Abstract
The appearance of proliferating bile ductular structures, which is called the “atypical ductular reaction” is frequently observed in various chronic liver diseases associated. However, the origin of these increased bile ductules has been a matter of controversy. In this study, we investigated the origin of ductular cells as an aspect of relation between epithelial to mesenchymal transition (EMT) and epithelial members of liver parenchyme, such as hepatocyte and cholangiocyte by immunohistochemical staining of human liver. Thirteen specimens of surgically resected liver with biliary cirrhosis were selected. Three sets of double immunohistochemical stains were done; Hep-Par 1 - cytokeratin 19 (CK19), Hep-Par 1 - α-sm ooth mus cle actin (α-SMA) and CK19 - α-SMA. As a result, we investigated the dual expression of the markers of hepatocyte and cholangiocyte in the same cell; in ductular cell and surrounding hepatocyte. However, there seems to be no dual expression of markers for EMT with epithelial markers. This study suggests a possibility of phenotypic change of mature hepatocyte into cholangiocyte. Future studies will be necessary to determine the role that proliferating cholangiocytes play in the pathogenesis of biliary fibrosis and how cholangiocytes interact with other cell types of the liver such as hepatic stellate cells or Kupffer cells.
Keywords: Liver, hepatocyte, bile duct cell, ductular reaction, transdifferentiation, immunohistochemistry
Introduction
The liver is mainly composed of two types of epithelial cells, hepatocytes and cholangiocytes. Cholangiocytes line the intrahepatic and extrahepatic bile duct system of the liver. The bile ductules and ducts are comprised of a branched system of interconnected tubes [1] which collect bile secreted at the canalicular membranes of hepatocytes [2], and deliver it to the gallbladder or the small intestine [3]. Although cholangiocytes represent a small proportion (3 to 5%) of the liver cells [3], these cells play an important pathophysiological role in the modification of the composition of bile during the transit through the bile ducts, which involves the secretion and absorption of water, electrolytes, and other organic solutes from hepatocellular bile [3].
Cholangiocytes are the target cells of a number of diseases termed cholangiopathies [4]. Cholangiopathies are predominantly characterized by a bile duct-directed inflammatory response that leads to bile duct injury associated with biliary proliferation in the early stage of the disease course [5]. If the biliary injury is chronic there will be an increased biliary fibrosis, loss of bile duct structures, and an increase in the incidence of bile duct cancer (i.e., cholangiocarcinoma) [5].
The appearance of proliferating bile ductular structures is frequently observed in various chronic liver diseases associated, including chronic hepatitis and cirrhosis [6,7]. This process is called the “atypical ductular reaction”. This ductular reaction gradually replaces the hepatic parenchyma and causes a gradual decrease in mature hepatocytes in the hepatic lobules or regenerative nodules [8].
It is important to precisely understand the origin and pathogenesis of the atypical ductular reaction because the prevention of its progression can be beneficial maintaining the proper functions of the liver [9]. However, the origin of these increased bile ductules has been a matter of controversy [8]. The cellular changes might be due to hepatic stem/progenitor cell activation, proliferation of preexisting cholangiocytes, or ductular metaplasia of mature hepatocytes [6,10]. While most investigators embrace the notion that the ductular reaction represents regenerative proliferation of bipotential hepatic stem/progenitor cells [11,12], there is no definitive evidence to support this [8].
Some recent studies have announced a new concept for the origin of ductular cells that the ductular reaction is one part of epithelial mesenchymal transition (EMT) process of hepatocyte or cholangiocyte during liver fibrosis. EMT is a process that is normally observed in embryonic stages of development, organ fibrosis, or wound healing, and it has recently been investigated as a mechanism of cancer cell migration and metastasis [13,14]. It is characterized by the loss of epithelial characteristics (E-cadherin) and the acquisition of a mesenchymal phenotype (vimentin, fibronectin and α-smooth muscle actin (α-SMA)) [15,16]. Because the liver is an organ prone to fibrosis and the origin of fibroblastic cells in fibrotic liver is still being debated, the possibility that liver epithelial cells participate in the fibrosis by EMT is appealing [17,18].
The aim of this study is to investigate the origin of ductular cells that might be transitioning from hepatocytes to cholangiocytes by immunohistochemical staining of human liver with ductular proliferation.
Materials and methods
Specimen selection
Biliary cirrhosis, known as cirrhotic liver with proliferation of bile ductular structure, was enrolled in this study. Cirrhosis was diagnosed by vascular septa formation and regenerative nodules. The vascular septum and ductular proliferation were confirmed by Masson’s trichrome stain and immunohistochemical stain of cytokeratin (CK)7, respectively. Fibrosis in subcapsular area and fibrous capsule formation of peritumoral lesions were excluded from this study.
Surgically resected liver specimen with no pathologic abnormality, such as traumatic lacerated liver, was used as negative control.
The histologic features from all specimens were reviewed by two pathologists. The histologic data were obtained from the pathologic report and microscopic review of Hematoxylin and Eosin (H & E) stained slides.
Altogether, 13 specimens of surgically resected liver were gathered from the Department of Surgical Pathology in Daegu Catholic Medical Center. The patients’ biological data and personal information were collected by reviewing the medical records such as sex, age, previous or present diagnoses of underlying liver disease, and date of operation.
The patient consent was not required because this research was a retrospective chart review and personal identifiable information were not included.
Immunohistochemistry
The formalin-fixed paraffin embedded tissues were cut into 4 μm sections and deparaffinized. Immunohistochemical staining using the BOND-MAX slide stainer (Leica Biosystems, Germany) was carried out in accordance with the manufacturer’s instructions. Hep-Par 1 (1:400, Dako-patts, Copenhagen, Denmark), CK19 (1:200, BioGenex, Netherland), CK7 (1:500, BioGenex, Netherland), and α-SMA (1:500, Dako-patts, Copenhagen, Denmark) were applied to the sections. Simple immunohistochemical stain of CK7 was done using Bond polymer refine detection kit (DAB, 3,3’-diaminobenzidine tetrahydrochloride, Leica Biosystems, Germany). Three sets of double immunohistochemical stains were done; Hep-Par 1 - CK19, Hep-Par 1 - α-SMA and CK19 - α-SMA. The double immunohistochemical stains were done by combination of Bond polymer refine detection kit and Bond primer Ap Red detection kit (fast red, Leica Biosystems, Germany).
Cytoplasmic staining of granular pattern was interpreted as positive for Hep-Par 1, and diffuse cytoplasmic staining was interpreted as positive for CK19, CK7, and α-SMA. Any other patterns of nuclear staining were regarded as false positive.
The stained sections were reviewed without any knowledge of the clinical data of patient cohort.
Results
Clinicopathological characteristics of patients
A total of 13 cases of biliary cirrhosis were analyzed from 11 men and 2 women with ages ranging from 36 to 64 years (mean, 54.1 years; median, 64.0 years). Etiologic factors of cirrhosis were hepatitis B viral (HBV) hepatitis in 11 patients, during the other 2 cases were caused by alcoholism and autoimmune response, respectively. Steatosis was observed in 2 cases; one case was associated with alcoholism and the other was associated with HBV.
Microscopic findings
All selected experimental cases showed well-defined regenerative nodules surrounded by thick fibrous septa with collagen deposition. The vascular septa bridge portal to central, which is normally connected only via the sinusoids (Figure 1A and 1B). The inflammatory infiltrates are variable and are predominantly composed of lymphoid and histiocytic cells in periportal, perilobular and sometimes centrilobular areas. The proliferation of flattened small cells with or without luminal space is observed in septal or intralobular area, which is called ductular reaction (Figure 1C and 1D). There is no visible bile in cytoplasm of the ductular cells.
Figure 1.
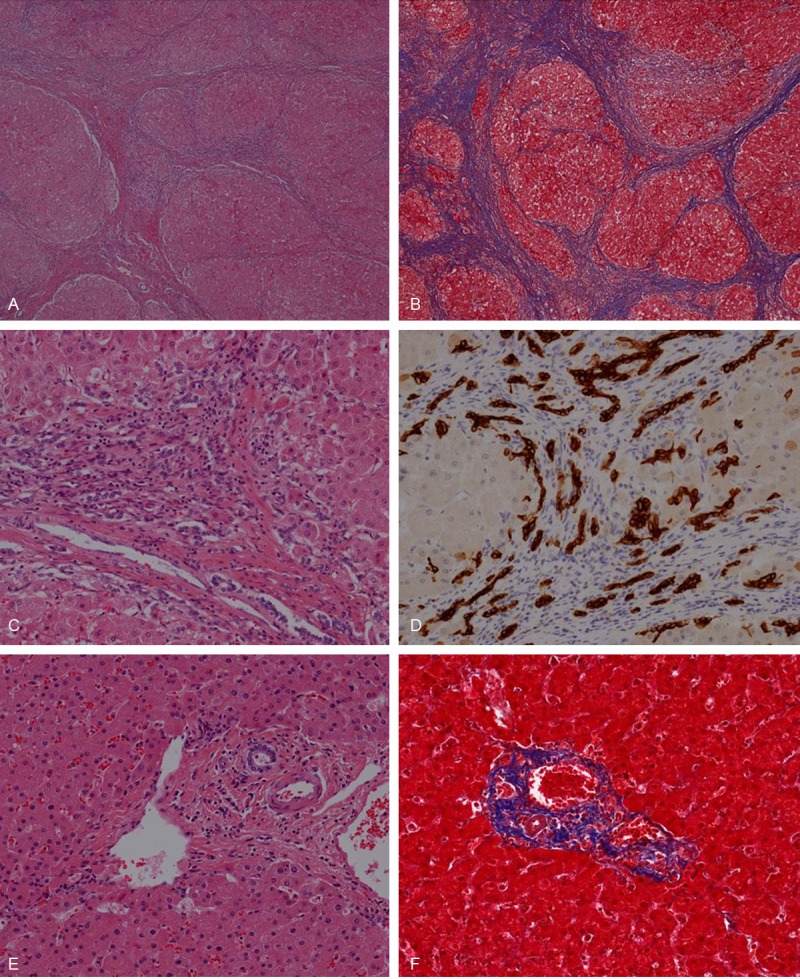
Microscopic findings well-defined regenerative nodules surrounded by thick fibrous septa with collagen deposition are seen in H & E (A) and Masson’s trichrome (B) stain. Cholangiocytes of bile ductule proliferate in septal area (C) demonstrated with CK7 immunohistochemically (D). In control cases, well-formed portal triads which consist of vein (portal vein), artery (hepatic artery) and biliary tract are seen (E). Collagen deposition is observed only in portal area and its close periphery (F). (A, C, E) Hematoxylin and eosin; (B, F) Masson’s trichrome; (A and B) x 4; (C to F) x 200.
In control cases, well-formed portal triads which consist of vein (portal vein), artery (hepatic artery) and biliary tract are seen (Figure 1E). Two or three bile ducts/ductiles exist in portal area. The ductular system is composed of short/low columnar or cuboidal cells with uniform round nuclei that are evenly spaced in basal nuclear location, which make round to oval or tubular luminal space. Collagen deposition is observed only in portal area and its close periphery (Figure 1F).
Expression of Hep-Par 1 and CK19
Most of the ductular cells show positive immunoreactivity for CK19. A few ductular cells have positivity for Hep-Par 1 and these cells are scattered in solitary or in small clusters of 2 or 3 cells. The cells which express CK19 and Hep-Par 1 are mixed together in mosaic pattern. Especially, the Hep-Par 1-positive ductular cells are found more frequently in perilobular or centrilobular area than in the intraseptal space. The dual expressions of Hep-Par 1 and CK19 in the same cell have also been observed (Figure 2).
Figure 2.
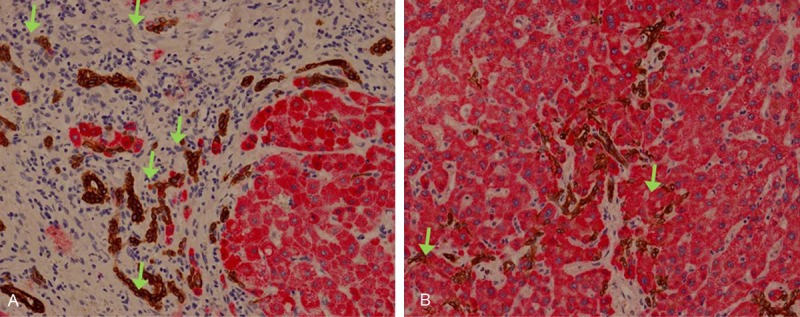
Expression of Hep-Par 1 and CK19. Ductular cells in perilobular (A) and centrilobular (B) area express Hep-Par 1 (red) or CK19 (brown), sometimes both in the same cell (arrows) (x 200).
Expression of α-SMA compared to Hep-Par 1 and CK19
The immunohistochemical stain of α-SMA was shown positive in myofibroblast, vascular wall of vein and artery in portal area and quiescent and reactive hepatic stellate cells (HSCs) in lobular space, in both experimental and control group. The hepatocytes, cholangiocytes or ductular cells show negative immunoreactivity for α-SMA. The expression of α-SMA is not overlapped with those of Hep-Par 1 or CK19 (Figure 3).
Figure 3.
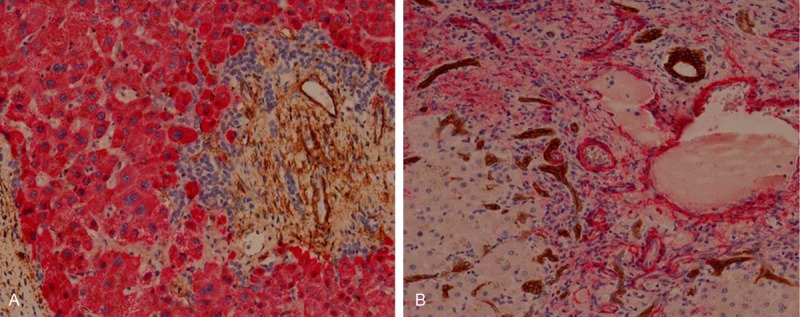
Expression of α-SMA compared to Hep-Par 1 and CK19. α-SMA is expressed in fibrous extracelluar space and in muscular cells of vascular wall, but not in ductular cells. The expression of α-SMA is not overlapped with Hep-Par 1 (A) or CK19 (B). (A) Hep-Par 1 (red), α-SMA (brown); (B) CK19 (brown), α-SMA (red), all x 200.
Discussion
A number of studies have defined three types of cholangiocyte proliferation: “typical”, “atypical”, and oval cell proliferation [19]. Firstly, “typical” cholangiocyte proliferation is a hyperplastic reaction, which induces an increase in the number of intrahepatic bile ducts (with a well-defined lumen) confined to portal areas [3,20]. In animal models, “typical” cholangiocyte proliferation is achieved by a number of experimental manoeuvres, including bile duct ligation (BDL) [3], partial hepatectomy [21], acute carbon tetrachloride (CCl4) treatment [22,23] and chronic feeding of alpha-naphthyl isothiocyanate (ANIT) [24], or bile salts [25]. “Typical” cholangiocyte proliferation is usually found in acute obstructive cholestatic liver disease, extrahepatic biliary atresia, or early phase of chronic cholestatic liver disease in human [26]. “Atypical” cholangiocyte proliferation, so called “(atypical) ductular reaction”, is an irregular proliferation of intrahepatic bile ducts sprouting into periportal and parenchymal regions and occasionally forming anastomosing cords with the adjacent hepatocytes [7,27]. These proliferated cholangiocytes are characterized by poorly formed lumen, edema and inflammatory infiltrates [26]. The “atypical” cholangiocyte proliferation/ductular reaction is commonly seen in patients with alcoholic liver disease, long standing extrahepatic bile duct obstruction, focal nodular hyperplasia [26], as well as prolonged cholestatic liver diseases such as primary biliary cirrhosis or primary sclerosing cholangitis [7,26,27]. Although there are some evidences that the “atypical” cholangiocyte proliferation is a course of metaplasia or transition from hepatic cord to bile ductule, and not a proliferation of ordinary ductule cells [28], most of the studies support the hypothesis that this is the proliferation of pre-existing bile ductules or putative hepatic progenitor cells [6]. Lastly, oval cell proliferation is observed in the early stage of carcinogenesis induced by many chemicals such as ethionine in rat liver [6].
The origin of ductular cell and pathogenesis of ductular reaction are not clearly established. In this study, we investigated the dual expressions of markers of hepatocyte (Hep-Par 1) and cholangiocyte (CK19) in one cell; ductular cell and surrounding (mature) hepatocyte. This result suggests a possibility of phenotypic change of a mature hepatocyte into a cholangiocyte. Although it has been postulated that the hepatocytic phenotype is fixed once the cells are terminally differentiated, some investigators have demonstrated that hepatocytes are able to transdifferentiate into bile ductule/duct-like cells [28-32]. Nishikawa and his coworkers reported that the mature hepatocytes could be differentiated into bile ductular cells by culturing in a type I collagen gel matrix with the presence of insulin and epidermal growth factor (EGF) or hepatocyte growth factor [29]. This phenotypic change is characterized by a loss of hepatocytic differentiation markers and the expression of bile ductular markers such as CK19, but it is not associated with the re-expression of markers of hepatic progenitor cells [8]. They also suggested that hepatocytic and bile ductular phenotypes may be mutually reversible [9]. They demonstrated that the transdifferentiated hepatocytes in vitro could recover the original hepatocytic phenotypes if they were placed in a more physiological environment [9]. The transdifferentiated cells retrieved from the collagen gel matrix spontaneously formed spheroidal aggregates on Matrigel-coated surfaces. In their preliminary experiments, cells plated with a serum-containing medium were spread across the surface without forming spheroids, and there was no recovery of DsRed2 fluorescence [9]. The recovery of albumin expression in transdifferentiated hepatocytes was enhanced by Dex, which has been shown to increase albumin and TAT expression in spheroidal aggregate cultures of newborn rat hepatocytes [33] and decrease CK19 mRNA expression in monolayer-cultured rat hepatocytes [28]. Other investigators have also reported similar findings regarding the ductular transdifferentiation of hepatocytes both in vitro and in vivo [30,32,34]. These studies demonstrated the phenotypic plasticity of mature hepatocytes and suggested that the bile ductular transdifferentiation might be involved in ductular reaction [8].
The hepatic progenitor cell is a bipotential cell that has ability to differentiate into both hepatocyte and cholangiocyte. While most investigators embrace the notion that the ductular reaction represents regenerative proliferation of bipotential hepatic stem/progenitor cells [11,12], there is no definitive evidence for this [8]. Noteboom et al [35] showed that the organization of the transplanted embryonic day 14 hepatocytes into the spleen of adult syngeneic rats was accompanied by the formation and maturation of bile ducts around these developing lobules. The morphological differentiation of the emerging bile ducts was accompanied by a gradual loss of hepatocyte markers and a gradual acquisition of cholangiocyte markers, with markers identifying a large-cholangiocyte phenotype appearing the latest. However, these evidences are only for the development of fetal cells in embryology, and not in adult tissue or human tissue. Because the methodology of our study was designed around a retrospective study which uses formalin fixed paraffin embedded specimen, the works for hepatic progenitor cells were not composed in our study.
Because the liver is an organ prone to fibrosis and the origin of fibroblastic cells in fibrotic liver is still being debated, the possibility that the liver epithelial cells participate in fibrosis by EMT is appealing [17,18]. However, there are conflicting data on whether or not EMT occurs in tissue fibrosis [36]. Many studies of EMT in fibrosis have failed to define EMT rigorously or to differentiate between the transition to a mesenchymal (EMT) versus a myofibroblast (EMyT) phenotype [16,37]. Nevertheless, surrogate fibroblast markers have often been used to identify EMT, most notably fibroblast-specific protein-1 (FSP-1), despite some data suggesting that it is nonspecific [38,39]. However the assumption that liver epithelial cells undergo EMT in liver fibrosis cannot be ruled out for biliary epithelial cells. Indeed, the biliary epithelial cell EMT could represent a cellular mechanism supporting histological observations [38]. “Ductular reaction” (i.e. “reactive cholangiocytes”) expresses a variety of pro-fibrogenic growth factors and cytokines and are likely to contribute to fibrosis and inflammation by promoting activation, proliferation, and collagen synthesis in the surrounding pro-fibrogenic cells [40-43]. Nevertheless, the possibility of a direct contribution of cholangiocytes to fibrosis via EMT was suggested by Omenetti and his colleagues [44], by showing in vitro a complete EMT in an immature cholangiocyte cell line treated with activated HSC conditioned medium. Irrespective of the underlying etiology, biliary epithelial cells from ducts associated with the ductular reaction were positive for FSP-1 and vimentin [45]. In biliary atresia, a disease defined by a destructive inflammatory obliterative cholangiopathy with portal tract fibrosis and ductular proliferation [46], biliary epithelial cells were shown to express FSP-1 and vimentin, while hepatocytes were not. Moreover, the authors of this study showed that the expression of mesenchymal markers in biliary epithelial cells is observed in all liver disease with a ductular proliferation component [47]. In mice submitted to BDL, which is the experimental liver fibrosis model that induces strong ductular reaction, the biliary epithelial cells undergo EMT as shown by α-SMA and type I collagen expression [48].
In our study, however, we were not able to observe any epithelial cells with the dual expressions of the epithelial cell marker (Hep-Par 1 or CK19) and mesenchymal cell marker (α-SMA). This finding is different from the previous studies. The previous studies were conducted with chronic obstructive cholestatic models or mature/immature cholangiocytes, and these models mainly showed type 1 typical cholangiocyte proliferation. It should be noted that type 2 atypical ductular proliferation was mostly observed in our study using chronic liver disease cases. This suggests a possibility of occurrence of different pathogenesis in atypical ductular reaction from typical proliferation, such as a phenotypic change of mature hepatocytes into ductular cells, not a process of EMT. The future work should focus on better understanding of the direct contribution of dysfunctional epithelial cells to liver fibrosis, as well as determining the mechanistic relationships between fibrogenesis and the progenitor cell activation, which are found in the ductular reaction. This will ultimately require the development of animal models of biliary fibrosis that will better reflect human diseases.
This ductular reaction gradually replaces the hepatic parenchyma and causes a gradual decrease in mature hepatocytes in the hepatic lobules or regenerative nodules [8]. It is typically seen in liver diseases associated with increased deposition of collagenous matrices produced by activated HSCs [11,49]. In such liver diseases, Kupffer cells are also activated to produce various inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), which is thought to play important pathogenetic roles [50,51]. Nishikawa et al showed that the inflammatory cytokines exert distinct effects on hepatocyte differentiation, indicating that TNF-α is unique among these cytokines in its ability to suppress the hepatocytic phenotype [8]. Their results indicated that TNF-α also has a profound influence on the differentiation status of hepatocytes [8]. They have shown, within a collagen-rich matrix, that TNF-α strongly enhances the bile ductular transdifferentiation of hepatocytes, especially through the suppression of hepatocytic differentiation and enhancement of ductular morphogenesis [8]. In transdifferentiated hepatocytes induced by TNF-α, there was an increase in the phosphorylation of JNK and c-Jun, but the phosphorylation of NFkB was almost unchanged [8]. The experiments using the specific JNK inhibitor (SP600125) suggested that the JNK-c-Jun pathway might be involved in branching morphogenesis [8]. Their results suggest that TNF-α might suppress the differentiation status of hepatocytes and stimulate their transformation into bile ductular cells, thereby contributing to the functional deterioration in chronic liver diseases. Its direct effects on hepatocyte differentiation might be involved in the pathogenesis of hepatic dysfunctional characteristic of the chronic liver injury associated with fibrosis [8].
In summary, we investigated the dual expressions of Hep-Par 1 and CK19 in ductular reaction (atypical ductular proliferation) of biliary fibrosis in human liver, so called ‘ductular cell’. This result demonstrated the phenotypic plasticity of mature hepatocytes and suggested that bile ductular transdifferentiation might be involved in ductular reaction. Future studies will be necessary to determine the role of proliferating atypical ductular cells in the pathogenesis of biliary fibrosis and how ductular cells interact with other cell types of the liver such as HSCs or Kupffer cells during cholestatic liver diseases in the aspects of microenvironmental effects of inflammation. Our developing knowledge of the fundamental factors that control ductular reaction will aid in the development of a new therapeutic approach targeted on ductular reaction in chronic liver diseases.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government [2012R1A1A401015639].
Disclosure of conflict of interest
None.
References
- 1.Sasaki H, Schaffner F, Popper H. Bile ductules in cholestasis: morphologic evidence for secretion and absorption in man. Lab Invest. 1967;16:84–95. [PubMed] [Google Scholar]
- 2.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 3.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaser SS, Onori P, Wise C, Yang F, Marzioni M, Alvaro D, Franchitto A, Mancinelli R, Alpini G, Munshi MK, Gaudio E. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig Liver Dis. 2010;42:245–252. doi: 10.1016/j.dld.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia X, Demorrow S, Francis H, Glaser S, Alpini G, Marzioni M, Fava G, Lesage G. Cholangiocyte injury and ductopenic syndromes. Semin Liver Dis. 2007;27:401–412. doi: 10.1055/s-2007-991516. [DOI] [PubMed] [Google Scholar]
- 6.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–524. doi: 10.1016/s0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa Y, Sone M, Nagahama Y, Kumagai E, Doi Y, Omori Y, Yoshioka T, Tokairin T, Yoshida M, Yamamoto Y, Ito A, Sugiyama T, Enomoto K. Tumor necrosis factor-alpha promotes bile ductular transdifferentiation of mature rat hepatocytes in vitro. J Cell Biochem. 2013;114:831–843. doi: 10.1002/jcb.24424. [DOI] [PubMed] [Google Scholar]
- 9.Sone M, Nishikawa Y, Nagahama Y, Kumagai E, Doi Y, Omori Y, Yoshioka T, Tokairin T, Yoshida M, Sugiyama T, Enomoto K. Recovery of mature hepatocytic phenotype following bile ductular transdifferentiation of rat hepatocytes in vitro. Am J Pathol. 2012;181:2094–2104. doi: 10.1016/j.ajpath.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 11.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Roskams T. Progenitor cell involvement in cirrhotic human liver diseases: from controversy to consensus. J Hepatol. 2003;39:431–434. doi: 10.1016/s0168-8278(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 13.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- 14.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000-5991. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 16.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 18.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 19.Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 20.Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Lesage G, Glaser SS, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 22.LeSage GD, Glaser SS, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 23.LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, Phinizy JL, Baiocchi L, Francis H, Lasater J, Ugili L, Alpini G. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–319. doi: 10.1002/hep.510290242. [DOI] [PubMed] [Google Scholar]
- 24.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 25.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 26.Sung HJ, Lee JT, Kum YS, Park JB, Park KK. Immunohistochemical Study about the Origin of Bile Ductules Proliferation in Obstructive Liver Disease. Korean J of Pathol. 2009;43:126–132. [Google Scholar]
- 27.Sirica AE, Gainey TW, Mumaw VR. Ductular hepatocytes. Evidence for a bile ductular cell origin in furan-treated rats. Am J Pathol. 1994;145:375–383. [PMC free article] [PubMed] [Google Scholar]
- 28.Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, Yoshioka T, Enomoto K. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166:1077–1088. doi: 10.1016/S0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix. Exp Cell Res. 1996;223:357–371. doi: 10.1006/excr.1996.0091. [DOI] [PubMed] [Google Scholar]
- 30.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalopoulos GK, Bowen WC, Mule K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Notenboom RG, van den Bergh Weerman MA, Dingemans KP, Vermeulen JL, van den Eijnde S, Reutelingsperger CP, Hut H, Willemsen R, Offerhaus GJ, Lamers WH. Timing and sequence of differentiation of embryonic rat hepatocytes along the biliary epithelial lineage. Hepatology. 2003;38:683–691. doi: 10.1053/jhep.2003.50365. [DOI] [PubMed] [Google Scholar]
- 36.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka K, Nouchi T, Marumo F, Sato C. Alpha-smooth-muscle actin expression in normal and fibrotic human livers. Dig Dis Sci. 1993;38:1473–1479. doi: 10.1007/BF01308606. [DOI] [PubMed] [Google Scholar]
- 38.Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology. 2010;51:737–740. doi: 10.1002/hep.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123:335–346. doi: 10.1007/s00418-005-0788-z. [DOI] [PubMed] [Google Scholar]
- 40.Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, Laffi G, Gentilini P. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152:423–430. [PMC free article] [PubMed] [Google Scholar]
- 41.Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 42.Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 43.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 44.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 46.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 47.Diaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, Russo PA, Rand EB, Furth EE, Wells RG. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 50.Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36:4–12. doi: 10.1007/s12016-008-8091-0. [DOI] [PubMed] [Google Scholar]
- 51.Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]


