Abstract
The tumor microenvironment has many roles involving tumor progression, invasion and metastasis. The tumor cells at the tumor border loose epithelial properties and acquire mesenchymal features. This, epithelial-to-mesenchymal transition (EMT) has been suggested to be an important process for tissue and lymphovascular invasion. Pulmonary tissue samples from 15 patients with primary adenocarcinoma were evaluated with using immunofluorescence multi-staining the EMT-associated markers including E-cadherin and alpha-smooth muscle actin (α-SMA), and transcription factors including E-SNAIL and SLUG, and ZEB1. The data were analyzed in specific area, such as tumor center and tumor border. In this study we show that the invasive adenocarcinoma differentially expressed SNAIL and SLUG, and Zeb1 and it was associated with the loss of epithelial marker (E-cadherin) and gaining of mesenchymal marker (α-SMA) at the invasive border of lung carcinoma. The positive rates of SNAIL and ZEB1 were 26.7% and 0% in the tumor center and 40% and 20% in tumor margin, respectively. In addition, the expression of both SNAIL and ZEB1 at the border of tumor was observed in two cases (2/10). These two cases were associated with lymph node metastasis and advanced stage. The process of EMT has been suggested to be of prime importance for tissue and lymphovascular invasion. The process of EMT may be activated in the tumor border of lung adenocarcinoma. Related transcription factors, such as SNAIL and SLUG, and ZEB1, might be induced by paracrine effects of surrounded inflammatory cells and fibroblasts.
Keywords: Epithelial mesenchymal transition, lung adenocarcinoma, SNAIL, SLUG, ZEB1
Introduction
Primary adenocarcinoma in lung is leading cause of cancer death in worldwide and it accounts for the most common histological type among non-small cell lung cancer. The tumor recurrence and metastasis are the most common events encountered after resection of tumor that lead to mortality [1,2]. The metastatic process starts with separation of cancer cells from the primary lesion, permeation of tumor cells into vessels, proliferation, and transmigration to the metastatic site [3]. Lymphovascular invasion represents the early phase of metastasis [4-6]. Therefore, the presence of lymphovascular invasion has been reported to be a predictor of recurrence and metastasis [7-9].
The epithelial-to-mesenchymal transition (EMT) is originally known as a process during embryonic development in which cells acquire mesenchymal phenotype and lose epithelial phenotype. However, EMT has emerged as a crucial phenomenon in carcinogenic process [10]. As EMT progresses, the tumor cells acquire motile and invasive phenotype [11-13].
The associations between EMT and local invasiveness as well as distant metastasis have been demonstrated in numerous studies [11-13]. Furthermore, a number of studies have reported that expression of molecules involved in EMT correlated with clinicopathological features in non-small cell lung cancer [14-22]. However, those studies cannot serve as a direct evidence of EMT because it is a transient process. The loss of epithelial marker is a hallmark of EMT but it could be a simple result from anaplasia [10]. Therefore, this issue needs to be examined by another approach to demonstrate epithelial and mesenchymal features according to the region of tumor cells in associations with histology and immunohistochemistry.
In this study, we used immunohistochemical and double immunofluorescence staining method to examine the expression of EMT-related proteins including E-cadherin, α-SMA, SNAIL, and ZEB-1 at the invasive front of the tumor, compare with the center of the tumor. The objectives of the study are: (1) to demonstrate the existence of EMT in lung adenocarcinoma and (2) evaluate the frequencies and expressions of EMT-related proteins associated with lymphovascular invasion and metastasis.
Materials and methods
From January 2012 to June 2013, 15 patients underwent surgical resection of lung adenocarcinoma in our medical center were enrolled in this study. Clinical and pathological data were evaluated by reviewing the reviewing the pathological records. Patient consent was not required as this research was a retrospective chart review and personal identifiable information was not included. The average age of patients was 65.2 years. Preoperative chemotherapy or radiotherapy was not performed to any patients. The resected specimens are from 4 men (26.7%) and 11 women (63.6%), and there were 2 cases (13.3%) of early stage cancer (stage I and II) and 13 cases (86.7%) of advanced stage (stage III) adenocarcinoma. There was no stage IV lung adenocarcinoma case included in this study. The diagnosis of adenocarcinoma was based on histological assessment of resected specimen by microscopy with H&E stain. The histological classification and grade were assessed with using the WHO classification. One case (6.7%) was well differentiated adenocarcinoma, twelve cases (80%) were moderately differentiated adenocarcinomas, and two cases (13.3%) were poorly differentiated adenocarcinomas. Four patients (26.7%) had lymphovascular invasion, two patients had neural invasion, and two patients had metastatic lung adenocarcinoma in lymph node. The clinical parameters and histological assessments of the respective cases are summarized in Table 1.
Table 1.
The clinicopathological features and differentially expressed of EMT-related markers in the tumor center and tumor margin of respective cases
| No. | Sex | Age | Size (cm) | Stage | Differentiation | L. V. I. | Neural invasion | Node metastasis | Center | Margin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| E-cad | SMA | SNAIL | ZEB1 | Ecad | SMA | SNAIL | ZEB1 | |||||||||
| 1 | F | 57 | 2.0 x 1.5 | IA | Moderately | + | - | - | + | - | + | - | + | + | + | - |
| 2 | M | 75 | 2.3 x 1.4 | IA | Moderately | + | - | - | + | - | - | - | + | - | + | - |
| 3 | F | 73 | 2.2 x 1.8 | IA | Moderately | - | + | - | + | - | - | - | + | - | - | - |
| 4 | F | 77 | 7.5 x 5.5 | IIB | Moderately | - | - | - | + | - | - | - | + | - | - | - |
| 5 | M | 82 | 2.8 x 2.2 | IA | Moderately | - | - | - | + | - | - | - | + | - | - | - |
| 6 | M | 71 | 1.7 x 1.5 | IA | Poorly | - | - | - | + | - | + | - | + | - | + | - |
| 7 | F | 78 | 2.3 x 1.9 | IA | Moderately | - | - | - | + | - | - | - | + | - | - | - |
| 8 | F | 45 | 1.5 x 1.3 | IA | Moderately | - | - | - | + | - | - | - | + | - | - | - |
| 9 | F | 51 | 2.7 x 2.2 | IIIA | Moderately | + | - | + | + | - | - | - | + | + | + | + |
| 10 | M | 76 | 2.8 x 2.5 | IA | Moderately | - | - | - | + | - | - | - | + | - | - | - |
| 11 | F | 46 | 1.8 x 1.4 | IA | Well | - | - | - | + | - | - | - | + | - | - | - |
| 12 | F | 69 | 1.9 x 1.5 | IA | Well | - | - | - | + | - | - | - | + | - | - | - |
| 13 | F | 51 | 1.7 x 1.5 | IA | Well | - | - | - | + | - | - | - | + | - | - | - |
| 14 | F | 56 | 4.8 x 2.1 | IIB | Poorly | - | - | - | + | - | - | - | + | - | - | - |
| 15 | F | 71 | 2.7 x 2.5 | IIIA | Moderately | - | + | + | + | - | - | - | + | + | + | + |
Abbreviation: L. V. I., Lymphovascular invasion.
Immunohistochemical staining of EMT markers
The formalin-fixed paraffin embedded tissues were cut into 4 μm sections and deparaffinized. The each section underwent immunohistochemical staining using rabbit polyclonal ZEB1 antibody (1:100, pH 6.0 Bethyl) and rabbit polyclonal anti-SNAIL + SLUG antibody (1:100, pH 6.0 Abcam). Only nuclear staining was assessed as positive or negative and the immunoreactivity in tumor center and tumor margin (invasion front) is analyzed separately.
Immunofluorescence staining of EMT markers
The formalin-fixed paraffin embedded tissues were incubated with anti-E-cadherin antibody (1:200, BD Biosciences, San Jose, CA, USA) and double staining one of the following antibodies: anti-Smooth muscle actin (1:400, Sigma-Aldrich, Saint Louis, MO, USA), anti-SNAIL + SLUG antibody (1:50, Abcam, Cambridge, MA, USA), and anti-ZEB1 antibody (1:50, Bethyl, Montgomery, TX, USA). To visualize the primary antibodies, slide were stained with secondary antibodies conjugated with FITC and TRITC. For each slide, counter stained with Hoechst to visualize the nuclei. All the stained slides were viewed and photographed under immunofluorescence microscope equipped with a digital camera.
Statistical analysis
All data collected from this study are analyzed with SPSS Amos 19.0 (IBM corp., Armonk, NY, USA). The data were expressed as mean ± standard deviation for continuous variables and P-value < 0.05 was considered to be statistically significant. The Fisher’s exact test or Pearson Chi-square test was used to compare differences in qualitative data.
Results
Fifteen patients who underwent surgical resection of adenocarcinoma of the lung are included in our study. The table shows the clinicopathological features including age, sex, differentiation of tumor, tumor size, neural invasion, lymphovascular invasion, lymph node metastasis, and stage of tumor.
Immunohistochemical staining of ZEB1
The cells with any degree of nuclear staining were scored as positive. The nuclear staining of ZEB1 tumor center (Figure 1C) and tumor margin (Figure 1D) was observed in all (100%) cases. However, the most positive cells with positive ZEB1 staining were spindle-shaped stromal element including endothelial, myofibroblast, fibroblast cells. Although some large cells with positive ZEB1 staining were observed, whether ZEB1 positive cells are from tumor cells or from stromal cells could not be differentiated only by immnuohistochemical stained slide.
Figure 1.
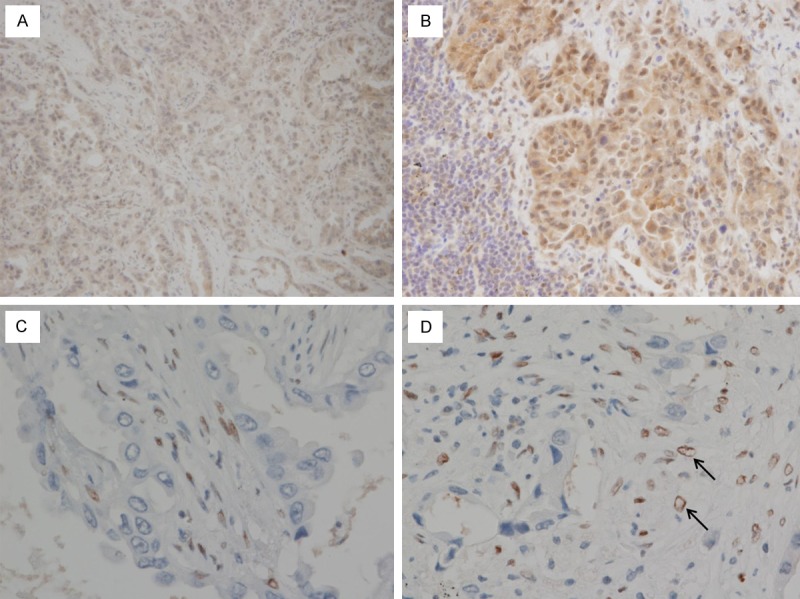
Immunohistochemical staining of SNAIL & SLUG (A, B) and ZEB1 (C, D). (A) In the center of tumor, SNAIL & SLUG is negative in both stromal cells and tumor. (B) The nuclear staining of SNAIL & SLUG is positive in tumor cells in tumor margin. (C) ZEB1 positive stromal cell are mostly observed in tumor center. (D) In tumor margin, ZEB1 is positive in tumor cells (marked with arrow), as well as in stromal cells.
Immunohistochemical staining of SNAIL and SLUG
Immunohistochemical staining of SNAIL & SLUG protein was detected at tumor center (Figure 1A) and tumor margin (Figure 1B) in all (100%) cases. However, some of the positive expression of SNAIL & SLUG was limited only in cytoplasm, not in nuclei. Cytoplasmic staining was disregarded. The nuclear expression of SNAIL & SLUG was observed in tumor center (n = 4, 26.7%) and tumor margin (n = 6, 40%). The staining patterns on nuclei were often focal and weakly stained. The nuclear staining was also identified in virtually all normal tissue of bronchial respiratory epithelium which was apart from the tumor.
Double immunofluorescence of E-cadherin and α-SMA in tumor center
In the center of the tumor, all the samples showed diffuse and strong expression of E-caherin on the cell membrane (Figure 2A). However, α-SMA expression was not detected on tumor cells. The signal was only focally detected at the stromal vascular structures.
Figure 2.
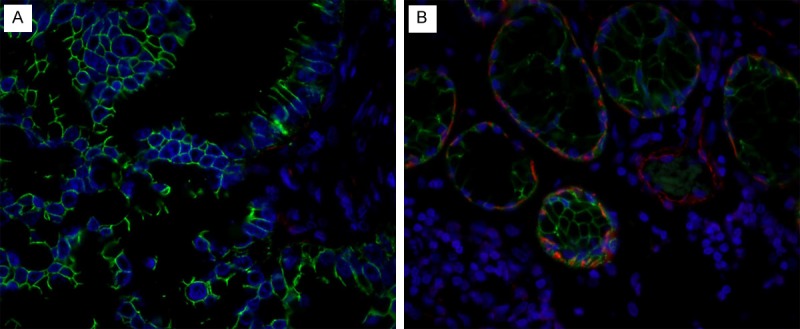
Double immunofluorescence staining of E-cadherin (detected in green) and α-SMA (detected in red) in the tumor tissue at the center (A) and the border (B) of the primary adenocarcinoma in lung. (A) E-cadherin positive cells are detected, but α-SMA is not detected in the center of the tumor; (B) Note that both E-cadherin positive and α-SMA positive cells are detected at infiltrating border of tumor. This is associated with the lymph node metastasis of the tumor cells. The blue staining represents the nuclei (original magnification X 400).
Double immunofluorescence of E-cadherin and α-SMA in tumor margin
Although the expression was less strongly and focally on the cell membrane, a similar pattern of E-cadherin expression was observed at the margin of the tumor as in the tumor center. The focal intracytoplasmic α-SMA expression was observed in the three cases (20%) in tumor cells at invasion front (Figure 2B). All in these three cases, the coexpression of E-cadherin is observed where the cell expressed α-SMA in cytoplasm. As it was seen in tumor center, the signal of α-SMA was also detected at the stromal vascular structures.
The patients with E-cadherin expression and α-SMA expression at the tumor margin were correlated with lymph node metastasis (P = 0.029) and advanced stage (P = 0.029) but were unrelated with lymphovascular invasion (P = 0.081) and neural invasion (P = 0.371).
Double immunofluorescence of E-cadherin and SNAIL and SLUG in tumor center
In the center of the tumor, two samples (13.3%) showed focal but strong expression of SNAIL & SLUG in the nuclei. All in this two cases, the coexpression of E-cadherin is observed in the cell membrane where the SNAIL & SLUG are expressed in nuclei.
The patients with E-cadherin expression and SNAIL & SLUG expression at the tumor center were not correlated with neural invasion (P = 1.000), lymphovascular invasion (P = 0.371), lymph node metastasis (P = 1.000), or advanced stage (P = 1.000).
Double immunofluorescence of E-cadherin and SNAIL & SLUG in tumor margin
Five samples (30%) showed expression of SNAIL & SLUG in nuclei at the invasion front of tumor. All in these five cases, the expression of E-cadherin on cell membrane is detected where the cells expressed SNAIL and SLUG in nuclei (Figure 3A-C). The patients with E-cadherin expression and SNAIL and SLUG expression at the margin of tumor were correlated with lymphovascular invasion (P = 0.022), but were not correlated with neural invasion (P = 1.000), lymph node metastasis (P = 0.095), and advanced stage (P = 0.095).
Figure 3.
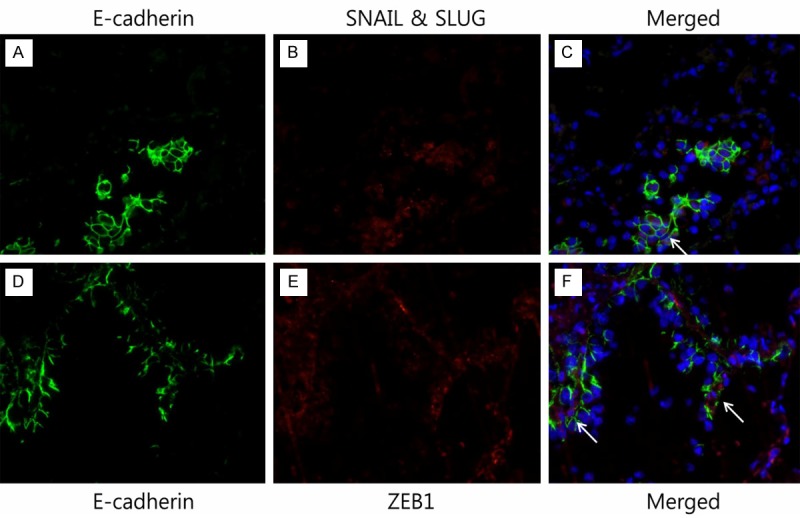
Merged images of double immunofluorescence multi-staining of the tumor tissue of lung adenocarcinoma. (A, D) anti-E-cadherin antibody, (B) anti-SNAIL antibody, and (C) merged image E-cadherin/SNAIL & SLUG. The coexpression of E-cadherin and SNAIL & SLUG is noted (marked with arrow). (E) Anti-ZEB1 antibody, and (F) merged image E-cadherin/ZEB1. The coexpression of E-cadherin and ZEB1 is noted (marked with arrow). The blue staining represents the nuclei (original magnification X 400).
Double immunofluorescence of E-cadherin and ZEB1 in tumor center
In the center of the tumor, all the samples showed diffuse and strong expression of E-caherin on the cell membrane. However, ZEB1 expression was not detected on the nuclei of tumor cells.
Double immunofluorescence of E-cadherin and ZEB1 in tumor margin
Two samples (11.3%) expressed ZEB1 protein in nuclei at the margin of tumor. All in these two cases, the expression of E-cadherin on cell membrane is observed as well as ZEB1 expression in nuclei (Figure 3D-F).
The patients with E-cadherin expression and ZEB1 expression at the invasion front were correlated with lymph node metastasis (P = 0.010) and advanced stage (P = 0.010), but not with neural invasion (P = 0.257) and lymphovascular invasion (P = 0.371).
Discussion
Primary lung adenocarcinoma is leading cause of cancer death in worldwide. The tumor invasion, recurrence, and metastasis are the most common events which lead to mortality of cancer patients. EMT has been recognized as crucial phenomenon during tumor cell invades the surrounding stroma. Recently, numerous studies reported that these complex processes are associated with EMT and it constitutes an important mechanism in the development of tumor invasiveness [23]. The clinical importance of SNAIL & SLUG expression is also well known for poor prognosis in various carcinomas, including ovarian carcinomas, urothelial carcinomas, hepatocellular carcinomas, breast cancer, and non-small cell lung carcinomas [22,24-27].
The downregulation of E-cadherin expression is the most crucial characteristic of EMT development and ZEB1, SNAIL, and SLUG are the major transcription inhibitor of E-cadherin [28]. The loss of E-cadherin may cause loss of contact inhibition and detachment of intercellular connections. Many studies showed that the loss of E-cadherin expression can lead to loss of contact inhibition, infinite proliferation, de-differentiation, and loose intercellular connections which enhanced invasion and migration feature of cancer cells [29-31].
Assuming that EMT should preferably occur at the invasion front of tumor, and at the site of vessel invasion, we assessed the tumor center and tumor margin separately for EMT related proteins and transcription factors [32,33].
On immunohistochemical study, our data showed that the nuclear expression of ZEB1 was observed in all cases both in tumor center and margin. The nuclear expression of SNAIL & SLUG was detected in tumor center (n = 4, 26.7%) and tumor margin (n = 6, 40%). Although the nuclear staining of SNAIL & SLUG was more frequently observed in the margin of tumor, the expression of transcription factors within the tumor was unexpectedly homogenous between both areas analyzed. This might be interpreted that distinguishing between stromal and tumor cells is necessity. ZEB1 was also highly expressed in tumor-associated stromal cells [34]. It is unclear whether ZEB1-positive stromal fibroblasts may be derived from epithelial cancer cells through a bona fide ZEB1-dependent EMT [34]. An attractive hypothesis of candidates for ZEB1 inducer is that tumor infiltrating macrophage, which are abundantly detected at the tumor-host interface, express cytokines that may induce ZEB1 expression [35]. Consistent with our observations in our study, the nuclear ZEB1, SNAIL & SLUG protein expression in lung adenocarcinoma-associated stromal cells are observed in other studies. The expression of E-cadherin transcription repressors previously reported in tumor associated stromal cells [24,34,36-38]. This raise the possibility that expression of transcription repressor in stromal cell may influence lung adenocarcinoma and it need to be investigated in further study.
In present double immunofluorescence analysis of lung cancer patients, we investigated the expression of transcriptional repressor and EMT-related protein in primary lung adenocarcinoma. Our results show that all of tumor cells in patients with lung adenocarcinoma had E-cadherin expression in both tumor center and tumor margin, but had less E-cadherin expression in tumor margin.
In addition to the loss of E-cadherin expression in the invasion front of tumor, we detected α-SMA which is coexpressed with E-cadherin. In this study α-SMA is used as a marker of mesenchymal differention, which may indicate the occurrence of EMT. In the center of tumor, there was no expression of α-SMA in tumor cells. On the other hand, in the margin of tumor, 20% of the cases had intracytoplasmic α-SMA expression and this was correlated with lymph node metastasis (P = 0.029) and advanced stage (P = 0.029) but were unrelated with lymphovascular invasion (P = 0.081) and neural invasion (P = 0.371).
In our study, expression of SNAIL & SLUG was observed in 30% of the cases at tumor margin and this was reduced by 13.3% at the tumor center. The expression of SNAIL & SLUG in tumor margin was significantly associated with lymphovascular invasion. On the other hand, the expression of SNAIL & SLUG did not correlated with any clinic parameter in tumor center.
We detected nuclear ZEB1 expression in 13.3% in tumor margin and no nuclear ZEB1 expression in tumor center. ZEB1 expression at the invasion front was correlated with lymph node metastasis (P = 0.010) and advanced stage (P = 0.010), but not with neural invasion (P = 0.257) and lymphovascular invasion (P = 0.371).
It has been demonstrated that increased α-SMA, ZEB1, and SNAIL & SLUG expression is observed in lung adenocarcinoma. The data suggest that expression of EMT related protein and transcription factor closely associated with enhanced invasion and lymph node metastasis of lung adenocarcinoma. However, it should be noted that this study examined in a relatively small sample of lung adenocarcinoma patients. Therefore, larger study is required to confirm the function of ZEB1, SNAIL & SLUG in lung adenocarcinoma.
Consistent with our observations, the data presented in this study suggest that EMT plays a role in lung adenocarcinoma and metastasis, Taken together, α-SMA, ZEB1, SNAIL and SLUG are overexpressed in the invasive front of tumors in some patients with lung adenocarcinoma suggesting that EMT occurs in the margin of tumor. Our data suggest that overexpression of α-SMA, ZEB1, and SNAIL & SLUG in tumor margin may play a critical role in EMT-induced invasion and metastasis in lung adenocarcinoma. Thus, ZEB1 and SNAIL & SLUG might be used as a predictive parameter for invasiveness and prognosis of lung adenocarcinoma. Furthermore, as our understanding of the underlying molecular processes in development of primary lung adenocarcinoma, elucidation of these interactions will be central to development of novel therapeutic strategies.
Acknowledgements
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009519), Rural Development Administration, Republic of Korea.
Disclosure of conflict of interest
None.
References
- 1.Williams BA, Sugimura H, Endo C, Nichols FC, Cassivi SD, Allen MS, Pairolero PC, Deschamps C, Yang P. Predicting postrecurrence survival among completely resected no small-cell lung cancer patients. Ann Thorac Surg. 2006;81:1021–1027. doi: 10.1016/j.athoracsur.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico TA. Molecular biologic staging of lung cancer. Ann Thorac Surg. 2008;85:S737–742. doi: 10.1016/j.athoracsur.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Kaseda K, Ishii G, Aokage K, Takahashi A, Kuwata T, Hishida T, Yoshida J, Kohno M, Nagai K, Ochiai A. Identification of intravascular tumor microenvironment features predicting the recurrence of pathological stage I lung adenocarcinoma. Cancer Sci. 2013;104:1262–1269. doi: 10.1111/cas.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler R, Gasser B, Massard G, Roeslin N, Meyer P, Wihlm JM, Morand G. Blood vessel invasion is a major prognostic factor in resected non-small cell lung cancer. Ann Thorac Surg. 1996;62:1489–1493. doi: 10.1016/0003-4975(96)00540-1. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 6.Rigau V, Molina TJ, Chaffaud C, Huchon G, Audouin J, Chevret S, Brechot JM. Blood vessel invasion in resected non small cell lung carcinomas is predictive of metastatic occurrence. Lung Cancer. 2002;38:169–176. doi: 10.1016/s0169-5002(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 7.Kroeger N, Rampersaud EN, Patard JJ, Klatte T, Birkhauser FD, Shariat SF, Lang H, Rioux-Leclerq N, Remzi M, Zomorodian N, Kabbinavar FF, Belldegrun AS, Pantuck AJ. Prognostic value of microvascular invasion in predicting the cancer specific survival and risk of metastatic disease in renal cell carcinoma: a multicenter investigation. J Urol. 2012;187:418–423. doi: 10.1016/j.juro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Lee AK, DeLellis RA, Silverman ML, Heatley GJ, Wolfe HJ. Prognostic significance of peritumoral lymphatic and blood vessel invasion in node-negative carcinoma of the breast. J Clin Oncol. 1990;8:1457–1465. doi: 10.1200/JCO.1990.8.9.1457. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Tateno H. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer. 1996;78:2313–2317. doi: 10.1002/(sici)1097-0142(19961201)78:11<2313::aid-cncr7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology. 2012;17:1048–1059. doi: 10.1111/j.1440-1843.2012.02173.x. [DOI] [PubMed] [Google Scholar]
- 11.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Al-Saad S, Al-Shibli K, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br J Cancer. 2008;99:1476–1483. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M, Allgayer H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res. 2010;8:1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 16.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with non-small cell lung carcinoma. Cancer. 2001;91:964–971. [PubMed] [Google Scholar]
- 18.Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and SNAIL expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082–1089. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- 19.Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–4796. [PubMed] [Google Scholar]
- 20.Nakata S, Sugio K, Uramoto H, Oyama T, Hanagiri T, Morita M, Yasumoto K. The methylation status and protein expression of CDH1, p16 (INK4A), and fragile histidine triad in nonsmall cell lung carcinoma: epigenetic silencing, clinical features, and prognostic significance. Cancer. 2006;106:2190–2199. doi: 10.1002/cncr.21870. [DOI] [PubMed] [Google Scholar]
- 21.Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, Lin SB, Liou GY, Lee ML, Chen JJ, Hong TM, Yang SC, Su JL, Lee YC, Yang PC. Transcription repressor SLUG promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11:8070–8078. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, Chia D, Goodglick L, Elashoff DA, Luo J, Magyar CE, Dohadwala M, Lee JM, St John MA, Strieter RM, Sharma S, Dubinett SM. SNAIL promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820–6829. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad AA, Awed NM, Abd Elkerim NN, El-Shennawy D, Alfons MA, Elserafy ME, Darwish YW, Barakat EM, Ezz-Elarab SS. Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol. 2010;17:3059–3067. doi: 10.1245/s10434-010-1151-8. [DOI] [PubMed] [Google Scholar]
- 24.Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. SNAIL and SLUG play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 25.Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia L, Wu Y, Zhou BP, Feng Y. SNAIL is critical for tumor growth and metastasis of ovarian carcinoma. Int J Cancer. 2010;126:2102–2111. doi: 10.1002/ijc.24901. [DOI] [PubMed] [Google Scholar]
- 26.Kosaka T, Kikuchi E, Mikami S, Miyajima A, Shirotake S, Ishida M, Okada Y, Oya M. Expression of SNAIL in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clin Cancer Res. 2010;16:5814–5823. doi: 10.1158/1078-0432.CCR-10-0230. [DOI] [PubMed] [Google Scholar]
- 27.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and SNAIL in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Lu C, Kang J, Cao C, Li M. Involvement of ZEB1 and E-cadherin in the invasion of lung squamous cell carcinoma. Mol Biol Rep. 2013;40:949–956. doi: 10.1007/s11033-012-2136-4. [DOI] [PubMed] [Google Scholar]
- 29.Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112:4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- 30.Parry DA. Hendecad repeat in segment 2A and linker L2 of intermediate filament chains implies the possibility of a right-handed coiled-coil structure. J Struct Biol. 2006;155:370–374. doi: 10.1016/j.jsb.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Prasad CP, Rath G, Mathur S, Bhatnagar D, Parshad R, Ralhan R. Expression analysis of E-cadherin, SLUG and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer. 2009;9:325. doi: 10.1186/1471-2407-9-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis GA, Cohen C. Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray-based study. Appl Immunohistochem Mol Morphol. 2010;18:433–441. doi: 10.1097/PAI.0b013e3181dddb20. [DOI] [PubMed] [Google Scholar]
- 34.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (deltaEF1) promotes tumor cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Franci C, Gallen M, Alameda F, Baro T, Iglesias M, Virtanen I, Garcia de Herreros A. SNAIL1 protein in the stroma as a new putative prognosis marker for colon tumours. PLoS One. 2009;4:e5595. doi: 10.1371/journal.pone.0005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franci C, Takkunen M, Dave N, Alameda F, Gomez S, Rodriguez R, Escriva M, Montserrat-Sentis B, Baro T, Garrido M, Bonilla F, Virtanen I, Garcia de Herreros A. Expression of SNAIL protein in tumor-stroma interface. Oncogene. 2006;25:5134–5144. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- 38.Graham TR, Yacoub R, Taliaferro-Smith L, Osunkoya AO, Odero-Marah VA, Liu T, Kimbro KS, Sharma D, O’Regan RM. Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat. 2010;123:139–147. doi: 10.1007/s10549-009-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


