Abstract
Current metabolomic studies of ischemic brain mainly attach importance on a certain ischemic period, are lack of data about dynamic metabolites in ischemic stroke process, especially early period. Thus, in this study, 1H NMR spectroscopy was used to investigate biochemical changes in the early stages of rats’ focal cerebral ischemia reperfusion (I/R) injury. Serum samples of 0, 0.5, 1, 3, 6, 12, 24 h of reperfusion, based on multivariate data analyses, were tested to analyze the changing of metabolites during the early disease process. Partial least squares-discriminant analysis scores plots of the 1H NMR data revealed clear differences among the experiment groups. Combination the results of loading plot and t-test, we found that 13 metabolites were changed significantly. Among that, malonic acid and glycine are the most noticeable variable metabolites. Dramatic changed malonic acid and glycine most probably served as biomarkers in this study. These findings help us understand the biochemical metabolite changes in early ischemic stroke stages, especially different periods. That may be conducive to distinguish at-risk individuals, benefit early diagnosis and understand the dynamic pathogenesis of early cerebral ischemia.
Keywords: Metabolomic, ischemic stroke, malonic acid, glycine, biomarkers
Introduction
Stroke is a major cause of death and disability worldwide [1]. In western countries, 80% to 85% of strokes among adults are ischemic, and the rest are hemorrhagic [2]. There are very limited treatments for stroke. Available intravenous and endovascular reperfusion techniques are restricted in the extent to which they achieve recanalization and avoid intracranial hemorrhage [3] even ischemic/reperfusion injury (I/R) [4]. The development of new therapeutics for ischemic stroke is imperative. More profound understanding about its mechanism, specially seeking novel biomarkers is sufficient beyond doubt to benefit ischemic patients.
Multiple processes are considered to cause ischemic stroke damage in terms of different angles, as we all known, e.g. excitotoxicity, acid toxicity and ionic imbalance, oxidative/nitrative stress, inflammation, peri-infarct depolarization and apoptosis [5-7]. Besides, metabolic disturbance serves as a crucial role in ischemic stroke injury, including an increase in anaerobic glycolysis, excessively abnormal release of glutamate and GABA, decrease of aspartate creatine, elevated extracellular lactate concentrations and so on [8-10].
As a powerful and comprehensive approach for characterizing the pathophysiological process and metabolic responses [11-13], recently, high-resolution nuclear magnetic resonance (NMR) based metabolomic has proved to be useful to study ischemic stroke injury [14-16]. These studies show numerous metabolites such as folic acid, cysteine, S-adenosyl homocysteine as well as oxidized glutathione, are found out as potential biomarkers. Based on these beneficial metabolomic studies, we found above studies mainly attach importance on a certain ischemic period, lack of data about dynamic metabolites in ischemic stroke process. Thus more further metabolomic researches on ischemic stroke, especially early ischemic stage as well as dynamic ischemic processes, are of consequence. From this, our previous study focused on metabolic profile of cerebrospinal fluid (CSF) from a rat model for ischemia/reperfusion (I/R) within 6 h.
However, the CSF samples are not being easy to get than the serum in clinic. Besides metabolomics approach has yet to be further used to explore complex pathophysiological process and identify biomarkers in ischemic stroke. Thus, we adopted 1H NMR spectroscopy to reveal dynamic metabolites in serum of I/R rats, at the period of ischemia 2 h and followed by reperfusion 0, 0.5, 1, 3, 6, 12, 24 h respectively. The aim of this study is to further define pathogenesis and metabolic features associated I/R injury, makes it possible to identify some potential biomarkers as well. That may be conducive to distinguish at-risk individuals, benefit early diagnosis and understand the pathogenesis of cerebral ischemia.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures performed in the experiments described here were in compliance with the relevant laws and under the permission of the Institutional Animal Care and Use Committee of Xuzhou Medical College. All surgery was performed under 10% chloral hydrate anesthesia, and all efforts were made to minimize suffering.
Animals
Sixty male Sprague-Dawley rats, aging 7 weeks and weighing 230~280 g, were obtained from the animal laboratory of Xuzhou Medical College (Xuzhou, China). Housed at 22°C, animals were maintained on a 12-hour light/dark cycle with unlimited access to food and water.
Surgical procedures
As described by Longa EZ et al and our previous studies [17,18]. Briefly, the rats were anesthetized with 10% chloral hydrate (300 mg/kg, i.p.) and the right common, internal, and external carotid arteries were exposed through a para-median incision of the neck. The external carotid artery was ligated. A 4-0 nylon surgical thread with round tip coated with poly-L-lysine was inserted about 18-20 mm through external carotid artery until the distal end met mild resistance, indicating the occlusion of the origin of the middle cerebral artery (MCAO). After 2 h occlusion, reperfusion was instituted by withdrawing intraluminal suture. The sham-operated (Sham) animals were treated identically but the middle cerebral artery was not occluded. In addition, the rats were supplemented with oxygen, during surgical procedures and the body temperature was maintained at 37.5°C using a heating blanket and a feedback temperature controller. Neurological evaluation was performed after MCAO according to a 5-points scale. Rats scored 2-3 were chosen following reperfusion.
Experimental groups
After surgical, the rats were randomly divided into the following groups (n=10, for each group): Sham group, which was had the surgical procedure without suture insertion (Sham). MCAO group was divided into some subgroups: 2 h MCAO followed by 0 h reperfusion (MCAO0), 0.5 h reperfusion (MCAO0.5), 1 h reperfusion (MCAO1), 3 h reperfusion (MCAO3), 6 h reperfusion (MCAO6), 12 h reperfusion (MCAO12), 24 h reperfusion (MCAO24).
Triphenyl tetrazolium chloride (TTC) staining [19] was used to assess the success/failure of the MCAO models. Briefly, after collecting serum (blood was centrifuged with 3000 r/min, 4°C for 10 min, and then stored at -80°C), 2 mm-thick coronal sections of brain tissue were stained in 2% TTC for 30 min at 37°C following 12 h immersion in 10% formalin. Normal cerebral tissue was stained red whereas the infarct tissue unstained.
1H NMR sample preparation
After being allowed to defrost at room temperature, the serum samples were centrifuged (14,000 r/min, 4°C for 10 min). Then 300 μl supernate, 100 μl TSP (3-trimethylsilyl-2H4-propionic acid) in D2O (1 mg/ml) and 200 μl D2O were mixed and centrifuged (12,000 r/min, 4°C) for 10 min. A 550 μl aliquot of this solution was pipetted into a 5-mm NMR tube. Samples were frozen at -80°C until NMR analysis [20].
1H NMR data acquisition
1H NMR spectra was acquired on a Bruker AVANCE 600 spectrometer (Bruker Biospin, Germany) operating at 600.13-MHz 1HNMR frequency and 300K using an NMRCASE for automatic sample delivery into a 5-mm TXI NMR probe. Gradient shimming was used immediately prior to spectral acquisition. 1H NMR spectra of the samples were acquired using a 1D NOESY pulse sequence and a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence. The CPMG sequence generates spectra edited by T2 relaxation times, i.e., with reduced signals from high molecular weight species or systems in intermediate chemical exchange. The 1D NOESY experiment generates a corresponding unedited spectrum with improved solvent peak suppression. For all spectra, 128 free induction decays were collected into 32K complex data points, using a spectral width of 5995.20 Hz, with a 2 s relaxation delay between pulses. A water presaturation pulse was applied throughout the relaxation delay [21]. For the 1D NOESY experiment, an additional presaturation pulse was applied during the mixing time (tm, 100 ms) and T1 was held constant at 3 μs. For the CPMG experiment, n=300 and τ=400 ms, for a total T2 relaxation time of 240 ms. Spectra were manually phased and baseline adjusted, and chemical shifts referenced to the TSP resonance at δ0.00.
Spectra were processed using the proprietary software package AMIX (Bruker Analytik, Rheinstetten, Germany). This divides the spectrum into a specified number of regions, and integrates the total signal area within each region to provide a numerical value. These values can then be used as variables for data analysis. In this case, spectra were divided into regions 0.04 ppm wide between δ10.0 and δ0.16, i.e., δ10.0-9.96, δ9.96-9.92, δ9.92-9.88, etc. Each variable throughout this study is referred to by the starting point of the chemical shift region to which it corresponds: i.e., the variable corresponding to the chemical shift region δ10.0-9.96 is called 10.0, δ9.96-9.92 is called 9.96, and so on. Variables between 5.00 and 4.68 were omitted, removing the area around the residual suppressed water resonance. This resulted in a total of 238 variables for data analysis. The data were then arranged in a matrix of n rows and m columns, in which n is the number of samples (i.e., individual rat) and m is the number of variables (chemical shift regions). Data were normalized by expressing each value in the nth row as a percentage of the sum value of the nth row, in order to prevent the separation of samples according to overall concentration [22].
Statistical analysis
The reduced and normalized NMR spectral data were imported into SIMCA-P (version 11.5, Umetrics AB, Umea Sweden) to partial least squares discriminant analysis (PLS-DA) [23]. SIMCA-P was used to generate all PLS-DA models and scores plots.
Partial least squares (PLS) techniques were used to assess correlation between the observed NMR data and other factors such as reperfusion times. A PLS model is expressed as a set of X-scores (NMR spectral regions) and Y-score vectors with corresponding X- and Y-weight vectors for a set of PLS model dimensions. Each dimension expresses a linear relation between an X-score and Y-score vector with the weight vectors describing how the X- and Y-variables are combined to give the X- and Y-score vectors. The model corresponds to fitting the lower dimensional line, plane or hyper plane simultaneously to the X and Y data as points in multidimensional space that best approximates the original data. The PLS model can be used to estimate the Y-variables corresponding to a given set of X-variables, (e.g., estimate the time of reperfusion for MCAO). In PLS-DA dummy variables representing the class (e.g., disease) of each sample form the ‘Y’ matrix. PLS-DA was used for classification of samples where the PCA models were dominated by effects such as disease or different disease process [24].
After metabolites were identified by PLS-DA loadings plots, unpaired t-tests [25] (comparison of means of two samples with equal variances) was performed to evaluate the responses of these specific metabolites besides the Sham and MCAO groups. In addition, the data were expressed as the means ± S.D. and the p-values of <0.05 was considered statistically significant.
Results
1H NMR spectra of the serum samples
The 1H NMR spectra of serum samples from the Sham and MCAO groups were acquired as described above and shown in Figure 1. Although the profiles of the 1H NMR spectra had many similar characteristic peaks, differences were observed in the major signals between the Sham and MCAO groups by close visual inspection of 1H NMR spectra. The signals were assigned on the basis of previously published data [26-29] and the Human Metabolomics Database (www.hmdb.com). What’s more, the PLS-DA method was applied to the 1H NMR data using total integral normalization method.
Figure 1.
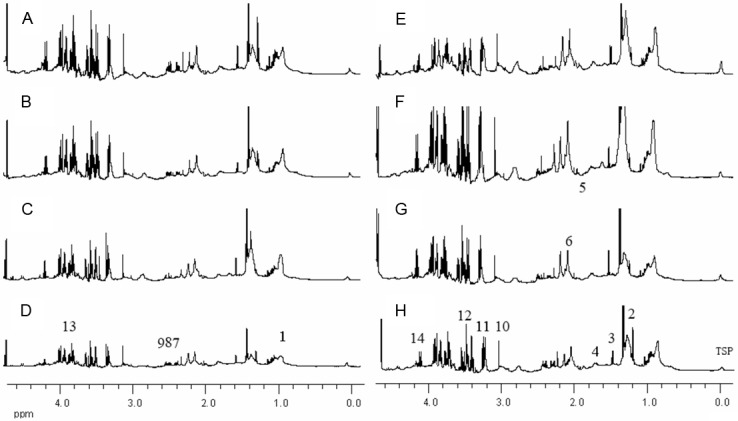
Partial 600 MHZ 1H NMR spectra of serum samples for rat with ischemia/reperfusion. Sham group (A), MCAO groups (B for MCAO0, C for MCAO0.5, D for MCAO1, E for MCAO3, F for MCAO6, G for MCAO12 and H for MCAO24.). The metabolites quantified in Table 1 are assigned as: 1. L-Valine; 2. L-Alanine; 3. L-Lactic acid; 4. Ornithine; 5. Acetic acid; 6. Glutamic acid; 7. Succinic acid; 8. Pyruvic acid; 9. Citric acid; 10. Malonic acid; 11. Betaine; 12. Glycine; 13. L-Serine.
Metabolites changing between sham and MCAO groups
The multivariate statistical (PLS-DA) and unpaired t-tests methods are used to uncover the metabolites that show a statistically significant concentration difference between the two observed sham group and each of the MCAO groups. The scores plots obtained from PLS-DA were shown in Figure 2. With the total integral normalization, the PC1 vs. PC2 plots show that the metabolite profiles of serum samples obtained from each of the combination were clearly separated along the first two PCs.
Figure 2.
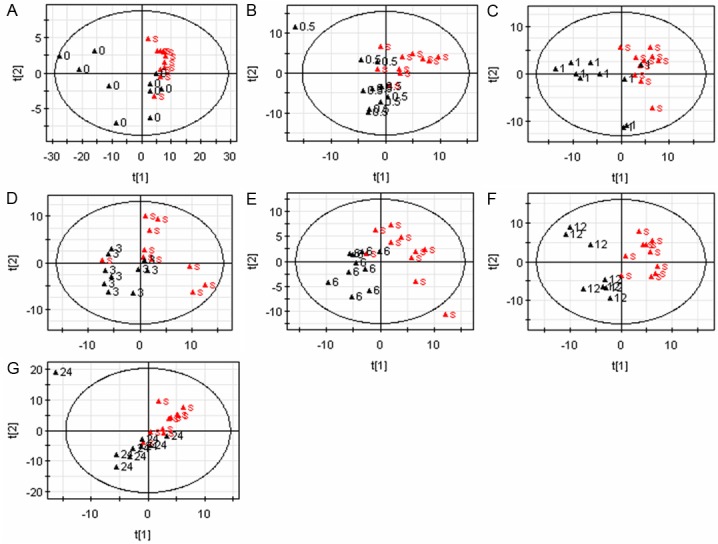
PLS-DA scores plots derived from the serum samples for rat with ischemia/reperfusion. Sham group (▲s) and the MCAO groups (▲0 for MCAO0, ▲0.5 for MCAO0.5, ▲1 for MCAO1, ▲3 for MCAO3, ▲6 for MCAO6, ▲12 for MCAO12 and ▲24 for MCAO24). Sham vs. MCAO0 (A), Sham vs. MCAO0.5 (B), Sham vs. MCAO1 (C), Sham vs. MCAO3 (D), Sham vs. MCAO6 (E), Sham vs. MCAO12 (F) and Sham vs. MCAO24 (G).
The loading plots of the first two PCs (Figure S1), were examined to determine the spectral regions (variables) and metabolites contributing to the separation on the PLS-DA maps. The spectral regions contributing to the separation and quantitative changes of the metabolites are listed in Table 1. By inspection of the loadings plots as well as the 1H NMR spectra, the most distinctive metabolic changes in MCAO groups seemed to be: ornithine, L-lactic acid, pyruvic acid, betaine, glutamic acid, glycine, L-serine, L-alanine, succinic acid, acetic acid, malonic acid, citric acid, L-valine (Table 1).
Table 1.
Metabolite NMR assignments and the changes of their relative concentrations in serum between Sham group and MCAO groups
| A. | |||||||||
|
| |||||||||
| Metabolites | Integrated spectral region | MACO0 | MACO0.5 | MACO1 | MACO3 | MACO6 | MACO12 | MACO24 | |
|
| |||||||||
| Ornithine | 1.72, 1.82, 1.93, 3.04 | ↑ | ↑ | ||||||
| L-Lactic acid | 1.32, 4.10 | ↓ | ↓ | ||||||
| Pyruvic acid | 2.46 | ↓ | ↓ | ↑ | |||||
| Betaine | 3.25, 3.89 | ↓ | ↓ | ||||||
| Glutamic acid | 2.12, 2.44, 3.76 | ↑ | ↑ | ↑ | |||||
| Glycine | 3.54 | ↑ | |||||||
| L-Serine | 3.96, 3.83 | ↓ | |||||||
| L-Alanine | 1.46, 3.76 | ↓ | |||||||
| Succinic acid | 2.39 | ↑ | |||||||
| Acetic acid | 1.91 | ↓ | ↓ | ↓ | ↑ | ||||
| Malonic acid | 3.11 | ↑ | ↑ | ↑ | |||||
| Citric acid | 2.65, 2.53 | ↑ | |||||||
| L-Threonine | 1.34, 3.58, 4.25 | ↓ | ↓ | ||||||
| L-Valine | 0.98, 1.03, 2.26, 3.6 | ↓ | ↓ | ↓ | |||||
|
| |||||||||
| B. | |||||||||
|
| |||||||||
| Metabolites | ppm | sham | MACO0 | MACO0.5 | MACO1 | MACO3 | MACO6 | MACO12 | MACO24 |
|
| |||||||||
| L-Valine | 1.03 | 0.0119±0.0029 | 0.0102±0.0037 | 0.0133±0.0038 | 0.0100±0.0023 | 0.0156±0.0026** | 0.0150±0.0036* | 0.0171±0.0042** | 0.0136±0.0031 |
| L-Lactic acid | 1.32 | 0.0468±0.0174 | 0.0416±0.0155 | 0.0455±0.0135 | 0.0641±0.0286 | 0.0471±0.0094 | 0.0387±0.0132 | 0.0425±0.0149 | 0.0370±0.0050 |
| L-Alanine | 1.46 | 0.0076±0.0020 | 0.0072±0.0022 | 0.0076±0.0019 | 0.0090±0.0027 | 0.0081±0.0022 | 0.0099±0.0012** | 0.0087±0.0015 | 0.0088±0.0026 |
| Ornithine | 1.72 | 0.0066±0.0014 | 0.0061±0.0016 | 0.0065±0.0015 | 0.0070±0.0021 | 0.0068±0.0012 | 0.0084±0.0010** | 0.0076±0.0009 | 0.0076±0.0016 |
| Acetic acid | 1.91 | 0.0028±0.0013 | 0.0020±0.0014 | 0.0018±0.0011 | 0.0021±0.0012 | 0.0024±0.0011 | 0.0044±0.0013** | 0.0031±0.0020 | 0.0038±0.0016 |
| Glutamic acid | 2.12 | 0.0149±0.0050 | 0.0163±0.0085 | 0.0197±0.0056* | 0.0115±0.0076 | 0.0214±0.0052** | 0.0187±0.0054 | 0.0207±0.0042** | 0.0180±0.0053 |
| Succinic acid | 2.39 | 0.0034±0.0010 | 0.0027±0.0015 | 0.0032±0.0014 | 0.0031±0.0012 | 0.0040±0.0012 | 0.0051±0.0013** | 0.0052±0.0010** | 0.0045±0.0015* |
| Pyruvic acid | 2.46 | 0.0042±0.0013 | 0.0030±0.0019 | 0.0037±0.0019 | 0.0029±0.0013* | 0.0047±0.0016 | 0.0057±0.0016** | 0.0061±0.0013** | 0.0056±0.0013* |
| Citric acid | 2.53 | 0.0009±0.0008 | 0.0014±0.0011 | 0.0017±0.0011* | 0.0012±0.0009 | 0.0011±0.0008 | 0.0016±0.0010 | 0.0009±0.0009 | 0.0013±0.0007 |
| Malonic acid | 3.11 | 0.0021±0.0017 | 0.0021±0.0016 | 0.0036±0.0022 | 0.0015±0.0013 | 0.0041±0.0024* | 0.0042±0.0027** | 0.0045±0.0019** | 0.0039±0.0023* |
| Betaine | 3.25 | 0.0176±0.0046 | 0.0138±0.0057 | 0.0164±0.0072 | 0.0108±0.0075** | 0.0172±0.0058 | 0.0155±0.0055 | 0.0122±0.0083* | 0.0184±0.0035 |
| Glycine | 3.54 | 0.0063±0.0038 | 0.0048±0.0025 | 0.0082±0.0041 | 0.0054±0.0051 | 0.0085±0.0031 | 0.0133±0.0090* | 0.0125±0.0066** | 0.0096±0.0042 |
| L-Serine | 3.83 | 0.0100±0.0027 | 0.0096±0.0032 | 0.0129±0.0035* | 0.0101±0.0039 | 0.0118±0.0022 | 0.0140±0.0051* | 0.0136±0.0041* | 0.0114±0.0024 |
| L-Threonine | 4.25 | 0.0041±0.0024 | 0.0033±0.0020 | 0.0051±0.0017 | 0.0050±0.0017 | 0.0034±0.0013 | 0.0039±0.0020 | 0.0046±0.0020 | 0.0036±0.0018 |
The symbols (↑ and ↓) indicate the increase and decrease of the metabolite levels. All metabolite levels represent mean ± SD. vs. Sham group t-test revealed the significant differences between each combination
p<0.05;
p<0.01.
Among that, the most obvious variable metabolites are malonic acid and glycine. During I/R, the level of malonic acid was increased at 3 h (P<0.05), then peaked at 12 h (P<0.01) after reperfusion. The level of glycine was peaked at 6 h (P<0.01), subsequently declined at 12 h after reperfusion, and finally dropped to the base line (P>0.05) (Figure 3). According to the results, 13 metabolites mentioned above, tended to reveal pathophysiological process of ischemic stroke. Moreover, malonic acid as well as glycine contributed to have the making of potential ischemic stroke biomarker.
Figure 3.
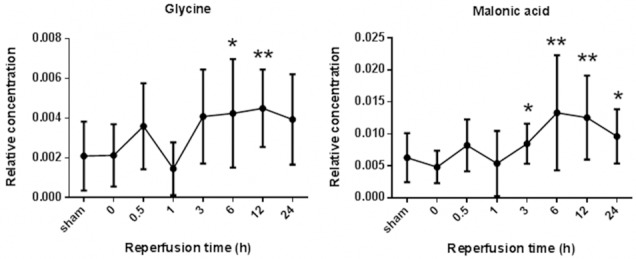
The dynamic changes of glycine and malonic acid relative concentrations between Sham group and MCAO groups.
Discussion
Nowadays, CT angiography, CT perfusion techniques and MRI-based techniques contribute to diagnose patients with stroke [30]. However, CT angiography and CT perfusion techniques exist insensitive to ischemic stroke. Most hospitals do not have these specialized MRI services available in the acute setting. Thus, searching useful biomarkers in ischemic stroke has been absorbing more attention. The ischemic period in particular is accompanied with significant dynamic alterations in the metabolites expression. Here, our study showed 13 metabolites changed significantly in the serum. In particular, by contrast, malonic acid and glycine are the most noticeable variable metabolites.
Among these metabolites, some were closed associated with energy metabolism. We observed significantly decreased but finally elevated levels of pyruvate from serum, as well as sustained reduced level of lactate. Lactate and pyruvate are known to involve in anaerobic glycolysis of survived cells, that proceed to metabolize glucose under local hypoxic conditions. Increased significantly but transiently level of citric acid was detected in our study, which participates in tricarboxylic acid (TCA) cycle. Sustainted declining level of valine was shown during from 0.5 h to 3 h after repersusion. The catabolic pathway of valine consists of several enzymatic steps and results in the formation of succinyl-CoA, a member of the TCA cycle [31]. The level of acetic acid was decreased initially, but elevated finally. Acetic acid can be converted to acetyl-CoA, indirectly associated with TCA cycle. As a neurotoxin, malonic acid acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain. From 0.5 h to 3 h after repersusion, the level of malonic acid was increased and may contribute to impaire energy metabolism. Strikingly, although the level of malonic acid was changed obviously in these metabolites, to our knowledge, few reports reveal the relationship between that with ischemic stroke. However, we have found no significant changed malonic acid in the cerebrospinal fluid, in our previous study.
Betaine, along with vitamins B6 and B12 and folic acid, helps reduce higher levels of homocysteine [32]. Having high levels of homocysteine is related to a higher risk of heart disease and stroke. As a protective substance made in the body, we revealed it was lessened at 0 h and 6 h after reperfusion. That suggested corresponding protective mechanism was destroyed by ischemic stroke [18].
Glutamic acid is a major excitatory neurotransmitter in the central nervous system, also as known as a prime example of an excitotoxin in ischemic stroke [33]. Metabolism of valine seems to be important in the process of glutamate translocation between astrocytes and neurons during glutamatergic signaling. Here, we found significant increased the level of glutamic acid exist in serum during 6 h to 24 h after reperfusion. Interestingly, in our previous study, the increased level of glutamic acid in the cerebrospinal fluid was culminated at 6 h, and decreased to the normal at 24 h after reperfusion. It seems that the changed glutamate in serum corresponds to the one in cerebrospinal fluid.
Similarly relationship between serum and cerebrospinal fluid occurs in glycine. We just found the level of glycine was elevated at 24 h after reperfusion during the whole I/R proceeding. In combination with our previous study, elevated level of glycine is liable to connect with its sustained decreased level in cerebrospinal fluid during the whole 24 h I/R. Glycine is biosynthesized in the body from serine via tetrahydrofolate. Glycine seemingly exhibits protection against ischemic damage [34,35], whereas some studies suggest glycine may contribute to the development of ischemic injury [36,37]. In any case, obvious changed glycine plays a constructive role in treatment of stroke.
In conclusion, our study reveals that dynamic metabolic alterations already emerge in the serum of an early stage of I/R rats. 13 significant changed metabolites were verified during different early I/R periods. Specially, dramatic changed malonic acid and glycine more exhibit possibility to be biomarkers in this study. That may be conducive to distinguish at-risk individuals, benefit early diagnosis and understand the pathogenesis of cerebral ischemia.
Acknowledgements
This work was supported by Xuzhou Medical College Found (NO. 2012KJZ21), Xianyang Basic Research Found (NO. 2011k13-03), Shannxi Nuclear Industrial Geological Bureau Research Found (NO. kj201117).
Disclosure of conflict of interest
The authors declare no conflict of interest.
Supporting Information
References
- 1.Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681–1692. doi: 10.1016/S0140-6736(11)60516-3. [DOI] [PubMed] [Google Scholar]
- 2.Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, Ferriero D, Jones BV, Kirkham FJ, Scott RM, Smith ER. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacco RL, Chong JY, Prabhakaran S, Elkind MS. Experimental treatments for acute ischaemic stroke. Lancet. 2007;369:331–341. doi: 10.1016/S0140-6736(07)60155-X. [DOI] [PubMed] [Google Scholar]
- 7.Unal-Cevik I, Kilinc M, Can A, Gursoy-Ozdemir Y, Dalkara T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke. 2004;35:2189–2194. doi: 10.1161/01.STR.0000136149.81831.c5. [DOI] [PubMed] [Google Scholar]
- 8.Sumbria RK, Klein J, Bickel U. Acute depression of energy metabolism after microdialysis probe implantation is distinct from ischemia-induced changes in mouse brain. Neurochem Res. 2011;36:109–116. doi: 10.1007/s11064-010-0276-2. [DOI] [PubMed] [Google Scholar]
- 9.Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci. 1999;19:34–39. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melani A, Pantoni L, Corsi C, Bianchi L, Monopoli A, Bertorelli R, Pepeu G, Pedata F. Striatal outflow of adenosine, excitatory amino acids, gamma-aminobutyric acid, and taurine in awake freely moving rats after middle cerebral artery occlusion: correlations with neurological deficit and histopathological damage. Stroke. 1999;30:2448–2454. doi: 10.1161/01.str.30.11.2448. discussion 2455. [DOI] [PubMed] [Google Scholar]
- 11.Issaq HJ, Van QN, Waybright TJ, Muschik GM, Veenstra TD. Analytical and statistical approaches to metabolomics research. J Sep Sci. 2009;32:2183–2199. doi: 10.1002/jssc.200900152. [DOI] [PubMed] [Google Scholar]
- 12.Dutta M, Joshi M, Srivastava S, Lodh I, Chakravarty B, Chaudhury K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol Biosyst. 2012;8:3281–3287. doi: 10.1039/c2mb25353d. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Wang S, Hao F, Li Y, Tang H, Shi X. NMR analysis of the rat neurochemical changes induced by middle cerebral artery occlusion. Talanta. 2012;88:136–144. doi: 10.1016/j.talanta.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Jung JY, Lee HS, Kang DG, Kim NS, Cha MH, Bang OS, Ryu do H, Hwang GS. 1H-NMR-based metabolomics study of cerebral infarction. Stroke. 2011;42:1282–1288. doi: 10.1161/STROKEAHA.110.598789. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Sun J, Liang Q, Cai Y, Li S, Huang Y, Wang Y, Luo G. A metabonomic approach applied to predict patients with cerebral infarction. Talanta. 2011;84:298–304. doi: 10.1016/j.talanta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang Y, Li M, Gu T, Xu P, Ma T, Gu S. 1H NMR-based Metabolomics Exploring Biomarkers in Rats Cerebralspinal Fluid After Cerebral Ischemia/Reperfusion. Mol Biosyst. 2013;9:431–9. doi: 10.1039/c2mb25224d. [DOI] [PubMed] [Google Scholar]
- 19.Berger C, Xia F, Maurer MH, Schwab S. Neuroprotection by pravastatin in acute ischemic stroke in rats. Brain Res Rev. 2008;58:48–56. doi: 10.1016/j.brainresrev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Khandelwal P, Beyer CE, Lin Q, Schechter LE, Bach AC 2nd. Studying rat brain neurochemistry using nanoprobe NMR spectroscopy: a metabonomics approach. Anal Chem. 2004;76:4123–4127. doi: 10.1021/ac049812u. [DOI] [PubMed] [Google Scholar]
- 21.Teahan O, Gamble S, Holmes E, Waxman J, Nicholson JK, Bevan C, Keun HC. Impact of analytical bias in metabonomic studies of human blood serum and plasma. Anal Chem. 2006;78:4307–4318. doi: 10.1021/ac051972y. [DOI] [PubMed] [Google Scholar]
- 22.Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, Smilde AK. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem. 2006;78:567–574. doi: 10.1021/ac051495j. [DOI] [PubMed] [Google Scholar]
- 23.Um SY, Chung MW, Kim KB, Kim SH, Oh JS, Oh HY, Lee HJ, Choi KH. Pattern recognition analysis for the prediction of adverse effects by nonsteroidal anti-inflammatory drugs using 1H NMR-based metabolomics in rats. Anal Chem. 2009;81:4734–4741. doi: 10.1021/ac9000282. [DOI] [PubMed] [Google Scholar]
- 24.Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, Nunez D, Sweatman BC, Haselden JN, Cox RD, Connor SC, Griffin JL. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics. 2007;29:99–108. doi: 10.1152/physiolgenomics.00194.2006. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Zheng C, Lanza IR, Nair KS, Raftery D, Vitek O. Interdependence of signal processing and analysis of urine 1H NMR spectra for metabolic profiling. Anal Chem. 2009;81:6080–6088. doi: 10.1021/ac900424c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu H, Pan Z, Xi B, Hainline BE, Shanaiah N, Asiago V, Gowda GA, Raftery D. 1H NMR metabolomics study of age profiling in children. NMR Biomed. 2009;22:826–833. doi: 10.1002/nbm.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreadou I, Papaefthimiou M, Zira A, Constantinou M, Sigala F, Skaltsounis AL, Tsantili-Kakoulidou A, Iliodromitis EK, Kremastinos DT, Mikros E. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009;22:585–592. doi: 10.1002/nbm.1370. [DOI] [PubMed] [Google Scholar]
- 28.Teng R, Junankar PR, Bubb WA, Rae C, Mercier P, Kirk K. Metabolite profiling of the intraerythrocytic malaria parasite Plasmodium falciparum by (1)H NMR spectroscopy. NMR Biomed. 2009;22:292–302. doi: 10.1002/nbm.1323. [DOI] [PubMed] [Google Scholar]
- 29.Viant MR, Lyeth BG, Miller MG, Berman RF. An NMR metabolomic investigation of early metabolic disturbances following traumatic brain injury in a mammalian model. NMR Biomed. 2005;18:507–516. doi: 10.1002/nbm.980. [DOI] [PubMed] [Google Scholar]
- 30.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, Schramm P, Juttler E, Oehler J, Hartmann M, Hahnel S, Knauth M, Hacke W, Sartor K. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 31.Murin R, Mohammadi G, Leibfritz D, Hamprecht B. Glial metabolism of valine. Neurochem Res. 2009;34:1195–1203. doi: 10.1007/s11064-008-9895-2. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson W, Elmslie J, Lever M, Chambers ST, George PM. Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am J Clin Nutr. 2008;87:577–585. doi: 10.1093/ajcn/87.3.577. [DOI] [PubMed] [Google Scholar]
- 33.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 34.Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, Bashkatova VG, Kovalenko AV, Kudrin VS, Yakovleva EV. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc Dis. 2000;10:49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- 35.Petrat F, Boengler K, Schulz R, de Groot H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br J Pharmacol. 2012;165:2059–2072. doi: 10.1111/j.1476-5381.2011.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T, Sato K, Narisawa A, Aoki Y, Matsubara Y, Omae T, Mizoi K, Kinouchi H. Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke. 2007;38:2157–2164. doi: 10.1161/STROKEAHA.106.477026. [DOI] [PubMed] [Google Scholar]
- 37.Yao W, Ji F, Chen Z, Zhang N, Ren SQ, Zhang XY, Liu SY, Lu W. Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke. 2012;43:2212–2220. doi: 10.1161/STROKEAHA.111.645994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


