Abstract
Histopathological subtyping of nonsmall cell lung cancer (NSCLC) is currently important in selecting specific therapeutic agents. It can be challenging in distinguishing poorly differentiated lung adenocarcinoma (AC) from squamous cell carcinoma (SCC) on small biopsy samples. This study was aimed to evaluate the utility of a panel of immunohistochemical markers consisting of ΔNp63 (p40), cytokeratins (CK) 5/6, thyroid transcription factor-1 (TTF-1) and napsin A (novel aspartic proteinase of the pepsin family) in subtyping poorly differentiated NSCLC. Forty-eight cases of NSCLC that could not be further classified by examination of hematoxylin-eosin (H&E)-stained slides on biopsy and had subsequent resection specimens were selected. Subtyping of the tumor was based on the resection specimen using the World Health Organization criteria. ΔNp63 was expressed in all 16 SCCs (100%), and was negative in all ACs and LCCs. CK5/6 was positive in 13 of 16 SCCs (81%), and was negative in all ACs and LCCs. TTF-1 was positive in 20 of 25 ACs (80%) and 3 of 7 LCCs (43%), but none of 16 SCCs. Napsin A was positive in 16 of 25 ACs (64%) and was negative in all SCCs and LCCs. Our study shows that a panel including ΔNp63, CK5/6, TTF-1, and napsin A allows correct subclassification of 39 of 48 cases of NSCLC on biopsy and may contribute to refine lung cancer classification in biopsy specimens, remarkably reducing the NSCLC-NOS (not otherwise specified) diagnostic category.
Keywords: Non-small cell lung carcinoma, adenocarcinoma, squamous cell carcinoma, immunohistochemistry, biopsy
Introduction
To date, lung cancer is the most common cause of cancer-related mortality in the world. Lung cancer is usually divided into small cell lung cancer and non-small cell lung cancer (NSCLC). The main histologic types of non-small cell lung carcinoma are squamous cell carcinoma (SCC), adenocarcinoma (AC), and large cell carcinoma (LCC) [1-3]. Historically, all subtypes of NSCLC received the same treatment. The histologic subclassification of non-small cell lung carcinoma was not clinically or therapeutically important, because of a lack of differential treatment options for NSCLC subtypes.
In contrast with past years, the availability of targeted therapies has created a need for precise subtyping of non-small cell lung carcinomas. For instance, gefitinib, the epidermal growth factor receptor inhibitor, is more likely to be effective in ACs than in SCCs [4]. Bevacizumab, the antivascular endothelial growth factor agent, is associated with uncommon but potentially fatal pulmonary hemorrhage in SCCs, and is therefore contraindicated in SCCs [5,6].
Although the majority of NSCLCs can be subtyped by examination of hematoxylin-eosin (H&E)-stained slides alone, Morphologic separation of poorly differentiated tumors can be difficult, especially in small biopsy specimens [7].
Therefore, there is an increasing need for additional diagnostic techniques such as immunohistochemistry (IHC) [8]. Several recent studies have demonstrated that different panels of IHC markers may be useful to distinguish adenocarcinoma (AC) from squamous cell carcinoma (SCC), especially on biopsy samples [9,10]. Our study focused on biopsy samples because most lung cancers are unresectable at diagnosis, the only available tissue sample in many cases being a biopsy. In addition, we included only poorly differentiated tumors because immunohistochemistry is most important in these cases. Our aim was to evaluate the role of IHC on poorly differentiated biopsy specimens to subtype NSCLCs, relying on the corresponding surgical specimens as the gold standard for diagnosis.
Materials and methods
Case selection
From the databases of the Pathology Divisions at the first people’s hospital of Changzhou, China, 245 cases of poorly differentiated NSCLCs diagnosed on small lung biopsies (endobronchial biopsies, transbronchial biopsies, and computed tomography-guided core biopsies) recorded over a 15-year period (January 1999 through December 2013) were collected retrospectively. In 63 of these cases, the tumor was subsequently resected at our hospital. Criteria for entering the investigation included: (1) review of the biopsy specimen confirmed a diagnosis of NSCLC; (2) the tumor in the biopsy showed no glandular or squamous differentiating features and could not be subtyped on the basis of H&E morphology. Upon revision of the original H&E morphology, 15 cases were excluded after they were reclassified as AC or SCC (13 cases), sarcomatoid carcinoma (2 cases). The 48 remaining cases were included in this study. The study comprised 31 endobronchial biopsies, 15 computed tomographyguided core biopsies and two transbronchial biopsies. The resected tumors were reviewed and subtyped on the basis of H&E morphology according to the 2013 World Health Organization (WHO) criteria by 2 experienced pathologists in lung cancer blinded to the immunohistochemical findings. Tumors were classified as AC if they showed gland formation, SCC if they contained intercellular bridges or showed keratinization, and large cell carcinoma (LCC) if they lacked glandular or squamous differentiation.
Immunohistochemistry
Immunohistochemical analysis was performed on 5-μm thick, formalin-fixed, paraffin-embedded tissue sections of each case and all slides were stained on a BECCHMARK Autostainer with the EnVision detection system. The antibodies used were ΔNp63 (p40, 1:200, Calbiochem), p63 (4A4, 1:200, Dako), CK5/6 (D5/16B4, 1:50, Dako), 34βE12 (34βE12, 1:100, Dako), TTF1 (8G7G3/1, 1:100, Dako), napsin A (MRQ-60, 1:200, Dako), CK7 (OV-TL12/30, 1:100, Dako), and CK8/18 (5D3, 1:200, Novocastra). Appropriate positive and negative controls were included with the study sections. For each stain, the percentage of positive cells was recorded. The presence of more than 10% expression of the marker within tumor cells was considered positive. Cases with less than 10% overall staining were considered negative.
Results
There were 16 SCCs s, 25 ACs, and 7LCCs in the resected samples.
Immunohistochemistry in biopsy specimens
The results of immunohistochemical expression on biopsy specimens for each histologic subtype of carcinoma are summarized in Table 1. ΔNp63 was expressed in all 16 SCCs (100%), and was negative in all ACs and LCCs. A strong and well-defined nuclear staining was considered a positive reaction. In SCCs, ΔNp63 was diffusely positive (50%-90% of tumor cells) in all cases (Figure 1). P63 staining was seen in all 16 SCCs (100%) and also seen in 4 of 25 ACs (16%). All 7 LCCs were negative for p63. In SCCs, p63 was diffusely positive (40%-90% of tumor cells) in all cases. Three cases of p63-positive AC were all positive for TTF-1 and napsin A. In these cases 20%-30% of tumor cells stained with p63 and 50%-90% of tumor cells stained with TTF-1. CK5/6 was positive in 13 of 16 SCCs (81%) (Figure 1), and was negative in all ACs and LCCs. All 3 cases that were negative for CK5/6 but positive for ΔNp63 lacked TTF-1 and napsin A expression. 34βE12 was positive in 15 of 16 SCCs (94%), in 15 of 25 ACs (60%), and in 2 of 7 LCCS (29%). TTF-1 was positive in 20 of 25 ACs (80%) (Figure 2) and 3 of 7 LCCs (43%) but none of 16 SCCs. Napsin A was positive in 16 of 25 ACs (64%) (Figure 2) and was negative in all SCCs and LCCs. All napsin A-positive cases were also positive for TTF-1. CK7 was positive in all 25 ACs, in 10 of 16 SCCs (63%), and in 5 of 7 LCCs (71%). CK8/18 was positive in all 25 ACs, in 11 of 16 SCCs (69%), and in 4 of 7 LCCs (57%).
Table 1.
Immunohistochemical reactions in lung biopsies of poorly-differentiated non-small cell carcinomas (positive/total stained)
| Tumor subtypes on resected specimens (No. of case) | |||
|---|---|---|---|
|
|
|||
| Marker | SCC (16) | AC (25) | LCC (7) |
| ΔNp63 | 16/16 | 0/25 | 0/7 |
| p63 | 16/16 | 4/25 | 0/7 |
| CK5/6 | 13/16 | 0/25 | 0/7 |
| 34βE12 | 15/16 | 15/25 | 2/7 |
| TTF1 | 0/16 | 20/25 | 3/7 |
| Napsin A | 0/16 | 16/25 | 0/7 |
| CK7 | 10/16 | 25/25 | 5/7 |
| CK8/18 | 11/16 | 25/25 | 4/7 |
| Biopsy diagnosis | SCC (16/16) | AC (20/25) | AC (3/7) |
| After IHC (No. of case) | NSCLC, NOS (5/25) | NSCLC, NOS (4/7) | |
Figure 1.

Immunoreactivity for ΔNp63 and CK5/6 in squamous cell carcinoma. A: Poorly differentiated non-small cell carcinoma in an endobronchial biopsy (HE, × 200). B: The tumor is positive for ΔNp63 (× 200). C: CK5/6 is also positive (× 200). D: The corresponding resected specimen showed focal keratinization in the tumor, confirming the diagnosis of squamous cell carcinoma (HE, × 200).
Figure 2.
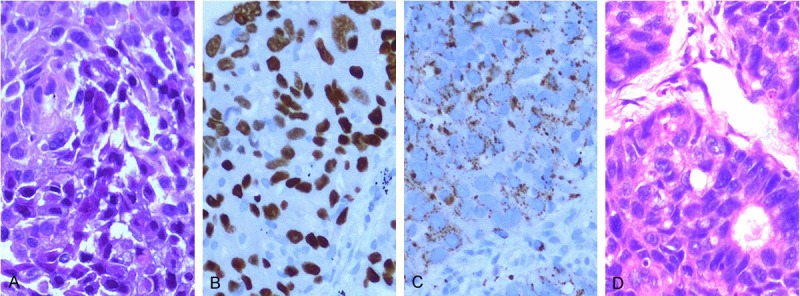
Immunoreactivity for TTF1 and napsin A in adenocarcinoma. A: Endobronchial biopsy shows poorly differentiated non-small cell carcinoma in (HE, × 200). B: The tumor is positive for TTF1 (× 200). C: Napsin A is also positive (× 200). D: The corresponding resected specimen showed glands in the tumor, confirming the diagnosis of adenocarcinoma (HE, × 200).
The sensitivity, specificity, positive predictive value, and negative predictive value of the markers for AC and SCC are presented in Table 2. For SCC, ΔNp63 is the only most sensitive and specific marker, and best negative predictor (NPV) (sensitivity: 100%, specificity: 100%, NPV: 100%). P63 is also the most sensitive marker but is not entirely specific, because it is also expressed occasionally in ACs (sensitivity: 100%, specificity: 88%). In contrast to p63, CK5/6 is entirely specific for SCC and the best positive predictor (specificity: 100%, PPV: 100%). 34βE12 is sensitive for SCC but is too nonspecific. For AC, both TTF1 and Napsin A are specific markers but Napsin A was less sensitive than TTF-1 (64% vs. 80%). CK7 and CK8/18 are the most sensitive markers for AC but is not specific.
Table 2.
Sensitivity, specificity, PPV and NPV of markers used in this study [% (positive/total stained)]
| Marker | Subtype | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| ΔNp63 | SCC | 100 (16/16) | 100 (32/32) | 100 (16/16) | 100 (32/32) |
| p63 | SCC | 100 (16/16) | 88 (28/32) | 80 (16/20) | 100 (28/28) |
| CK5/6 | SCC | 81 (13/16) | 100 (32/32) | 100 (13/13) | 91 (32/35) |
| 34βE12 | SCC | 94 (15/16) | 47 (15/32) | 47 (15/32) | 94 (15/16) |
| TTF1 | AC | 80 (20/25) | 87 (20/23) | 87 (20/23) | 80 (20/25) |
| Napsin A | AC | 64 (16/25) | 100 (23/23) | 100 (16/16) | 72 (23/32) |
| CK7 | AC | 100 (25/25) | 35 (8/23) | 63 (25/40) | 100 (8/8) |
| CK8/18 | AC | 100 (25/25) | 35 (8/23) | 63 (25/40) | 100 (8/8) |
Sensitivity = TP/TP+FN; Specificity = TN/TN+FP; Positive predictive value (PPV) = TP/TP+FP; Negative predictive value (NPV) = TN/TN+FN. FN indicates false negatives; FP, false positives; TN, true negatives; TP, true positives.
Algorithm for interpreting immunohistochemistry in biopsy specimens
On the basis of above results, an algorithm for interpreting immunohistochemistry in poorly differentiated NSCLC on biopsy specimens was devised and outlined in Table 3. Because ΔNp63 provides a higher specificity than p63 and equal sensitivity for the diagnosis of squamous cell carcinoma of the lung, we include ΔNp63 to substitute with p63 in our panel. On account of the low specificity of CK7 and CK8/18 for AC and 34βE12 for SCC respectively, these markers were excluded from our panel. So the panel of choice is composed of p40, CK5/6, TTF1, and Napsin A. Positivity for the combination of p40 and CK5/6 indicated SCC. Tumors positive for ΔNp63 but negative for CK5/6 were diagnosed as SCC also. Positivity for the combination of TTF1 and Napsin A indicated AC. Tumors positive for TTF1 but negative for Napsin A were diagnosed as AC also. According to above criteria, we accurately subtype 39 of 48 (81%) poorly differentiated NSCLCs on biopsy specimens, including 16 SCCs and 25 ACs. Nine tumors could not be further classified on biopsies by this immunohistochemical panel.
Table 3.
Algorithm for subtyping of poorly-differentiated non-small cell lung carcinomas according to immunohistochemical staining in lung biopsies
| ΔNp63 | CK5/6 | TTF1 | Napsin A | Diagnosis |
|---|---|---|---|---|
| + | + | - | - | Squamous cell carcinoma |
| + | - | - | - | Squamous cell carcinoma |
| - | - | + | + | Adenocarcinoma |
| - | - | + | - | Adenocarcinoma |
| - | - | - | - | Poorly-differentiated |
| non-small cell carcinoma |
Discussion
For many years, lung cancer was subdivided into small cell carcinoma and non-small cell carcinoma (NSCLC). Until recently, the differentiation of AC and SCC from non-small cell carcinoma had an academic and not a therapeutic implication, because all variants of NSCLC were treated with similar chemotherapy regimens with or without combined radiation therapy. Because of recent advances in targeted therapies, the subclassification of the NSCLC category to AC and SCC is becoming increasingly critical. Notably, gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, is currently recommended as the first-line treatment for adenocarcinoma with EGFR mutations [4]. Bevacizumab is contraindicated in squamous cell carcinoma because of potentially life-threatening side effects [11].
The WHO has published guidelines for the classification of lung cancer in resection specimens based primarily on H&E morphology of the entire tumor. However, most of lung cancers present at advanced stages and are unresectable. In these situations, differentiating between AC and SCC subtypes of NSCLC on biopsies is important in predicting response to targeted therapies. In fact, most NSCLCs can be easily subtyped as AC or SCC on biopsy specimens without using immunohistochemistry. However, difficulty in classification may be encountered due to the presence of only a small amount of tumor that may not show features of differentiation. Our study shows that the use of a panel of immunohistochemical stains, including ΔNp63, CK5/6, TTF-1, and napsin A in such specimens allows correct subclassification in more than 80% of cases.
The human p63 gene is located on chromosome 3q27-29 and comprises 15 exons. It contains 2 promoters that generate 2 classes of proteins: the full-length protein TAp63, which contains the N-terminal transactivation domain, and the N-terminal-truncated protein isoform ΔNp63, which lacks this transactivation domain [12].
In previous reports, the sensitivity of p63 ranges from 75% to more than 95% whereas specificity is between 70% and 90% [13-15]. In our study, p63, although highly sensitive for SCC, was not entirely specific, because it was also expressed occasionally in ACs. Problems may arise in biopsy samples taken from p63-positive areas with solid growth pattern of poorly differentiated AC, which is often referred to as SCC. ΔNp63 expression in lung carcinomas has been scarcely reported [16,17]. Nonaka [16] reported that ΔNp63 was not positive in any adenocarcinomas, but p63 expression was observed in 15% of adenocarcinomas. Righi [17] reported that ΔNp63 was expressed in all 15 SCCs, whereas it was negative in 28 of 29 adenocarcinomas. Our results are similar to those of the above-mentioned study. Given that ΔNp63 is completely negative in adenocarcinomas, it appears to provide a high specificity and sensitivity for the diagnosis of squamous cell carcinoma of the lung superior to p63.
In our hands, CK5/6 is typically a specific marker for SCC. The high specificity of CK5/6 for SCC in our study (100%) is similar to earlier studies [18-20]. Despite the high specificity and sensitivity of ΔNp63 for SCC, we recommend inclusion of CK5/6 in our immunohistochemical panel because positive staining may be helpful when ΔNp63 staining is equivocal, especially because it is a cytoplasmic rather than nuclear stain. The high molecular weight keratin marker, 34βE12, is also a highly sensitive marker for SCC, but is too nonspecific to be diagnostically useful. Similar findings have been reported by other investigators, suggesting that 34βE12 should not be used to diagnose SCC [21,22].
TTF1 is a highly specific, but not very sensitive marker for ACs, The high specificity of TTF-1 for ACs has been noted by earlier studies [23-25]. Napsin A is a new marker for diagnose pulmonary ACs. In our study, napsin A was more specific but slightly less sensitive than TTF-1 for AC. Because napsin A provided excellent specificity for predicting an adenocarcinoma histotype [26-28], we recommend that napsin A could be added in the immunohistochemical panel because positive staining may be highly useful for separating primary lung ACs from SCCs, especially other investigators have reported rare cases of TTF-1-negative but napsin A-positive pulmonary AC [25,26].
Three biopsy samples in our study were positive for TTF-1 but negative for napsin A, and the resected tumors fulfilled the WHO morphologic criteria for LCC. Recent studies have demonstrated that many LCCs show features of AC and because TTF-1 is highly specific for AC, we believe that such tumors should be classified as AC [26,29].
Two of the markers we evaluated for ACs, CK7 and CK8/18, were highly sensitive but too nonspecific to be diagnostically helpful. Our findings were similar to those reported by other investigators, suggesting that CK7 and CK8/18 should not be used to differentiate between AC and SCC [30,31].
Lung carcinomas are a heterogenous group of tumors and this heterogeneity is particularly prominent in the adenocarcinoma category. Heterogeneity confounds accurate diagnosis, as sampling of a poorly differentiated area cannot, by definition, differentiate ADC or SCC. In the cases in which morphologic subtype is reported as NSCLC NOS, panels of immunohistochemical markers to differentiate AC from SCC are especially useful. Loo et al [20] reported that they were able to subtype 30 of 42 (71%) poorly differentiated NSCLCs as AC or SCC. They used a slightly different panel than ours, including TTF-1, p63, CK5/6, and Alcian blue-periodic acid Schiff. Nicholson et al [21] reported that a combination of PAS with diastase, p63, TTF1, and CK5/6 is able to classify most small biopsies of NSCLC into SCC or ADC when the subtype is not definitive by morphology alone. Our findings in this study are similar and support these earlier reports. In our study, poorly differentiated NSCLCs can be accurately subtyped on small lung biopsies in more than 80% of cases (81%, 39 of 48 cases) using a panel composed of TTF-1, Napsin A, ΔNp63, and CK5/6.
In conclusion, on the basis of the results of our study and a comprehensive review of the literature, we suggest that immunohistochemical staining should be carried out in all cases in which no glandular morphologic features or squamous morphological features are identified by examination of hematoxylin-eosin (H&E)-stained slides. The immunohistochemical profile we recommend is composed of TTF-1, Napsin A, ΔNp63, and CK5/6.
Disclosure of conflict of interest
None.
References
- 1.Burnett RA, Swanson Beck J, Howatson SR, Lee FD, Lessells AM, McLaren KM, Ogston S, Robertson AJ, Simpson JG, Smith GD, et al. Observer variability in histopathological reporting of malignant bronchial biopsy specimens. J Clin Pathol. 1994;47:711–713. doi: 10.1136/jcp.47.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera MP, Detterbeck F, Mehta AC. Diagnosis of lung cancer: the guidelines. Chest. 2003;123:129S–136S. doi: 10.1378/chest.123.1_suppl.129s. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123:115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Spreafico A, Novello S, Wood MD, Simms L, Papotti M. The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol. 2008;3:1468–1481. doi: 10.1097/JTO.0b013e318189f551. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J. Clin. Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grilley-Olson JE, Hayes DN, Moore DT, Leslie KO, Wilkerson MD, Qaqish BF, Hayward MC, Cabanski CR, Yin X, Socinski MA, Stinchcombe TE, Thorne LB, Allen TC, Banks PM, Beasley MB, Borczuk AC, Cagle PT, Christensen R, Colby TV, Deblois GG, Elmberger G, Graziano P, Hart CF, Jones KD, Maia DM, Miller CR, Nance KV, Travis WD, Funkhouser WK. Validation of interobserver agreement in lung cancer assessment: hematoxylin-eosin diagnostic reproducibility for non-small cell lung cancer: the 2004 World Health Organization classification and therapeutically relevant subsets. Arch Pathol Lab Med. 2013;137:32–40. doi: 10.5858/arpa.2012-0033-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosai J. Why microscopy will remain a cornerstone of surgical pathology. Lab Invest. 2007;87:403–408. doi: 10.1038/labinvest.3700551. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho L. Reclassifying bronchial-pulmonary carcinoma: differentiating histological type in biopsies by immunohistochemistry. Rev Port Pneumol. 2009;15:1101–1119. [PubMed] [Google Scholar]
- 10.Edwards SL, Roberts C, McKean ME, Cockburn JS, Jeffrey RR, Kerr KM. Preoperative histological classification of primary lung cancer: accuracy of diagnosis and use of the non-small cell category. J Clin Pathol. 2000;53:537–540. doi: 10.1136/jcp.53.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Candi E, Cipollone R, Rivetti di Val Cervo P, Gonfloni S, Melino G, Knight R. p63 in epithelial development. Cell Mol Life Sci. 2008;65:3126–3133. doi: 10.1007/s00018-008-8119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au NH, Gown AM, Cheang M, Huntsman D, Yorida E, Elliott WM, Flint J, English J, Gilks CB, Grimes HL. P63 expression in lung carcinoma: a tissue microarray study of 408 cases. Appl Immunohistochem Mol Morphol. 2004;12:240–247. doi: 10.1097/00129039-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Jorda M, Gomez-Fernandez C, Garcia M, Mousavi F, Walker G, Mejias A, Fernandez-Castro G, Ganjei-Azar P. P63 differentiates subtypes of nonsmall cell carcinomas of lung in cytologic samples: implications in treatment selection. Cancer. 2009;117:46–50. doi: 10.1002/cncy.20015. [DOI] [PubMed] [Google Scholar]
- 15.Khayyata S, Yun S, Pasha T, Jian B, McGrath C, Yu G, Gupta P, Baloch Z. Value of P63 and CK5/6 in distinguishing squamous cell carcinoma from adenocarcinoma in lung fine-needle aspiration specimens. Diagn Cytopathol. 2009;37:178–183. doi: 10.1002/dc.20975. [DOI] [PubMed] [Google Scholar]
- 16.Nonaka D. A study of DeltaNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol. 2012;36:895–899. doi: 10.1097/PAS.0b013e3182498f2b. [DOI] [PubMed] [Google Scholar]
- 17.Righi L, Graziano P, Fornari A, Rossi G, Barbareschi M, Cavazza A, Pelosi G, Scagliotti GV, Papotti M. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology: a retrospective study of 103 cases with surgical correlation. Cancer. 2011;117:3416–3423. doi: 10.1002/cncr.25830. [DOI] [PubMed] [Google Scholar]
- 18.Camilo R, Capelozzi VL, Siqueira SA, Del Carlo Bernardi F. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol. 2006;37:542–546. doi: 10.1016/j.humpath.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Downey P, Cummins R, Moran M, Gulmann C. If it’s not CK5/6 positive, TTF-1 negative it’s not a squamous cell carcinoma of lung. APMIS. 2008;116:526–529. doi: 10.1111/j.1600-0463.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 20.Loo PS, Thomas SC, Nicolson MC, Fyfe MN, Kerr KM. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol. 2010;5:442–447. doi: 10.1097/JTO.0b013e3181d40fac. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson AG, Gonzalez D, Shah P, Pynegar MJ, Deshmukh M, Rice A, Popat S. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010;5:436–441. doi: 10.1097/JTO.0b013e3181c6ed9b. [DOI] [PubMed] [Google Scholar]
- 22.Viberti L, Bongiovanni M, Croce S, Bussolati G. 34betaE12 Cytokeratin Immunodetection in the Differential Diagnosis of Small Cell Tumors of Lung. Int J Surg Pathol. 2000;8:317–322. doi: 10.1177/106689690000800410. [DOI] [PubMed] [Google Scholar]
- 23.Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15:415–420. doi: 10.1097/PAI.0b013e31802fab75. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura N, Miyagi E, Murata S, Kawaoi A, Katoh R. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol. 2002;15:1058–1067. doi: 10.1097/01.MP.0000028572.44247.CF. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654–661. doi: 10.1038/modpathol.2010.38. [DOI] [PubMed] [Google Scholar]
- 26.Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41:20–25. doi: 10.1016/j.humpath.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, Shijubo N, Yamada G, Ichimiya S, Satoh M, Abe S, Sato N. Napsin A is useful to distinguish primary lung adenocarcinoma from adenocarcinomas of other organs. Pathol Res Pract. 2005;201:579–586. doi: 10.1016/j.prp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Hirano T, Gong Y, Yoshida K, Kato Y, Yashima K, Maeda M, Nakagawa A, Fujioka K, Ohira T, Ikeda N, Ebihara Y, Auer G, Kato H. Usefulness of TA02 (napsin A) to distinguish primary lung adenocarcinoma from metastatic lung adenocarcinoma. Lung Cancer. 2003;41:155–162. doi: 10.1016/s0169-5002(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 29.Pardo J, Martinez-Penuela AM, Sola JJ, Panizo A, Gurpide A, Martinez-Penuela JM, Lozano MD. Large cell carcinoma of the lung: an endangered species? Appl Immunohistochem Mol Morphol. 2009;17:383–392. doi: 10.1097/PAI.0b013e31819bfd59. [DOI] [PubMed] [Google Scholar]
- 30.Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN, Oien KA. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 31.Johansson L. Histopathologic classification of lung cancer: Relevance of cytokeratin and TTF-1 immunophenotyping. Ann Diagn Pathol. 2004;8:259–267. doi: 10.1016/j.anndiagpath.2004.07.001. [DOI] [PubMed] [Google Scholar]


