258–264. doi: 10.3978/j.issn.2072-1439.2013.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunez J, Nunez E, Bodi V, Sanchis J, Minana G, Mainar L, Santas E, Merlos P, Rumiz E, Darmofal H, Heatta AM, Llacer A. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 29.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]


