Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most common types of tumors worldwide, particularly in China, and human papillomavirus (HPV) is thought to be a potential risk factor for this cancer. To determine whether this is true, we collected 177 formalin-fixed and paraffin-embedded ESCC samples from two hospitals. We screened for 23 different HPV genotypes using a human papillomavirus genotyping kit, which allowed us to amplify the L1 gene by polymerase chain reaction (PCR) and test for 23 HPV subtypes by reverse dot blot (RDB) on a single membrane. We also used immunohistochemistry (IHC) to detect the P16INK4a protein, the expression of which is linked to HPV E7 activity and which is used to diagnose cervical intraepithelial neoplasia. The genotyping results showed that only six samples were weakly positive for HPV: two for HPV16, two for HPV11 and two for HPV35, with no samples showing strong positive signals. The IHC results showed only five samples with diffuse positive staining, with the other samples being completely negative or having only focal positive signals, which were considered as negative. This study demonstrates that the HPV infection rate in ESCC samples is very low, suggesting that HPV is not the etiological cause of ESCC.
Keywords: Human papillomavirus, esophageal squamous cell carcinoma, PCR, RBD, P16INK4a
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most prevalent tumor types worldwide, particularly in China and East Asia [1]. Although the major risk factors for ESCC are not well characterized, they are thought to include alcohol, tobacco, hot beverages, and a low intake of fruits and vegetables [2,3]. In addition, since Syrjanen et al. first identified flat condylomous lesions in invasive squamous cell carcinomas of the esophagus in 1982 [4], human papillomavirus (HPV) has been considered as a potential risk factor for ESCC.
Over the last three decades, many studies around the world have attempted to verify this proposal, although the results have been inconclusive. The majority of studies have tested for the presence of the four most common HPV types, namely 6, 11, 16 and 18. In particular, the infection rates determined by polymerase chain reaction (PCR) ranged from zero in Japan [5], Kenya [6], Italy [7] and Germany [8] to extremely low in America [9] and Sweden [10]. Therefore, these studies suggest that HPV is not a pathogenic factor for ESCC. In contrast, other studies suggest that HPV may play an important role in ESCC, with measured infection rates ranging from 24.2% to 44.7% (using the same detection method) in India [11], South Africa [12], the United Kingdom [13] and Chile [14]. It is possible that these results reflect true regional disparities in infection rates, which may be low in developed countries and high in developing countries. However, contradictory results have also been reported. For example, one study reported that the infection rate in Japan was 42% [15], whereas another study using the same method claimed that it was zero [5]. Considering this, Miyagi et al. [16] noted that infection rates can change over time, even within the same area and we thought it might have potential HPV subtype infection besides the four common subtypes.
Studies carried out in China have also yielded conflicting results. The majority of studies have identified HPV in ESCC samples, with infection rates ranging from 17.1% [17] to 78.11% [18], detected via amplification of the L1 gene. One study reported an infection rate of 100%, as determined by detection of the HPV16 E6 and E7 genes in early cancer cases in a high-risk area using PCR and ISH [19]. Furthermore, some studies [20] have suggested that HPV may play as important a role in ESCC as it does in cervical carcinoma, whereas other studies suggest the opposite. Gao et al. [21] identified no positive cases of HPV among invasive ESCC samples in a cross-sectional study using a multivalent HPV hybridization probe and the Digene Hybrid Capture II method, and Peixoto et al. [22] found only two infections among 32 cases in a high-risk region using PCR-based Southern blot hybridization. In addition, Lam et al. and He et al. [23,24], who were from the same research group, reported different infection rates in Hong Kong and Sichuan using PCR, single-strand conformational polymorphism (SSCP) analysis, and DNA sequencing, suggesting that HPV may indeed play different roles in low- and high-incidence areas. However, we believe these discrepancies might be attributable to differences in the methodologies and subjects between the various studies.
To reliably measure the infection rate for HPV in Shanghai, we screened 177 cases for 23 different HPV types using PCR and reverse dot blot analysis, testing for the most common HPV subtypes associated with abnormal cytology [25]. To rule out methodological differences, we tested all samples in two isolated labs by blind assignment, and we also detected P16INK4a expression by immunohistochemistry (IHC). In particular, these two methods were used to determine the HPV infection rates for cervical carcinoma and cervical intraepithelial neoplasia samples encountered during routine work.
Materials and methods
Study subjects
Considering potential contamination from exposure to the oral cavity, time since sampling and the various sampling procedures, 74 samples were obtained from all patients who underwent surgical esophagectomy at the Fifth People’s Hospital of Shanghai, Fudan University, between 1999 and 2010, as well as 103 samples from the Shanghai Chest Hospital, Shanghai Jiaotong University, between 2008 and 2011. All patients gave written informed consent for their participation. This study has been approved by the Ethics Committees of the two institutions, and it conforms to the principles outlined in the Declaration of Helsinki. All the samples were formalin-fixed and paraffin-embedded. Two senior pathologists who were blinded to the HPV DNA and P16INK4a results diagnosed and graded all samples using hematoxylin and eosin (H&E)-stained sections. Well-differentiated carcinomas were graded as having a minor component of nonkeratinizing basal-like cells and prominent keratinization, particularly with squamous pearl formations akin to non-neoplastic squamous epithelia and tumor cells arranged in sheets (Figure 1A). Poorly differentiated carcinomas were graded as consisting of predominantly basal-like cells forming large and small nests with frequent central necrotic regions (Figure 1C). Moderately differentiated carcinomas were graded between the well-differentiated and poorly differentiated carcinomas, lacking the pearl formations akin to non-neoplastic squamous epithelia (Figure 1B). The depth of tumor infiltration was staged as follows: T1) invading the lamina propria or submucosa; T2) invading the muscularis propria; T3) invading the adventitia; and T4) invading adjacent structures.
Figure 1.
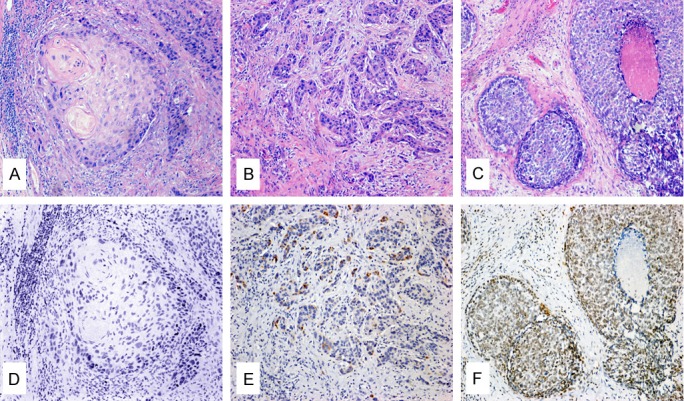
Images of typical H&E and IHC staining. Images were collected using a light microscope at 100 × magnification. The tissue sections were scored using the following criteria: panel A: Well-differentiated ESCC with squamous pearl formations; panel B: Moderately differentiated ESCC; panel C: Poorly differentiated ESCC with predominantly basal-like cells forming nests with central necrotic regions; panel D: Completely negative P16INK4a expression; panel E: Negative expression of P16INK4a with focal staining; and panel F: Positive expression of P16INK4a with intense staining. Panels A-C were prepared by H&E staining. Panels D-F were prepared by IHC staining.
The age of the patients ranged from 43 to 83, with a mean age of 62.7 (Std. Deviation 8.53) and a median age of 63. In total, 144 patients were male and 33 were female, yielding a sex ratio of 4.36:1. Of the total 177 patients, the majority (127, 72.3%) were from Shanghai, 16 (9%) were from Jiangsu, 14 (7.9%) were from Anhui and 12 (6.8%) were from Zhejiang, with the remaining 7 (4%) patients coming from other provinces; the majority of the provinces were located in east China, with the exception of one patient from Sichuan.
HPV genotype screening using the human papillomavirus genotyping kit
The Human Papillomavirus Genotyping kit for 23 Types was produced by Yaneng Bioscience co., LTD (Shen Zhen, China), and the kit was applied for with permit number 3400994 in 2008 by the Fresh Armed State Drug Administration, China. The kit was used to perform Polymerase chain reaction (PCR) to amplify the L1 gene in conjunction with reverse dot blot (RDB) analysis to identify the HPV subtypes. This method offers a simple testing strategy involving a membrane chip that can detect infections from multiple HPV subtypes, including 18 high-risk types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 83 and MM4) and 5 low-risk types (HPV 6, 11, 42, 43 and 44). This method had a sensitivity of 103 copies/ml and a specificity of 99%; β-globin was used as an internal positive control.
DNA extraction and polymerase chain reaction (PCR) conditions
DNA was extracted from 4-5 serial sections (4 μm thick) by hot dehiscing using the Human Papillomavirus Genotyping kit for 23 Types, according to the manufacturer’s instructions. The tissues prepared for extraction included representative tumor tissues and the adjacent normal tissue. The quality of the extracted DNA was verified using a spectrophotometer (260 nm ultraviolet light). The extracted DNA was concentrated by high-speed centrifugation at 4°C. For each PCR reaction, 5 μL extracted concentrated DNA was used in a final reaction volume of 25 μL. The PCR amplification conditions were as follows: preheating at 95°C for 10 min, followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 42°C for 90 sec, and extension at 72°C for 30 sec, with a final extension at 72°C for 5 min. The amplicons were then denatured and subjected to hybridization.
To test the quality of the DNA, the kit was also used to amplify the housekeeping gene β-globin within the same reactions as an internal positive control. We also used a verified HPV multiple infection cervical intraepithelial neoplasia sample as a positive control and distilled water as a negative control; all control samples were subject to the same treatments and processed at the same time as the experimental samples. To ensure the samples were not contaminated within the lab, every test was carried out with fresh wash buffer and all samples were independently tested in two isolated labs by blind assignment.
HPV detection and typing
We use the reverse dot blot (RDB) method for HPV detection and typing. The 25 μL reaction volumes containing the amplified fragments were hybridized to the dot blot membrane in 6 mL hybridization solution (2×SSC, 0.1%SDS) at 51°C for 2 hours. After a stringent wash, the hybridized membrane was probed by adding a streptavidin-horseradish peroxidase conjugate (which binds to the biotinylated PCR products) and a substrate (3,3’,5,5’-Tetramethylbenzidine) to generate a blue precipitate at the site of the probe dot. The results were inspected by macroscopic observation, and the results were deemed reliable when the PC (positive control) dot appeared as a clear round blue dot. A clear round blue dot was scored as positive for the corresponding HPV subtype, a dilute blue dot was scored as weakly positive, and the absence of a dot was scored as negative.
Immunohistochemical staining
Lesion sections (3 μm thick) were cut and immunostained for P16INK4a using the monoclonal antibody 16P04/JC2 and the EnVision Detection Kit (Dako, Denmark); a known positive cervical intraepithelial neoplasia tissue was used as a positive control, and lack of antibody staining was used as a negative control. After dewaxing, rehydration and rinsing in PBS buffer, all sections were treated for 20 minutes at 95°C in a high pH (pH = 9.0) EDTA-Tris buffer for antigen retrieval. Endogenous enzymatic activity in the sections was inhibited with 3% hydrogen peroxide for eight minutes. The sections were incubated at 37°C with the P16INK4a antibody for one hour and the secondary antibody for 30 minutes. The sections were incubated with DAB (3,3’-diaminobenzidine) and counterstained with hematoxylin to increase contrast. PBS buffer was used to rinse the sections between each step. All positive and negative control samples were treated using the same procedures.
Evaluation of immunohistochemical staining
Cells with specific cytoplasmic or/and nuclear brown staining were scored as positive. By counting the proportion of positive tumor cells (a lack of positive cells was recorded as complete negative), the samples were scored as follows: less than 10% as focal positive, over 80% as diffuse positive and between 10% and 80% as partial positive. All immunostained slides were evaluated by two senior pathologists, and only three types of positive patterns were observed: complete negative, focal positive and diffuse positive.
Data analysis and statistics
All data were analyzed using the SPSS (version 13.0) statistical software program (SPSS Inc., Chicago, IL) by chi-square test or Fisher exact test. For all tests, a P value less than 0.05 was considered statistically significant.
Results
Re-diagnosis of study subject
Of the 177 total patients that were re-diagnosed and graded, only 24 (13.56%) samples were graded as poorly differentiated carcinomas, predominantly showing basal-like cells and nests with frequent central necrotic areas. Fifty-five (31.07%) samples were graded as well-differentiated carcinomas, showing prominent keratinization akin to what is observed in squamous pearl formations or non-neoplastic squamous epithelia. Over half of the samples (98, 55.37%) were graded as moderately differentiated carcinomas, representing the largest of the three groups. Only four (2.3%) well-differentiated carcinoma cases showed invasion of the submucosa and were staged as T1; none of the poorly or moderately differentiated carcinomas were staged as T1. Forty (22.6%) cases showed invasion of the muscularis propria and were staged as T2. Finally, 133 (75.1%) cases showed invasion of the adventitia and were staged as T3. Histological patterns typical of differentiated ESCC are shown in Figure 1A-C. Table 1 lists the relationships between the histological gradations and the general conditions of the patients, although only one statistically significant relationship was identified by our analysis (P > 0.05), which was tumor infiltration depth (P = 0.009). In particular, all four T1 tumors were evaluated as well-differentiated carcinomas, and all moderately and poorly differentiated carcinomas were staged T2 or higher.
Table 1.
Relationships between histological gradations and general conditions
| General conditions | Histological grading | Numbers of cases | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Well-differentiated | Moderately differentiated | Poorly differentiated | |||
| Gender | |||||
| Male | 43 | 79 | 22 | 144 | |
| Female | 12 | 19 | 2 | 33 | |
| Total | 55 | 98 | 24 | 177 | 0.353 |
| Age | |||||
| <63 | 24 | 50 | 11 | 85 | |
| ≥63 | 31 | 48 | 13 | 92 | |
| Total | 55 | 98 | 24 | 177 | 0.663 |
| Tumor infiltration depth | |||||
| T1 | 4 | 0 | 0 | 4 | |
| T2 | 15 | 17 | 8 | 40 | |
| T3 | 36 | 81 | 16 | 133 | |
| Total | 55 | 98 | 24 | 177 | 0.009 |
| Province | |||||
| Shanghai | 26 | 81 | 21 | 128 | |
| Jiangsu | 9 | 6 | 1 | 16 | |
| Anhui | 7 | 6 | 1 | 14 | |
| Zhejiang | 9 | 2 | 1 | 12 | |
| Jiangxi | 2 | 0 | 0 | 2 | |
| Fujian | 0 | 1 | 0 | 1 | |
| Guangdong | 1 | 0 | 0 | 1 | |
| Hubei | 0 | 1 | 0 | 1 | |
| Shandong | 1 | 0 | 0 | 1 | |
| Sichuan* | 0 | 1 | 0 | 1 | |
| Total | 55 | 98 | 24 | 177 | |
T1: tumor invades the lamina propria or submucosa; T2: tumor invades the muscularis propria; T3: tumor invades the adventitia.
Sichuan is the western province in China.
Detection of HPV-specific DNA by PCR connected with reverse bot blot (RBD)
Of the 177 samples we screened, only six (3.4%) samples were weakly positive for HPV, and no samples were intensely positive. Of the six weakly positive samples, two were positive for HPV16, two for HPV11 and two for HPV35. Both HPV35-positive samples were well-differentiated and one of the HPV11-positive samples was poorly differentiated, although no statistically significant differences were observed for the positive samples. The surgery dates for the six samples were between 2000 and 2010. Typical results are shown in Figure 2, and Table 2 lists the conditions of all six weakly positive samples.
Figure 2.
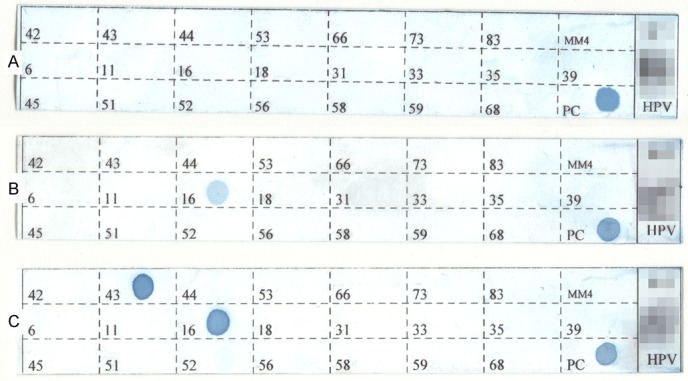
Images of typical HPV genotyping results. Clear round blue dots on the labeled grid were considered as positive signals for the corresponding HPV subtype; the PC grid shows the internal β-globin positive control. Membrane (A) was only positive for PC and was therefore scored as negative. Membrane (B) showed a clear light blue dot at position 16 on the grid and was therefore considered weakly positive for HPV16. Membrane (C) showed two dots at positions 16 and 43 on the grid and was therefore considered positive for HPV 16 and 43, which was from the HPV multiple infection cervical intraepithelial neoplasia sample. The patients’ information was mosaicked.
Table 2.
Related conditions of the six weakly positive HPV samples
| Case No. | Year | HPV subtype | Gender | Age | Histologic gradation | Expression of P16INK4a | Tumor infiltration depth |
|---|---|---|---|---|---|---|---|
| 1 | 2006 | 35 | Female | 62 | Well-Differentiated | Negative | T3 |
| 2 | 2010 | 35 | Male | 63 | Well-Differentiated | Negative | T3 |
| 3 | 2000 | 16 | Female | 45 | Well-Differentiated | Negative | T2 |
| 4 | 2008 | 16 | Male | 70 | Moderately Differentiated | Negative | T2 |
| 5 | 2010 | 11 | Male | 63 | Moderately Differentiated | Negative | T2 |
| 6 | 2004 | 11 | Male | 67 | Poorly Differentiated | Focal Positive | T3 |
T2: tumor invades the muscularis propria; T3: tumor invades the adventitia.
Analysis of P16INK4a expression by immunohistochemical staining
All 177 samples were also immunostained for P16INK4a and scored by the percentage of positive cells. The results could be grouped into three categories. Over half of the samples (104, 58.76%) showed no positive tumor cells and were considered as completely negative (Figure 1D). Nearly four in ten samples (68 samples, 38.42%) showed less than ten percent positive tumor cells and were considered as focal positive (Figure 1E), only one sample was weakly positive for HPV11. The other five (2.82%) samples showed over eighty percent positive tumor cells and were considered as diffuse positive (Figure 1F), although none of these samples were scored as positive or weakly positive by HPV subtype screening. With respect to the cervical intraepithelial neoplasia (CIN) samples [26], only intense diffuse positive tumor cells were scored as P16INK4a-positive; focal positive cells were scored as negative. There was no statistically significant difference between the two groups (P > 0.05); P16INK4a expression levels are described in Table 3, and related conditions are listed in Table 4.
Table 3.
P16INK4a expression in esophageal squamous cell carcinoma
| Related conditions | Negative | Intensely Positive | Total | P value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Completely negative | Focal Positive | Total | ||||
| Histologic grading | ||||||
| Poorly Differentiated | 13 | 10 | 23 | 1 | 24 | |
| Moderately Differentiated | 60 | 37 | 96 | 1 | 98 | |
| Well-Differentiated | 31 | 21 | 52 | 3 | 55 | |
| Total | 104 | 68 | 172 | 5 | 177 | 0.567 |
| Gender | ||||||
| Male | 83 | 57 | 140 | 4 | 144 | |
| Female | 21 | 11 | 32 | 1 | 33 | |
| Total | 104 | 68 | 172 | 5 | 177 | 0.801 |
| Age | ||||||
| <63 | 54 | 28 | 82 | 3 | 85 | |
| ≥63 | 50 | 40 | 90 | 2 | 92 | |
| Total | 104 | 68 | 172 | 5 | 177 | 0.333 |
| Tumor infiltration depth | ||||||
| T1 | 3 | 1 | 4 | 0 | 4 | |
| T2 | 26 | 13 | 39 | 1 | 40 | |
| T3 | 75 | 54 | 129 | 4 | 133 | |
| Total | 104 | 68 | 172 | 5 | 177 | 0.839 |
| Province | ||||||
| Shanghai | 78 | 48 | 126 | 2 | 128 | |
| Jiangsu | 8 | 6 | 14 | 2 | 16 | |
| Anhui | 8 | 6 | 14 | 0 | 14 | |
| Zhejiang | 9 | 3 | 12 | 0 | 12 | |
| Jiangxi | 0 | 2 | 2 | 0 | 2 | |
| Fujian | 1 | 0 | 1 | 0 | 1 | |
| Guangdong | 0 | 0 | 0 | 1 | 1 | |
| Hubei | 1 | 0 | 1 | 0 | 1 | |
| Shandong | 0 | 1 | 1 | 0 | 1 | |
| Sichuan | 0 | 1 | 1 | 0 | 1 | |
| Total | 104 | 68 | 172 | 5 | 177 | |
T1: Tumor invades the lamina propria or submucosa; T2: tumor invades the muscularis propria; T3: tumor invades the adventitia.
Table 4.
Related conditions of the five positive P16INK4a samples
| Case number | HPV Subtype | Gender | Age | Histologic grading | Tumor infiltration depth |
|---|---|---|---|---|---|
| 1 | Negative | Female | 69 | Well-Differentiated | T3 |
| 2 | Negative | Male | 80 | Poorly Differentiated | T2 |
| 3 | Negative | Male | 61 | Well-Differentiated | T3 |
| 4 | Negative | Male | 60 | Well-Differentiated | T3 |
| 5 | Negative | Male | 53 | Moderately Differentiated | T3 |
T2: tumor invades the muscularis propria; T3: tumor invades the adventitia.
Discussion
More than one hundred HPV subtypes have been described [27], over forty of which have been shown to be involved in human disease, and these subtypes are traditionally characterized by the sequence of their L1 gene. Moreover, specific HPV subtypes have been confirmed as the major pathogenic factors for cervical tumors [28-30], and recently, clinical trials [31] showed that HPV vaccines could prevent high-risk virus infection.
However, as described above, it is still unclear whether HPV plays a role in ESCC. Esophageal tumors are one of the most common tumors in the Shanghai region [32], and the majority of these are ESCC. Considering the large number of studies showing that the HPV infection rate varies worldwide [11-13,15,17,18], we suspected that HPV could be a potential pathogenic factor for ESCC and that the HPV vaccine might be effectively on ESCC patients in the future. The current study involved a simple test used to screen ESCC samples for the most common HPV subtypes, although our results showed no samples that were strongly positive and only six samples that were weakly positive, aside from cross-contaminated samples. Therefore, counting the weakly positive samples, the measured infection rate was only 3.4%, similar to other studies reporting rates of zero [5-7] or very low rates [9,10]. Considering that viral infection rates can vary over 10-20-year periods [25], we tested all ESCC samples from the Fifth People’s Hospital of Shanghai, Fudan University collected over a period of 12 years. However, the extremely low infection rate and random distribution of the surgical dates for the positive samples suggests that HPV is not the major etiology for ESCC. The current study also identified two samples that were weakly positive for HPV35, which has not been previously describe for ESCC, although there was no evidence that HPV35 was involved in the etiology of ESCC.
Some studies [19,33] have suggested that while the HPV L1 region may be disrupted or lost, the HPV16 E7 gene can lead to the immortalization of normal esophageal epithelial cells [34]. Therefore, we tested the E7-related protein P16INK4a using an immunohistochemical staining method. P16INK4a is a cyclin-dependent kinase (CDK) inhibitor that slows the cell cycle by inactivating CDKs that phosphorylate the retinoblastoma (Rb) protein. In high-risk HPV subtypes, such as HPV16, the E7 protein can inactivate Rb function and induce the overexpression of P16INK4a protein [35]. The overexpression of P16INK4a protein, particularly intense positive expression, has been used as a marker in high-grade cervical intraepithelial lesions [26,36], cervical squamous cell carcinomas [35] and endocervical adenocarcinoma [37,38]. However, in this study, we only observed P16INK4a-positive signals in five (2.8%) samples; the other samples showed negative or focal staining. Furthermore, none of the samples that were strongly positive for P16INK4a were also scored as positive by HPV genotyping, and only one sample scored as weakly positive for HPV was also focal positive for P16INK4a. The reasons for the observed disagreement between the HPV genotyping and the P16INK4a immunohistochemical results may be twofold. Positivity for HPV and negativity for P16 could be due to methylation of the P16 gene promoter. For example, Kawakami et al. [39] found that approximately 20% of HPV DNA-positive oropharyngeal squamous cell carcinomas were negative for P16, which was due to the fact that most of these tumors showed DNA methylation at the P16 gene promoter. Similarly, Salam et al. [40] found that over seventy percent of esophageal squamous cell carcinoma cases were showed a loss of P16 expression that was linked to promoter methylation. In contrast, positivity for P16 and negativity for HPV could be indicative of activation of an alternative pathway for P16 overexpression. Riethdorf et al. [41] and Lerma et al. [42] suggested that the P16/Rb pathway may not be the only way pathway that causes vulvar neoplasia and carcinoma. Several pieces of evidence suggest the existence of a non-HPV-dependent P16 expression pathway, including the HPV-negative cell line C33A [43], HPV-negative/P16-positive cervical adenocarcinoma [44], penile squamous cell carcinoma [45] and the observance of a higher positivity rate for P16 immunohistochemistry than for HPV DNA testing [46]. We show that function of the high-risk HPV E7 gene is not related to the etiology of ESCC, as very few ESCC samples were positive for P16INK4a. In addition, it appears that the E7/Rb/P16 pathway is not necessary for P16 activation, suggesting an alternative pathway for P16 overexpression.
Using sensitive molecular biology methods, we determined that the infection rates for 23 HPV subtypes in 177 ESCC samples were close to zero, and we also show that expression of the related protein P16INK4a was extremely low in these same samples using a simple IHC method. Therefore, we suggest that HPV is not the etiological agent of ESCC, at least not within the last 10 years in East China, particularly in Shanghai.
Acknowledgements
This scientific research project was supported by the Minhang district Public Health Bureau, Shanghai (project number 2009 MW03).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wu M, Liu AM, Kampman E, Zhang ZF, Van’T VP, Wu DL, Wang PH, Yang J, Qin Y, Mu LN, Kok FJ, Zhao JK. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. Int J Cancer. 2009;124:1907–1913. doi: 10.1002/ijc.24142. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Yuan JM, Wang R, Gao YT, Yu MC. Alcohol, tobacco, and diet in relation to esophageal cancer: the Shanghai Cohort Study. Nutr Cancer. 2008;60:354–363. doi: 10.1080/01635580701883011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjanen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1982;52:283–292. [PubMed] [Google Scholar]
- 5.Saegusa M, Hashimura M, Takano Y, Ohbu M, Okayasu I. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in esophageal and gastric carcinomas in Japan. Mol Pathol. 1997;50:101–104. doi: 10.1136/mp.50.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White RE, Mungatana C, Mutuma G, Robert ME, Daniel RW, Topazian MD, Shah KV. Absence of human papillomavirus in esophageal carcinomas from southwestern Kenya. Dis Esophagus. 2005;18:28–30. doi: 10.1111/j.1442-2050.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 7.Talamini G, Capelli P, Zamboni G, Mastromauro M, Pasetto M, Castagnini A, Angelini G, Bassi C, Scarpa A. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. Int J Cancer. 2000;86:874–878. doi: 10.1002/(sici)1097-0215(20000615)86:6<874::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Awerkiew S, Bollschweiler E, Metzger R, Schneider PM, Holscher AH, Pfister H. Esophageal cancer in Germany is associated with Epstein-Barr-virus but not with papillomaviruses. Med Microbiol Immunol. 2003;192:137–140. doi: 10.1007/s00430-002-0128-z. [DOI] [PubMed] [Google Scholar]
- 9.Kamath AM, Wu TT, Heitmiller R, Daniel R, Shah KV. Investigation of the association of esophageal carcinoma with human papillomaviruses. Dis Esophagus. 2000;13:122–124. doi: 10.1046/j.1442-2050.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 10.Lagergren J, Wang Z, Bergstrom R, Dillner J, Nyren O. Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. J Natl Cancer Inst. 1999;91:156–162. doi: 10.1093/jnci/91.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Katiyar S, Hedau S, Jain N, Kar P, Khuroo MS, Mohanta J, Kumar S, Gopalkrishna V, Kumar N, Das BC. p53 gene mutation and human papillomavirus (HPV) infection in esophageal carcinoma from three different endemic geographic regions of India. Cancer Lett. 2005;218:69–79. doi: 10.1016/j.canlet.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Matsha T, Donninger H, Erasmus RT, Hendricks D, Stepien A, Parker MI. Expression of p53 and its homolog, p73, in HPV DNA positive esophageal squamous cell carcinomas. Virology. 2007;369:182–190. doi: 10.1016/j.virol.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Morgan RJ, Perry AC, Newcomb PV, Hardwick RH, Alderson D. Human papillomavirus and esophageal squamous cell carcinoma in the UK. Eur J Surg Oncol. 1997;23:513–517. doi: 10.1016/s0748-7983(97)92981-4. [DOI] [PubMed] [Google Scholar]
- 14.Castillo A, Aguayo F, Koriyama C, Torres M, Carrascal E, Corvalan A, Roblero JP, Naquira C, Palma M, Backhouse C, Argandona J, Itoh T, Shuyama K, Eizuru Y, Akiba S. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World J Gastroenterol. 2006;12:6188–6192. doi: 10.3748/wjg.v12.i38.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa M, Ohoka I, Yamazaki K, Hanami K, Sugano I, Nagao T, Asoh A, Wada N, Nagao K, Ishida Y. Expression of p21/WAF-1, status of apoptosis and p53 mutation in esophageal squamous cell carcinoma with HPV infection. Pathol Int. 2002;52:442–450. doi: 10.1046/j.1440-1827.2002.01373.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyagi J, Tsuhako K, Kinjo T, Iwamasa T, Hirayasu T. Recent striking changes in histological differentiation and rate of human papillomavirus infection in squamous cell carcinoma of the lung in Okinawa, a subtropical island in southern Japan. J Clin Pathol. 2000;53:676–684. doi: 10.1136/jcp.53.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Villiers EM, Lavergne D, Chang F, Syrjanen K, Tosi P, Cintorino M, Santopietro R, Syrjanen S. An interlaboratory study to determine the presence of human papillomavirus DNA in esophageal carcinoma from China. Int J Cancer. 1999;81:225–228. doi: 10.1002/(sici)1097-0215(19990412)81:2<225::aid-ijc10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Cao B, Tian X, Li Y, Jiang P, Ning T, Xing H, Zhao Y, Zhang C, Shi X, Chen D, Shen Y, Ke Y. LMP7/TAP2 gene polymorphisms and HPV infection in esophageal carcinoma patients from a high incidence area in China. Carcinogenesis. 2005;26:1280–1284. doi: 10.1093/carcin/bgi071. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929–934. doi: 10.1093/carcin/22.6.929. [DOI] [PubMed] [Google Scholar]
- 20.Ding GC, Ren JL, Chang FB, Li JL, Yuan L, Song X, Zhou SL, Guo T, Fan ZM, Zeng Y, Wang LD. Human papillomavirus DNA and P16 (INK4A) expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol. 2010;16:5901–5906. doi: 10.3748/wjg.v16.i46.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao GF, Roth MJ, Wei WQ, Abnet CC, Chen F, Lu N, Zhao FH, Li XQ, Wang GQ, Taylor PR, Pan QJ, Chen W, Dawsey SM, Qiao YL. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. Int J Cancer. 2006;119:1354–1359. doi: 10.1002/ijc.21980. [DOI] [PubMed] [Google Scholar]
- 22.Peixoto GD, Hsin LS, Snijders P, Wilmotte R, Herrero R, Lenoir G, Montesano R, Meijer CJ, Walboomers J, Hainaut P. Absence of association between HPV DNA, TP53 codon 72 polymorphism, and risk of esophageal cancer in a high-risk area of China. Cancer Lett. 2001;162:231–235. doi: 10.1016/s0304-3835(00)00643-1. [DOI] [PubMed] [Google Scholar]
- 23.Lam KY, He D, Ma L, Zhang D, Ngan HY, Wan TS, Tsao SW. Presence of human papillomavirus in esophageal squamous cell carcinomas of Hong Kong Chinese and its relationship with p53 gene mutation. Hum Pathol. 1997;28:657–663. doi: 10.1016/s0046-8177(97)90174-x. [DOI] [PubMed] [Google Scholar]
- 24.He D, Zhang DK, Lam KY, Ma L, Ngan HY, Liu SS, Tsao SW. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 gene mutation. Int J Cancer. 1997;72:959–964. doi: 10.1002/(sici)1097-0215(19970917)72:6<959::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Hou R, Xu C, Zhang S, Wu M, Zhang W. Distribution of human papillomavirus genotype and cervical neoplasia among women with abnormal cytology in Beijing, China. Int J Gynaecol Obstet. 2012;119:257–261. doi: 10.1016/j.ijgo.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Dijkstra MG, Heideman DA, de Roy SC, Rozendaal L, Berkhof J, van Krimpen K, van Groningen K, Snijders PJ, Meijer CJ, van Kemenade FJ. p16 (INK4a) immunostaining as an alternative to histology review for reliable grading of cervical intraepithelial lesions. J Clin Pathol. 2010;63:972–977. doi: 10.1136/jcp.2010.078634. [DOI] [PubMed] [Google Scholar]
- 27.de Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur HH. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Millikan RC. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1994;86:392–393. doi: 10.1093/jnci/86.5.392. [DOI] [PubMed] [Google Scholar]
- 29.Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, Scott DR, Sherman ME, Kurman RJ, Wacholder S, Et A. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 30.Tase T, Okagaki T, Clark BA, Twiggs LB, Ostrow RS, Faras AJ. Human papillomavirus DNA in adenocarcinoma in situ, microinvasive adenocarcinoma of the uterine cervix, and coexisting cervical squamous intraepithelial neoplasia. Int J Gynecol Pathol. 1989;8:8–17. doi: 10.1097/00004347-198903000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Paavonen J. Baseline demographic characteristics of subjects enrolled in international quadrivalent HPV (types 6/11/16/18) vaccine clinical trials. Curr Med Res Opin. 2008;24:1623–1634. doi: 10.1185/03007990802068151. [DOI] [PubMed] [Google Scholar]
- 32.Jin F, Devesa SS, Chow WH, Zheng W, Ji BT, Fraumeni JJ, Gao YT. Cancer incidence trends in urban shanghai, 1972-1994: an update. Int J Cancer. 1999;83:435–440. doi: 10.1002/(sici)1097-0215(19991112)83:4<435::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Jin Y, Chen X, Jin C, Law S, Tsao SW, Kwong YL. Cytogenetic aberrations in immortalization of esophageal epithelial cells. Cancer Genet Cytogenet. 2006;165:25–35. doi: 10.1016/j.cancergencyto.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayed K, Korourian S, Ellison DA, Kozlowski K, Talley L, Horn HV, Simpson P, Parham DM. Diagnosing cervical biopsies in adolescents: the use of p16 immunohistochemistry to improve reliability and reproducibility. J Low Genit Tract Dis. 2007;11:141–146. doi: 10.1097/01.lgt.0000265777.36797.e7. [DOI] [PubMed] [Google Scholar]
- 37.Carico E, Fulciniti F, Giovagnoli MR, Losito NS, Botti G, Benincasa G, Farnetano MG, Vecchione A. Adhesion molecules and p16 expression in endocervical adenocarcinoma. Virchows Arch. 2009;455:245–251. doi: 10.1007/s00428-009-0811-1. [DOI] [PubMed] [Google Scholar]
- 38.Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW, Hsu JD, Ruan A, Chao KC, Han CP. Scoring mechanisms of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med. 2009;7:25. doi: 10.1186/1479-5876-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakami H, Okamoto I, Terao K, Sakai K, Suzuki M, Ueda S, Tanaka K, Kuwata K, Morita Y, Ono K, Nishio K, Nishimura Y, Doi K, Nakagawa K. Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer Med. 2013;2:933–941. doi: 10.1002/cam4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salam I, Hussain S, Mir MM, Dar NA, Abdullah S, Siddiqi MA, Lone RA, Zargar SA, Sharma S, Hedau S, Basir SF, Bharti AC, Das BC. Aberrant promoter methylation and reduced expression of p16 gene in esophageal squamous cell carcinoma from Kashmir valley: a high-risk area. Mol Cell Biochem. 2009;332:51–58. doi: 10.1007/s11010-009-0173-7. [DOI] [PubMed] [Google Scholar]
- 41.Riethdorf S, Neffen EF, Cviko A, Loning T, Crum CP, Riethdorf L. p16INK4A expression as biomarker for HPV 16-related vulvar neoplasias. Hum Pathol. 2004;35:1477–1483. doi: 10.1016/j.humpath.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Lerma E, Esteller M, Herman JG, Prat J. Alterations of the p16/Rb/cyclin-D1 pathway in vulvar carcinoma, vulvar intraepithelial neoplasia, and lichen sclerosus. Hum Pathol. 2002;33:1120–1125. doi: 10.1053/hupa.2002.129415. [DOI] [PubMed] [Google Scholar]
- 43.Milde-Langosch K, Hagen M, Bamberger AM, Loning T. Expression and prognostic value of the cell-cycle regulatory proteins, Rb, p16MTS1, p21WAF1, p27KIP1, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol. 2003;22:168–174. doi: 10.1097/00004347-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Murphy N, Ring M, Killalea AG, Uhlmann V, O’Donovan M, Mulcahy F, Turner M, McGuinness E, Griffin M, Martin C, Sheils O, O’Leary JJ. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56–63. doi: 10.1136/jcp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannweiler S, Sygulla S, Winter E, Regauer S. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol. 2013;69:73–81. doi: 10.1016/j.jaad.2012.12.973. [DOI] [PubMed] [Google Scholar]
- 46.Perez C, Castillo M, Alemany L, Tous S, Klaustermeier J, de Sanjose S, Velasco J. Evaluation of p16 (INK4a) overexpression in a large series of cervical carcinomas: concordance with SPF10-LiPA25 PCR. Int J Gynecol Pathol. 2014;33:74–82. doi: 10.1097/PGP.0b013e3182774546. [DOI] [PubMed] [Google Scholar]


