Abstract
The specific mechanism underlying the role of putative stem cell marker aldehyde dehydrogenase 1 (ALDH1) playing in development and progression of breast cancer is currently unclear. Transforming growth factor β (TGFβ) signaling pathway is reported to be activated in most cancers. Thus a study was initiated to explore possible differences and correlation of ALDH1 and TGFβ2 expression in the most common malignant and benign tumors of the breast in Chinese women. Samples of 75 breast cancer tissues, 30 paracancerous normal tissues, and 39 fibroadenoma breast tissues were investigated for the expression of ALDH1 and TGFβ2 using immunohistochemistry. The positive rates of ALDH1 and TGFβ2 protein were 62.67% and 66.67%, respectively, in breast cancer tissues, which were significantly higher than that in normal fibroadenoma breast (P<0.05) and paracancerous tissues (P<0.01). ALDH1 and TGFβ2 status were significantly associated with tumor histological grade and receptor status (P<0.05). Expression of ALDH1 was found to be positively correlative to TGFβ2 in breast cancer (r = 0.33, P<0.01). Expression of both proteins remained significantly associated with reduced overall survival (OS) by univariate analysis (P<0.05). Multivariate Cox regression analysis showed that ALDH1 expression, tumor stage, and lymph node status are independent prognostic factors in invasive breast cancer patients. Thus ALDH1 and TGFβ2 play important roles in the development of breast cancer. The ALDH1 phenotype is an independent predictor of poor prognosis, and TGFβ2 signaling pathway activation might be involved in the pathological regulation of ALDH1 in breast cancer.
Keywords: ALDH1, TGFβ2, breast cancer, fibroadenoma, stem-like cells, prognosis
Introduction
Breast cancer is a common breast malignancy and a major cause of cancer mortality in women worldwide [1]. Despite earlier detection and developments in new treatment protocols, a subset of patients still display poor prognosis and early metastasis and might die from the disease within five years of diagnosis [2,3]. The cancer stem cell hypothesis was proposed to explain breast cancer heterogeneity and risk of recurrence. These small subpopulations of cells within malignant breast tumors have the capacity to self-renew, proliferate and differentiate into multiple cell types, and may contribute to the failure of chemotherapy and promote tumor recurrence or metastasis [4]. A candidate stem-like cell marker aldehyde dehydrogenase 1 (ALDH1), a detoxifying enzyme responsible for the oxidation of intracellular aldehydes, has attended much attention in recent years [5-7]. Mounting evidences have shown that the breast carcinoma cells with ALDH1 phenotype participate in the acquisition of progenitor features [5,8]. Increased ALDH1 activity was also found to play a critical role in mediating the clinically aggressive behavior of breast cancer, and thus led to early metastasis and poor clinical outcome [9,10]. However, the mechanisms by which the ALDH1 phenotype contributes to malignant cell proliferation or metastatic behavior in breast cancer remain to be elucidated. Meantime, most prior studies have focused on studying ALDH1 expression in malignant tumors with a lack of benign tumors. A parallel study of ALDH1 expression in benign versus malignant tumors might provide new important insight into differences between the two categories. Therefore, breast cancer and fibroadenoma, which represent the most frequently encountered malignant and benign breast neoplasm among women, were both involved in the study.
In exploring the mechanisms of ALDH1 activity, we focused our study on transforming growth factor (TGF) signaling pathway. TGFβ2, belonging to a superfamily of polypeptide growth factors, is ubiquitously expressed and has been detected in a variety of different cell types. It is considered to be a hallmark of various malignant tumors [11] including pancreatic carcinoma, glioma, melanoma, and colorectal carcinoma, due to its pivotal role playing in multiple biological processes such as cell proliferation, differentiation, apoptosis, angiogenesis and immune response [12,13]. Moreover, studies have also indicated a molecular link between TGFβ2 signaling and CD44, another putative marker of breast cancer stem cells [14,15], and further demonstrated that activation of TGFβ2 is an essential CD44-downstream event required for breast cancer invasion and metastasis [16-18]. However, whether TGFβ2 signaling pathway is associated with ALDH1 expression in breast cancer development and progression is nascent.
The purpose of this study was to investigate the expression characteristics of ALDH1 and TGFβ2 in benign and malignant breast tumors using an immunohistochemical method, to explore their relationship as well as to investigate the correlations among their expressions, clinicopathological parameters and prognosis.
Materials and methods
Cases and clinical data
We examined paraffin blocks with samples from 75 breast cancer patients, and 30 paracancerous normal counterparts (defined as more than 5 cm away from the carcinoma tissue), who underwent surgery at the First Affiliated Hospital of Bengbu Medical College between January 2006 and June 2007. 39 samples of fibroadenoma breast tissues were also included in the study. Patients with bilateral tumors or a prior history of cancer (other than basal cell carcinoma or cervical carcinoma in situ) were excluded. Approval was obtained from the medical ethics committee of our institute, and women have signed a written informed consent to participate in the study.
All tumor materials were available for histological examination. Primary surgery was completed on all breast cancer cases that included complete resection of tumor (modified radical mastectomy) and axillary lymph node dissection, and no patient had received radiation, chemotherapy or hormone therapy preoperatively. The median age of breast cancer patients was 47 years old (range, 20 to 80 years). 65 patients received adjuvant chemotherapy (regimen CEF or AT), and 51 patients received adjuvant hormonal treatment (tamoxifen, most cases). Five-year follow-up data for all 65 invasive breast cancer patients was obtained, and the follow-up ended in September 2012. Patient outcomes were defined as overall survival (OS). The duration of OS was determined as the time between the start of surgery and the date of death.
Pathologic examination and assessment
All malignant tumors were classified according to the criteria of WHO. Histological types included 10 non-invasive carcinomas and 65 invasive carcinomas, with ductal 83.08% (54 samples), lobular 9.23% (6 samples), tubular 3.08% (2 samples), medullary 3.08% (2 samples), and mucoid 1.53% (1 sample). The histological grade of invasive carcinoma was determined using current histological standards of Common Malignant Tumors Diagnosis and Treatment Guidelines in China. Histological grade I-II accounted for 60% (39 samples), and grade III accounted for 40% (26 samples). Staging at the time of diagnosis was based on the tumor-lymph node metastasis (TNM) classification. Tumors of stage I accounted for 12% (9 samples), tumors of stage II accounted for 45.33% (34 samples), and tumors of stage III accounted for 42.67% (32 samples). Tumor size and lymph node status were evaluated separately. The clinicopathological parameters are summarized in Table 1.
Table 1.
Relationships between ALDH1 or TGFβ2 expression and clinicopathological parameters for the 75 breast cancer patients
| Variable | n | ALDH1 | P value | TGFβ2 | P value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| - | + | - | + | ||||
| Age (years) | |||||||
| ≤35 | 5 | 2 | 3 | 1.000 | 2 | 3 | 1.000 |
| >35 | 70 | 26 | 44 | 23 | 47 | ||
| Menstrual status | |||||||
| Pre-menopausal | 52 | 22 | 30 | 0.181 | 20 | 32 | 0.157 |
| Post-menopausal | 23 | 6 | 17 | 5 | 18 | ||
| Histological type | |||||||
| Non-invasive | 10 | 6 | 4 | 0.111 | 4 | 6 | 0.723 |
| Invasive | 65 | 22 | 43 | 21 | 44 | ||
| Grade (for invasive carcinoma) | |||||||
| I-II | 39 | 18 | 21 | 0.015* | 17 | 22 | 0.017* |
| III | 26 | 4 | 22 | 4 | 22 | ||
| Tumor size (cm) | |||||||
| ≤2 | 29 | 14 | 15 | 0.311 | 13 | 16 | 0.116 |
| 2-5 | 37 | 11 | 26 | 8 | 29 | ||
| >5 | 9 | 3 | 6 | 4 | 5 | ||
| Lymph node metastasis | |||||||
| Negative | 35 | 15 | 20 | 0.355 | 13 | 22 | 0.513 |
| Positive | 40 | 13 | 27 | 12 | 28 | ||
| Clinical stage | |||||||
| I | 9 | 6 | 3 | 0.150 | 5 | 4 | 0.154 |
| II | 34 | 11 | 23 | 8 | 26 | ||
| III | 32 | 11 | 21 | 12 | 20 | ||
| Triple negativity features (for invasive carcinoma) | |||||||
| Present | 13 | 1 | 12 | 0.022* | 1 | 12 | 0.023* |
| Absent | 52 | 23 | 29 | 22 | 30 | ||
P<0.05.
Hormone receptor, and human epidermal growth factor receptor 2 status
Based on the status of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), tissue samples of all carcinomas were classified into triple negative breast cancer (TNBC) and non-TNBC. ER and PR status was defined as positive when ≥1% of tumor cells showed positive immunohistochemical staining (antibodies for ER and PR, Santa Cruz, USA). Immunohistochemical staining for HER2 was scored into 4 grades (0, 1+, 2+, or 3+). Tumors with scores of 3+ were considered HER2-positive. Tumors with scores of 2+ were further determined by fluorescence in situ hybridization (FISH) using PathVysion HER2 DNA Probe kits (Vysis, Downers Grove, IL). Cancer nuclei were scored for the centromere enumeration probe (CEP) 17 and HER2 signals. Specimens with a HER2:CEP17 ratio of >2.0 were considered positive for gene amplification [19]. Tumors with scores of 1+ or 0+ were considered HER2-negative. A pathologic non-TNBC was defined as the absence of either above receptor in the primary lesion. According to the immunohistochemical analysis, 15 were classified as TNBC and 60 as non-TNBC.
Immunohistochemistry and evaluation
Rabbit polyclonal anti-human ALDH1 antibody, at a dilution of 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse polyclonal anti-human TGFβ2 antibody at a dilution of 1:200 (Santa Cruz Biotechnology), were used for immunohistochemistry. All formalin-fixed specimens were embedded in paraffin and cut by a microtome into 4 μm sections. Immunohistochemical staining was performed according to our previously described standard protocols [20]. The negative control slides were processed by omitting the primary antibody but including all other steps of the procedure. Microscopic analyses of ALDH1 and TGFβ2 were assessed independently by two observers in a blinded manner. There was no discrepancy between the two investigators. ALDH1 and TGFβ2 staining were detected mainly in the cytoplasm, and subjective estimation was judged according to the criteria described by Ginestier et al [5]. Immunohistochemical staining of ALDH1 and TGFβ2 was classified as negative (<5% positive cells), 1+ (5%-10% positive cells), 2+ (10%-50% positive cells), or 3+ (≥50% positive cells).
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 statistical software (Chicago, IL, USA). Correlations between molecular makers and clinicopathological parameters were evaluated by χ2 test, Fisher’s exact test, Wilcoxon rank test where appropriate. Spearman’s correlation was used to analyze the association between ALDH1 and TGFβ2 protein expression. The OS duration was calculated using the Kaplan-Meier method and was compared using log-rank tests. A Cox proportional hazards regression model was used for multivariate analyses. The results were considered to be statistically significant at P<0.05.
Results
Expression of ALDH1 and TGFβ2 among different groups
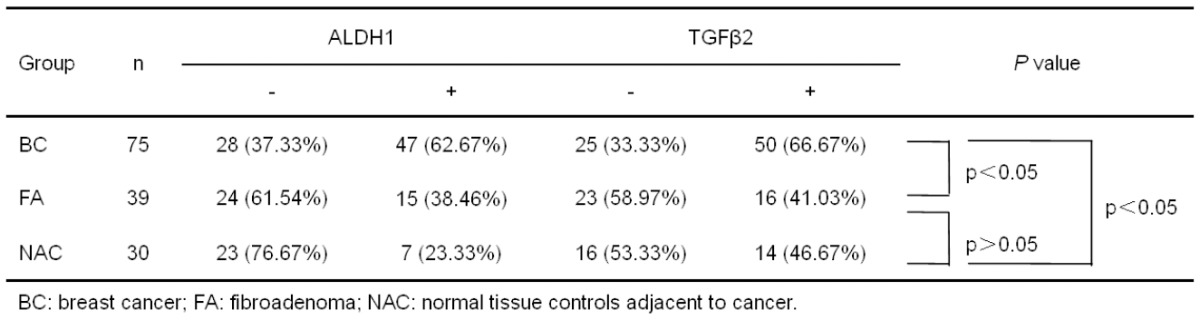
When comparing ALDH1 protein expression in the different clinical groups (i.e. BC: breast cancer; FA: fibroadenoma; NAC: normal tissue controls adjacent to cancer), statistically significant differences were observed among the groups (Table 2). In BC group, the expression of ALDH1 was confined to the cellular cytoplasm and occurred in 47 cases (62.67%), whereas it was negative in 28 cases (37.33%) (Figure 1A). Of all 39 FA cases, 15 cases (38.46%) were observed as cytoplasmic positive for ALDH1 expression (Figure 1B). However, most of the NAC samples had negative ALDH1 staining (23/30, 76.67%) (Figure 1C). There was no difference in staining intensity among all categories. For ALDH1 immunohistochemical analysis between either two groups, no statistically significantly difference was observed between FA and NAC (P>0.05). Of special interest was the difference in the positive rate of ALDH1 between BC and FA or NAC (P = 0.014 and P = 0.000, respectively).
Table 2.
ALDH1 and TGFβ2 expression in the different groups
|
Figure 1.
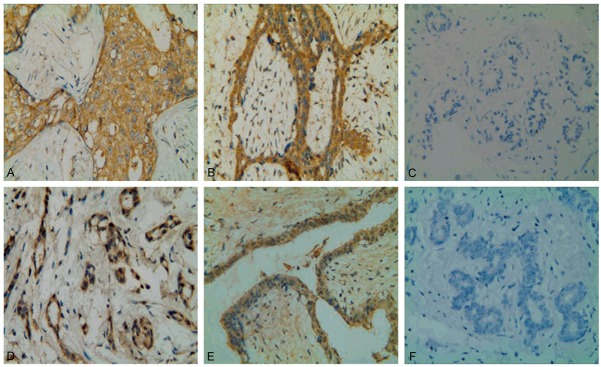
Immunohistochemical analyses of ALDH1 and TGFβ2 expression in sections of different breast tissues. A: Breast cancer cells showed extensive cytoplasmic staining for ALDH1. B: Cytoplasmic positive staining for ALDH1 in benign fibroadenoma. C: ALDH1-negative staining in non-cancerous normal tissue adjacent to cancer. D: Strong expression of TGFβ2 in the cytoplasm of breast cancer cells. E: Diffuse cytoplasmic staining for TGFβ2 in fibroadenoma tissue. F: TGFβ2-negative staining in paracancerous normal tissue. Representative immunohistochemical examples of staining were shown (original magnification, ×400).
For TGFβ2 immunohistochemical analysis, we found it displayed similar cytoplasmic expression patterns in all sorts of breast tissues (Figure 1D-F). As illustrated in Table 2, the positive rate of TGFβ2 protein expression in breast cancer cells was 66.67%, which was also significantly higher than that in fibroadenoma breast tissues (P = 0.009) and paracancerous tissues (P = 0.002).
Association between ALDH1 or TGFβ2 expression and clinicopathologic factors
For all 75 breast carcinomas, the expressions of ALDH1 in different subgroups were compared and summarized in Table 1. It showed that ALDH1 status was only associated with tumor histological grade (P = 0.015) and receptor status (P = 0.022). No significant correlation was observed between ALDH1 expression and patient age, menstruation status, histological type, tumor size, clinical stage and lymph node status (P>0.05; Table 1). Similar results were also observed for TGFβ2 expression (Table 1).
Correlation between ALDH1 or TGFβ2 staining intensity and tumor histological grade
To explore more detailed correlation between protein level and tumor histological grade, scoring grades analysis for ALDH1 and TGFβ2 were further performed in 65 invasive breast carcinomas. As demonstrated in Table 3, there was a significant correlation between ALDH1-positive staining intensity and histological grade (P = 0.039). For TGFβ2 immunostaining, the expression intensity was also stronger in carcinomas with a high histological grade (III) compared to carcinomas with a relative low histological grade (I-II) (P = 0.004, Table 3).
Table 3.
Relationships between ALDH1 or TGFβ2 protein staining intensity and tumor histologic grade
| Staining intensity | ALDH1 | TGFβ2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | I-II | III | P value | n | I-II | III | P value | |
| - | 22 | 18 | 4 | 0.039* | 21 | 17 | 4 | 0.004* |
| + | 14 | 6 | 8 | 10 | 7 | 3 | ||
| ++ | 24 | 13 | 11 | 30 | 14 | 16 | ||
| +++ | 5 | 2 | 3 | 4 | 1 | 3 | ||
| Total | 65 | 39 | 26 | - | 65 | 39 | 26 | - |
P<0.05.
Correlation between ALDH1 or TGFβ2 staining intensity and tumor receptor status
The associations between staining intensity of established biologic markers and receptor status were shown in Table 4. Among 13 TNBC invasive carcinomas, 12 samples were observed to be positive for both proteins. There was a significant difference in the staining intensity of ALDH1 between TNBC and non-TNBC (P = 0.017). The differences in TGFβ2-positive staining intensity displayed a homogenous pattern: TNBC revealed a strong staining intensity, while non-TNBC revealed a moderate staining intensity (P = 0.021). These results indicated differences in amount of protein in the positive cells between these two groups.
Table 4.
Relationships between ALDH1 or TGFβ2 protein staining intensity and tumor receptor status
| Staining intensity | ALDH1 | TGFβ2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | TNBC | non-TNBC | P value | n | TNBC | non-TNBC | P value | |
| - | 24 | 1 | 23 | 0.017* | 23 | 1 | 22 | 0.021* |
| + | 16 | 4 | 12 | 14 | 3 | 11 | ||
| ++ | 22 | 7 | 15 | 21 | 7 | 14 | ||
| +++ | 3 | 1 | 2 | 7 | 2 | 5 | ||
| Total | 65 | 13 | 52 | - | 65 | 13 | 52 | - |
P<0.05.
Correlation of ALDH1 and TGFβ2 expression in breast carcinoma
Positive expression of ALDH1 and TGFβ2 was detected in 37 (52.11%) of 75 breast cancer samples and a complete lack of either protein was found in 15 (20%) of the tumors. There was a significant positive correlation between ALDH1 and TGFβ2 protein expression (r = 0.33, P = 0.004) (Table 5).
Table 5.
Correlation of ALDH1 and TGFβ2 protein expression in 75 breast carcinomas
| ALDH1 | TGFβ2 | Total | r | P value | |
|---|---|---|---|---|---|
|
| |||||
| - | + | ||||
| - | 15 | 13 | 28 | 0.33 | 0.004* |
| + | 10 | 37 | 47 | ||
| Total | 25 | 50 | 75 | ||
P<0.05.
Survival analysis of breast cancer patients
Of all 75 breast cancer patients, 10 cases of non-invasive carcinoma had no special treatment after surgery and exhibited good prognosis, thus it is not included in the survival analysis. Among 65 invasive carcinomas with routine postoperative therapy (i.e. adjuvant chemotherapy, radiotherapy and endocrine treatment), 20 cases (30.77%) had already died by the time the study was completed. Univariate analysis by the log-rank test demonstrated lymph node status, clinical stage, receptor status, ALDH1 and TGFβ2 status to be significant prognostic parameters (Table 6). Analysis of the impact of ALDH1 status was shown in Figure 2A. Patients with high ALDH1 expression tended to have poorer prognosis than patients with low ALDH1 expression (P = 0.012, log-rank test). Kaplan-Meier curves of OS stratified by TGFβ2 status were shown in Figure 2B. Similarly, cases with positive TGFβ2 expression had poorer 5-year survival rate (P = 0.023, log-rank test). We further performed a Cox multivariate analysis to identify independent prognostic markers for OS. Our data revealed that ALDH1 expression, lymph node metastasis and clinical stage were statistically significant as independent negative prognostic factors for survival (Table 7).
Table 6.
Univariate analysis for prognostic parameters in 65 invasive breast carcinomas for 5-year survival rate
| Variable | n | 5-year survival rate (%) | P value |
|---|---|---|---|
| Age (years) | 0.644 | ||
| ≤35 | 5 | 60.00 | |
| >35 | 60 | 70.00 | |
| Menstrual status | 0.411 | ||
| Pre-menopausal | 44 | 72.73 | |
| Post-menopausal | 21 | 61.90 | |
| Tumor size (cm) | 0.525 | ||
| ≤2 | 22 | 63.64 | |
| >2 | 43 | 72.09 | |
| Lymph node metastasis | 0.048* | ||
| Negative | 32 | 84.38 | |
| Positive | 33 | 54.55 | |
| Clinical stage | 0.007* | ||
| I-II | 33 | 81.82 | |
| III | 32 | 56.25 | |
| Receptor status | 0.012* | ||
| TNBC | 13 | 36.36 | |
| Non-TNBC | 52 | 77.78 | |
| ALDH1 status | 0.012* | ||
| ALDH1+ | 40 | 57.50 | |
| ALDH1- | 25 | 88.00 | |
| TGFβ2 status | 0.023* | ||
| TGFβ2+ | 42 | 59.52 | |
| TGFβ2- | 23 | 86.96 | |
P<0.05.
Figure 2.
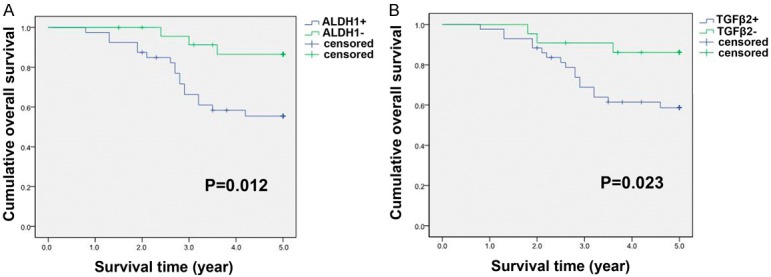
Kaplan-Meier overall survival (OS) curves for 65 patients with invasive breast carcinoma. A: Patients with ALDH1 positive expression had a significantly worse OS compared with those with ALDH1 negative expression. B: The survival time of patients with TGFβ2 positive expression was significantly shorter than that of patients with negative expression. P-values were calculated using the log-rank test.
Table 7.
Multivariate analysis (Cox regression model) of prognostic parameters for overall survival in 65 invasive breast carcinomas
| Variable | Beta | Standard Error | Wald | Relative Risk | 95% CI | P value |
|---|---|---|---|---|---|---|
| ALDH1 | 1.559 | 0.633 | 6.072 | 4.756 | 1.376-16.439 | 0.014* |
| ALDH1- vs. ALDH1+ | ||||||
| Lymph node metastasis | -1.130 | 0.492 | 5.266 | 0.323 | 0.123-0.848 | 0.022* |
| N- vs. N+ | ||||||
| Clinical stage | -1.179 | 0.518 | 5.184 | 0.308 | 0.111-0.849 | 0.023* |
| I-II vs. III |
P<0.05.
Discussion
ALDH1 and TGFβ2 are biologic markers which have shown importance for tumorigenesis. In the present study we have performed comparative analyses of their expressions in cancerous and paracancerous tissues of women operated for breast cancer, and normal fibroadenoma breast tissue of women operated for non malignant condition. The impact of the two proteins on the clinical outcome in breast cancer patients was also investigated.
When comparing immunohistochemical determination for ALDH1 among different sources of tissue, we observed a highly statistical difference in protein positive rate. Expression of ALDH1 was more frequently detected in cancerous tissues compared to that in normal paracancerous or fibroadenoma tissues. As for TGFβ2, it followed a similar pattern except for the immunoreactive intensity, indicating that the two proteins associated with tumor occurrence and development is already detected in the normal tissue, leading to higher risk for development of a malignant disease in the breast. Therefore, the combined detection of ALDH1 and TGFβ2 in tissue sample before surgery might be helpful in diagnosis of malignant breast tumor.
For relationship between protein expression and clinicopathological parameters for the breast cancer subgroup, results revealed only histological grade and receptor status were significant. Among 65 invasive carcinomas, ALDH1 over-expression was strongly associated with a high histological grade. Cancer cells with high histological grade were considered to be more likely to metastasize to the axillary lymph nodes, however, no correlation between protein expression and nodal status was observed in the present study. These results were somewhat in accordance with previous meta-analysis [21], by showing the presence of cancer stem cells positive for ALDH1 was significantly associated with high histological grade, ER negativity, PR negativity, and HER2 positivity, but not tumor size or nodal status.
Recently researches have focused on TNBC due to its high risk of distant recurrence and poor clinical outcome [22,23]. Previous meta-analysis has established a link between receptor status and ALDH1 expression [21]. Moreover, Nalwoga et al [24] demonstrated a high prevalence of ALDH1 expression among breast carcinomas with basal-like markers and features from an African population. Considering that TNBC represents the main subset of basal-like breast cancer, thus it is not surprising that the ALDH1 expression is significant difference between TNBC and non-TNBC patients as revealed in the present study. Stem cell-like populations in breast cancer are characterized by the expression of ALDH1 [5], and the amount of cancer stem cells within breast tumors may correspond to the risk of distant metastases [25,26]. Thus, ALDH1 status might represent an indicator of aggressive breast cancer with features such as ER/PR negativity, similar to what others have reported [5,27]. However, no interaction was also found between ALDH1 expression and receptor status in other studies [10,28]. These differences among the studies may be attributed to the methodological discrepancies, and different samples under investigation.
Over-expression of TGFβ has been reported in most cancers [29], and these high TGFβ levels in tumor tissues correlate with markers of a more metastatic phenotype [30]. Moreover, treatment of mice with TGFβ neutralizing antibodies strongly inhibits development of either lung- or bone metastases [31]. In consistent with these findings, we observed a higher frequency of TGFβ2 expression (66.67%) in breast cancer tissues compared to that in fibroadenoma (41.03%) and paracancerous (46.67%) samples, from a Chinese population. Furthermore, high expression of TGFβ2 was associated with features of aggressive tumors such as high histological grade and ER/PR/HER2 negativity. Therefore, quite similar to the well accepted dominant role of TGFβ1 in breast cancer development [30], our study reveals an important role of another TGFβ family member, TGFβ2, in this process.
Here, investigations of ALDH1 and TGFβ2 in different subgroups, especially in TNBC subgroup were of particular interest as their expression patterns were highly consistent. For example, in tumors with either low grade I-II or high grade III, the distribution of ALDH1-positive staining intensity is similar to that of TGFβ2 (Table 3). Our results also showed that coexpression of ALDH1 and TGFβ2 was observed in almost all samples of TNBC (both 12/13, 92.31%). All TNBC with ALDH1 positivity were positive for TGFβ2 staining. The incidences of high expression (grade 2+-3+) were 61.54% (8 samples) for ALDH1 and 69.23% (9 samples) for TGFβ2 in 13 TNBC tissues, respectively, strongly suggesting an interaction between ALDH1 and TGFβ2, and that an enrichment of protein-positive cancer cells in more aggressive tumors of breast. Previous studies reported that high levels of ALDH1 [5] and TGFβ2 [30] in breast cancer tissues might correlate with poor patient outcome. Our results were similar: both ALDH1 and TGFβ2 were over-expressed in breast cancer tissues; and positive expression of either ALDH1 or TGFβ2 was further found to be significantly correlated with reduced 5-year survival of invasive carcinoma patients by univariate analysis (P = 0.012 for ALDH1; and P = 0.023 for TGFβ2).
To the best of our knowledge, we are the first to show that expression of ALDH1 in breast cancer is positively correlated with TGFβ2. ALDH1 has been demonstrated as a putative marker of human mammary stem cells and a predictor of poor prognosis [5], however, the biologic mechanism underlying is not well established. Understanding the cross-talk between cell populations mediated by ALDH1 may be important when designing strategies to manage human breast cancer development and progression. At present, the explanation for positive correlation between these two proteins is unknown. To speculate, our findings might be related to a directly linkage between ALDH1 and TGFβ signaling pathways. TGFβ has the potential to act directly on a small TGFβ-responsive progenitor cell population. For example, CD44+ cell-specific genes included many known stem-cell markers and correlated with decreased patient survival [32]. The TGFβ pathway was specifically active in CD44+ mammary cancer cells with stem-cell-like properties, and treatment of these CD44+ cells with a TGFβ receptor kinase inhibitor caused markedly decreased metastatic potential [14]. Furthermore, previous studies also provided evidence that activation of TGFβ2 is an essential CD44-downstream event required for tumor cell survival and metastasis [17,18]. Thus, it is quite possible that TGFβ2 directly interacted with ALDH1, like CD44, in regulating the dynamics of cell populations within normal and malignant breast tissues by favoring stem-cell self renewal, and, consequently, promoting breast cancer development and progression. However, other mechanisms such as epithelial-to-mesenchymal transition (EMT) also remains as potential. EMT is associated with both anchorage-independent growth and a stem cell-like phenotype. Meantime, it has been known for some time that TGFβ can induce benign mammary epithelial cells to undergo EMT [33,34], postulating a link between TGFβ activation and ALDH1 over-expression in breast cancer development. In support of this, Mani et al. [35] has provided further evidence for a key association between mesenchymal and stem cell phenotypes and the role of TGFβ therein with a series of elegant studies. Therefore, considering the essential role in maintaining the mammary epithelial (cancer) stem cell pool, our data suggest that TGFβ2 signaling pathway activation might be involved in ALDH1-mediating breast tumor development and progression, though further studies to confirm the direct interaction between these two factors are warranted.
In summary, we have in the present study demonstrated for the first time that the expression of ALDH1 and TGFβ2, are different in various clinical categories (malignant breast tumors, paracancerous normal counterparts or benign fibroadenomas). These two proteins were positively correlated and associated with reduced survival in breast cancer patients. Therefore, combined detection of ALDH1 and TGFβ2 may be applicable to clinical management of breast cancer, contributing to both stratify women at risk for development of malignant disease and the identification of patients with poor prognosis.
Acknowledgements
This work was supported by grant from the Natural Science Foundation of Anhui Province, China (No. 1408085QH166), and internal grant from Bengbu Medical College (No. Bykf13A12).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 3.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–41. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 7.Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakshatri H, Srour EF, Badve S. Breast cancer stem cells and intrinsic subtypes: controversies rage on. Curr Stem Cell Res Ther. 2009;4:50–60. doi: 10.2174/157488809787169110. [DOI] [PubMed] [Google Scholar]
- 9.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Y, Lin Y, Shen S, Zhou Y, Mao F, Guan J, Sun Q. Expression of ALDH1 in breast invasive ductal carcinoma: an independent predictor of early tumor relapse. Cancer Cell Int. 2013;13:60. doi: 10.1186/1475-2867-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlingensiepen KH, Fischer-Blass B, Schmaus S, Ludwig S. Antisense therapeutics for tumor tartreatment: the TGF-beta2 inhibitor AP 12009 in clinical development against malignant tumors. Recent Results Cancer Res. 2008;177:137–50. doi: 10.1007/978-3-540-71279-4_16. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AB, Flanders KC, Heine UI, Jakowlew S, Kondaiah P, Kim SJ, Sporn MB. Transforming growth factor-beta: multifunctional regulator of differentiation and development. Philos Trans R Soc Lond B Biol Sci. 1990;327:145–54. doi: 10.1098/rstb.1990.0050. [DOI] [PubMed] [Google Scholar]
- 13.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–62. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 14.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Kuo YC, Su CH, Liu CY, Chen TH, Chen CP, Wang HS. Transforming growth factor-beta induces CD44 cleavage that promotes migration of MDA-MB-435s cells through the up-regulation of membrane type 1-matrix metalloproteinase. Int J Cancer. 2009;124:2568–76. doi: 10.1002/ijc.24263. [DOI] [PubMed] [Google Scholar]
- 16.Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C. Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Cancer Res. 2004;10:491–8. doi: 10.1158/1078-0432.ccr-0320-03. [DOI] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–12. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 18.Ouhtit A, Madani S, Gupta I, Shanmuganathan S, Abdraboh ME, Al-Riyami H, Al-Farsi YM, Raj MH. TGF-beta2: A Novel Target of CD44-Promoted Breast Cancer Invasion. J Cancer. 2013;4:566–72. doi: 10.7150/jca.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressler LG, Berry DA, Broadwater G, Cowan D, Cox K, Griffin S, Miller A, Tse J, Novotny D, Persons DL, Barcos M, Henderson IC, Liu ET, Thor A, Budman D, Muss H, Norton L, Hayes DF. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23:4287–97. doi: 10.1200/JCO.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 20.He XD, Wang Y, Wu Q, Wang HX, Chen ZD, Zheng RS, Wang ZS, Wang JB, Yang Y. Xuebijing Protects Rats from Sepsis Challenged with Acinetobacter baumannii by Promoting Annexin A1 Expression and Inhibiting Proinflammatory Cytokines Secretion. Evid Based Complement Alternat Med. 2013;2013:804940. doi: 10.1155/2013/804940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Jiang Y, Yan T, Di G, Shen Z, Shao Z, Lu J. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122:795–801. doi: 10.1007/s10549-010-0999-4. [DOI] [PubMed] [Google Scholar]
- 22.Li CY, Zhang S, Zhang XB, Wang P, Hou GF, Zhang J. Clinicopathological and prognostic characteristics of triple- negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev. 2013;14:3779–84. doi: 10.7314/apjcp.2013.14.6.3779. [DOI] [PubMed] [Google Scholar]
- 23.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 24.Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer. 2010;102:369–75. doi: 10.1038/sj.bjc.6605488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–9. [PubMed] [Google Scholar]
- 26.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–21. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, Tamaki Y, Terada N, Noguchi S. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–8. doi: 10.1111/j.1349-7006.2009.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakakibara M, Fujimori T, Miyoshi T, Nagashima T, Fujimoto H, Suzuki HT, Ohki Y, Fushimi K, Yokomizo J, Nakatani Y, Miyazaki M. Aldehyde dehydrogenase 1-positive cells in axillary lymph node metastases after chemotherapy as a prognostic factor in patients with lymph node-positive breast cancer. Cancer. 2012;118:3899–910. doi: 10.1002/cncr.26725. [DOI] [PubMed] [Google Scholar]
- 29.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs. 2003;21:21–32. doi: 10.1023/a:1022951824806. [DOI] [PubMed] [Google Scholar]
- 30.Dumont N, Arteaga CL. Transforming growth factor-beta and breast cancer: Tumor promoting effects of transforming growth factor-beta. Breast Cancer Res. 2000;2:125–32. doi: 10.1186/bcr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM, Fisher LW, Fedarko NS, Jain A, Pinkas J, Lonning S, Wakefield LM. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res. 2006;66:6327–35. doi: 10.1158/0008-5472.CAN-06-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, Schreiner G, Barnard N, Reiss M. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315–30. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 35.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


