Abstract
The aim of this study was to explore the relationship between the expression of p53, p21 and Cdc2 in the early laryngeal cancer with negative pathological margins and its local recurrence. During 2004-2010, a total of 85 patients with early laryngeal cancer were selected in Tangshan Union Hospital, Hebei, China, and immunohistochemical method was used to detect the expression of p53, p21 and Cdc2 in the negative pathological margin tissues. All patients were followed up for two years to collect pathological data for evaluating the survival and tumor recurrence. Two years after surgery 14 of 85 patients with laryngeal cancer presented with recurrence (recurrent group), while 71 patients without recurrence (none recurrent group). The positive rate of p53, p21 and Cdc2 protein in laryngeal cancer tissues was 60.0% (51/85), 38.8% (33/85) and 70.6% (60/85), respectively, while that of the three proteins in the cancer adjacent tissues was 36.5% (31/85), 21.2% (18/85) and 29.4% (25/85), respectively. The differentiation and TNM stage of tumor had no correlation with the three proteins. The positive rate of p53 in the surgical margin of the recurrent group and non recurrent group was 71.4% (10/14) and 29.6% (21/71) (P = 0.003), that of p21 was 50.0% (7/14) and 15.5% (11/71), (P = 0.011) and Cdc2 was 57.1% (8/14) and 23.9% (17/71) (P = 0.030), respectively. In conclusion, p53, p21 and Cdc2 may be involved in the occurrence, development and recurrence of laryngeal squamous cell carcinoma. Overexpression of p53, p21 and Cdc2 in the surgical margin of early laryngeal cancer is closely related to local recurrence of tumor.
Keywords: Laryngeal squamous cell carcinoma, p53, p21, Cdc2, immunohistochemistry, local recurrence
Introduction
Laryngeal squamous cell carcinoma is the most common histological type of head and neck cancer, its occurrence and development is a multi-factor, multi-stage process. Of all the factors that affecting the progressing of laryngeal cancer, the cell cycle proteins may play a more important role in the development of the tumor. The cell cycle is the cascade events that promote a growing cell to duplicate all its components to split into two daughter cells. In all the regulators that control the progression of cell mitosis, the cyclins and cyclin-dependent kinases (CDKs) are the two critical classes of molecules. These proteins form a heterodimer in which cyclins are the regulatory subunits and CDKs are the catalytic subunits; when the complex is activated from external signals, CDKs activate or inactivate downstream target proteins to orchestrate coordinated entry into the next phase of the cell cycle. CDK is divided into two categories which are activated by cyclin CDK binding protein or by CDK inhibitors CKIs. CKI inhibitors are divided into two families: one is INK4 family, including p15INK4B, p16INK4A, p18INK4C and p19INK4D, which can bind CDK4 and CDK6 specifically; the other is the kinase inhibitor protein (KIP) or CDK interacting proteins family (CIP), including p21, p27Kip1 and p57Kip2, all these CKIs can binds to and inhibits cyclin proteins binding [1,2]. Cyclin-dependent kinase 1 (CDK1) is a member of the Ser/Thr protein kinase family coded by cell division cycle (cdc) gene 2 whose relative molecular weight is 34KD. In the late stage of G2, Cdc2 and cyclin B combined into Cdc2-cyclinB kinase complex, which is called mitosis promoting factor or maturation promoting factor (MPF), can prompt cells from G2 phase into M phase [3]. Less report was found about the alterations of p53, p21 and Cdc2 in laryngeal carcinoma currently. We thus used immunohistochemical method to detect the expression of p53, p21 and Cdc2 proteins in 85 cases of laryngeal cancer and adjacent tissues to analyze its relationship of the pathological changes and prognosis of tumor.
Material and methods
Cases description
The study involved 85 patients who underwent CO2 laser surgery at Tangshan Union Hospital, China, from 2004 to 2010, Tissues 5 mm away from cancer were used as control. The patients aged from 48 to 79 years with a mean age of 52.3 ± 9.8 years, including 78 males and 7 females, and according to the AJCC Cancer Staging, they were all belonged to stage I that was subdivided into T1a and T1b, including 58 cases in stage T1a and 27 cases in stage T1b. All patients were treated without radiotherapy and chemotherapy before surgery. Pathological classification of tumor was graded as well-, moderately- and poorly-differentiated squamous cell carcinoma of the larynx. The study was approved by the Ethics Committee of Tangshan Union Hospital.
Immunohistochemical staining
We studied the correlation of the proteins to tumor recurrence by detecting the expression of p53, p21 and Cdc2. All specimens obtained from patients were fixed in 10% neutral formalin, embedded in paraffin routinely and one representative block from each patient was sectioned at 4 μm, stained with hematoxylin and eosin (HE) and evaluated by immunohistochemistry according to the protocol described in the manufacturer’s guide accompanying the kit. Known positive expression samples of p53, p21 and Cdc2 were used as positive immunostaining controls. For the negative control, the primary antibodies were replaced with phosphate-buffered saline (PBS). The mouse monoclonal antibody against human p53 (Santa Cruz, USA, 1:100) and Cdc2 were purchased from Santa Cruz Biotechnology (Santa Cruz, USA, 1:300), and p21 was obtained from Invitrogen Biotechnology (Invitrogen, USA, 1:100). SP immunohistochemistry kit was purchased from New Biotechnology Development Co, Fuzhou, China.
Evaluation of immunohistochemical analysis
Positive signals of the immunohistochemical staining were brown or brownish yellow in color. Nuclear staining was considered positive on the immunostaining for p53, and the cytoplasmic and/or nuclear immunostaining were considered positive for cdc2 and p21. Staining was observed in 10 representative high-power fields, the brown nuclear and/or cytoplasmic staining cells were observed and counted [4-7]. Positive staining in more than 10% of the cells was considered positive, photographed under × 100 magnification, while less than 10% or colorless were defined as negative. The scoring results were divided into two categories, either negative or positive. The uninterpretable results were eliminated from further consideration.
Statistical analysis
The statistical analyses were performed with PASW Statistics 20.0 (SPSS Inc., Chicago, IL, USA). The relationships between the expression of p53, p21 and Cdc2 and the various clinicopathological findings were evaluated using the Chi-square test, and the Spearman Chi-square test rank correlation between the expressions of two proteins. P values less than 0.05 were considered to be statistically significant.
Results
Clinical and follow-up data of patients with early laryngeal cancer
The clinical data of the patients and the relationships between the clinicopathologic parameters and the p53, p21 and Cdc2 expression in 85 early laryngeal cancer are summarized in Table 1. Three pathologists examined the cases independently and if there was any disagreement, we had a discussion with another pathologist to reach consensus. Pathologists were responsible for the postoperative follow-up. Among 85 laryngeal cancer patients followed up for 2 years there were 14 cases of local recurrence (recurrence group) while 71 patients without recurrence (recurrence-free group). As patients in the group of laryngeal cancer were all stage T1 without lymph node metastasis, there was no correlation of the expression of the three proteins with tumor differentiation or TNM stage (Table 1).
Table 1.
Expression of p53, p21 and Cdc2 proteins in laryngeal carcinoma tissue (No. of patients)
| Clinical Features | Total No. | p53 | P | p21 | P | Cdc2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| + | - | + | - | + | - | |||||
| Total No. | 85 | 51 | 34 | 33 | 52 | 60 | 25 | |||
| Differentiation | ||||||||||
| Well-moderately Differentiated | 63 | 35 | 28 | 0.157 | 23 | 40 | 0.459 | 42 | 21 | 0.179 |
| Poorly differentiated | 22 | 16 | 6 | 10 | 12 | 18 | 4 | |||
| TNM staging | ||||||||||
| Stage T1a | 58 | 32 | 26 | 0.183 | 20 | 38 | 0.229 | 40 | 18 | 0.630 |
| Stage T1b | 27 | 19 | 8 | 13 | 14 | 20 | 7 | |||
Correlation of the expression of p53, p21 and Cdc2 with outcomes of patients with laryngeal carcinoma
The expression rates of p53 protein in laryngeal carcinoma and its surgical margin were 60.0% (51/85) and 36.5% (31/85) respectively (Figure 1A, 1B), whereas those of p21 protein were 38.8% (33/85) and 21.2% (18/85) (Figure 1C, 1D) and Cdc2 were 70.6% (60/85) and 29.4% (25/85), respectively, (Figure 1E, 1F). The p53 expression rate was 71.4% (10/14) and 27.9% in the cases of recurrent and none recurrent groups in the surgical margins of the cancer, respectively, with statistically significant difference (P = 0.003), whereas those of p21 were 50.0% (7/14) and 15.5% (11/71), respectively, and these difference was also statistically significant (P = 0.003). Furthermore, the positive rates of Cdc2 expression were 57.1% (8/14) and 23.9% (17/71) in the cases of recurrent and none recurrent group in the surgical margins of the cancer, respectively, with statistically significant difference (P > 0.05) (Table 2). There was no correlation between the expression of p53 and p21, and between p21 and Cdc2 in the surgical margins of the cancer (P > 0.05) (Tables 3, 4).
Figure 1.
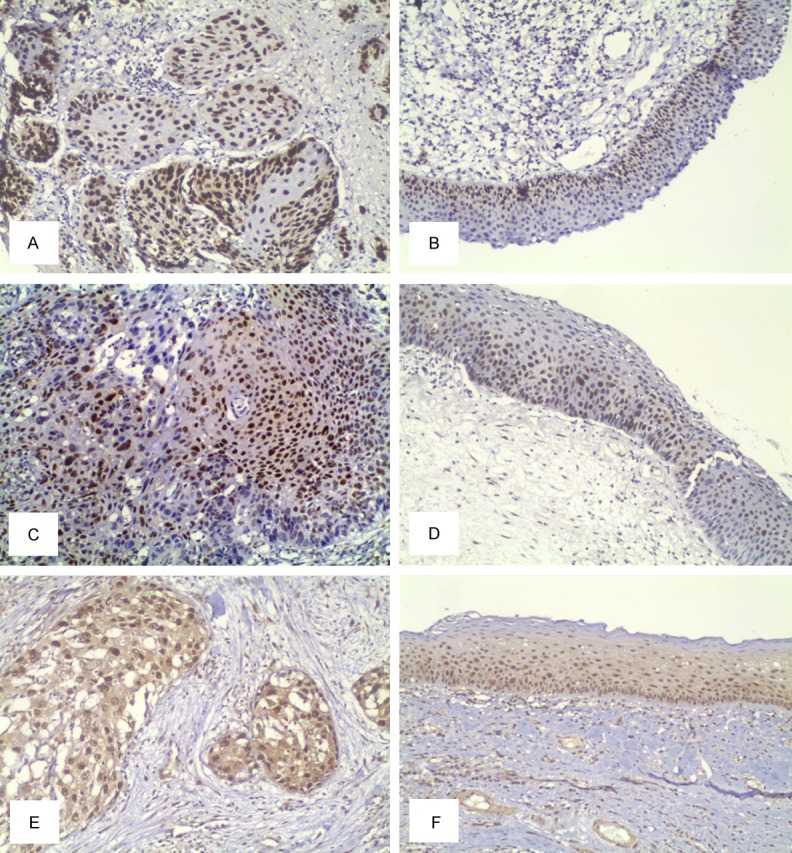
Expression of p53, p21 and Cdc2 in laryngeal squamous cell carcinoma and its surgical margins. A. Immunohistochemical detection of p53 in laryngeal carcinoma. B. Immunohistochemical detection of p53 in surgical margin of laryngeal tumor tissue. C. Immunohistochemical detection of p21 in laryngeal carcinoma tissue. D. Immunohistochemical detection of p53 in surgical margin of laryngeal tumor tissue. E. Immunohistochemical detection of Cdc2 in laryngeal carcinoma tissue. F. Expression of Cdc2 in surgical margin of laryngeal tumor tissue. SP method, original magnification × 100.
Table 2.
Relationship between the expression of p53, p21 and Cdc2 and prognosis in laryngeal tumor resection marginal tissue (No. of patients)
| Prognosis | Total No. | p53 | P | p21 | P | Cdc2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| + | - | + | - | + | - | |||||
| Recurrence | 14 | 10 | 4 | 0.003 | 7 | 7 | 0.011 | 8 | 6 | 0.030 |
| Non recurrence | 71 | 21 | 50 | 11 | 60 | 17 | 54 | |||
Table 3.
Relationship between expression of p53 and p21 in laryngeal tumor resection marginal tissue (No. of patients)
| p53 (+) | p53 (-) | P | |
|---|---|---|---|
| p21 (+) | 8 | 10 | 0.429 |
| p21 (-) | 23 | 44 |
Table 4.
Relationship between expression of p21 and Cdc2 in laryngeal tumor resection marginal tissue (No. of patients)
| p21 (+) | p21 (-) | P | |
|---|---|---|---|
| Cdc2 (+) | 7 | 18 | 0.320 |
| Cdc2 (-) | 11 | 49 |
Discussion
CO2 laser is one of the most common treatment modalities for early laryngeal cancer, especially for the patients of stages T1 and T2. The goals of treatment are preserving laryngeal voice and avoiding the pain of surgical laryngeal splitting so as to improve patients’ quality of life [4]. Additional goals include minimizing the risk of serious complications and cost of treatment. Recently the 5-year survival rate of laryngeal cancer was ranged from 40% to 80%. Recurrence after surgery is one of the main factors that affecting prognosis and whether the surgical margin had residual tumor tissue or not was significantly associated with recurrence. Local recurrence rate in patients with laryngeal negative margin of surgical pathology is rather high [5]. So molecular biology research about the laryngeal carcinoma and its surgical marginal tissue is still a hotspot nowadays.
Wild-type p53 tumor suppressor gene is one of the most common tumor suppressors yhat plays roles of suppressing tumor grows by a variety of ways and known for its “molecular policeman” by maintaining the stability of the human genome. In contrast, mutant p53 gene plays a role of proto-oncogenes who can promote the occurrence and development of tumors. Mutation of p53 gene was found in almost all human tumors. Wild-type p53 protein can form a tetramer in vivo and its half-life is only 6-20 minutes, thus it cannot be detected by immunohistochemistry. While half-life of mutant p53 protein was prolonged; and then it can be detected by immunohistochemistry [6]. It has been reported that positive rate of p53 protein in primary laryngeal squamous cell carcinoma was 40-60%, which was the most closely related oncogenes discovered by so far with the occurrence of laryngeal carcinoma and correlated significantly with the degree of malignancy of laryngeal cancer [7]. p53 protein was strongly positive in laryngeal squamous cell carcinoma tissues and was also detected in adjacent tissues of the tumor. Other study suggested that pathologists should detect p53 protein in the surgical margins routinely after surgery that was meaningful for the determination of local tumor recurrence [8]. Jalali MM et al [9] have shown that p53 expression is associated closely with laryngeal cancer recurrence; the recurrence and mortality rate of p53 positive group was significantly higher than that of the negative group. In this study, the positive rate of p53 protein in laryngeal cancer and surgical margin of tumor adjacent tissues was 60.0% (51/85) and 36.5% (31/85), respectively. In recurrent group and non recurrent group of this cohort, the positive rate of p53 protein in the cancer surgical margin was 71.4% (10/14) and 29.6% (21/71) (P = 0.003), respectively. Therefore, it is implicated that detecting the expression of p53 protein in the laryngeal surgical margin can predict the risk of tumor recurrence.
p21 is one of the CIP family members of CKI, and it can inhibit the cell cycle progression mainly through inhibition of the function of CDK2. Relationship of p21 gene and tumor is mainly synergies with other related genes in the expression levels, which acts in a p53-dependent pathway or p53-independent pathway [10]. Clinical reports of p21 expression and tumor prognosis are not identical. Kapranos et al [11] have shown that p21 expression is a better prognostic factor in stage III head and neck squamous cell carcinoma. Bandoh et al [12] studied the expression of p21 in 70 cases of squamous cell carcinoma of the maxillary sinus and found that the missing of p53-dependent p21 expression was found in patients with high proliferative tumor cells. Kaplan-Meier analysis showed that the expression of p21 was not correlated with longer disease-free survival of patients. Fischer et al [13] studied the relationship between the expression of p21 and prognosis in 144 cases of hypopharynx squamous cell carcinoma and found that p21 expression was correlated with clinical lymph node staging (positive rate of p21 in group N0 and group N1-2 was 66.7% and 93.7%, respectively; P < 0.001), local recurrence (p21 positive rate of recurrence group and non-recurrence group was 69.6% and 50%, respectively; P = 0.031) and poor prognosis (5-year survival rates of p21 positive group and p21 negative group were 35.3% and 54.7%, respectively; P = 0.016). Expression of p21 is an important marker of poor prognosis in head and neck squamous cell carcinoma (HNSCC). Our study found that the recurrence rate in patients with p21 positive expression is higher than that of patients with negative one, and the difference was statistically significant. Analysis shows that expression of p21 has two pathways: p53-dependent and p53-independent. It is a complicated relationship between the expression of p21 and p53 in tumor tissues, and the expression of p21 and p53 was heterogenous in different cells and tumors [14]. p21 expression was heterogeneous in various tumors, so that it is still controversial that p21 is a tumor suppressor gene or an oncogene. Early studies have shown that when p21 was answering a variety of environmental stimuli, cell cycle arrests, which supported the opinion that p21 is a tumor suppressor gene. In addition, biochemical and genetic studies have shown that p21 does not rely on classical signaling pathway of p53, and acts as a major effector of tumor suppressor genes in a variety of antiproliferative activities. Despite its main role is to inhibit cell proliferation and promote cell differentiation and senescence, but new studies have shown that under certain circumstance p21 can promote cell proliferation and plays a carcinogenic role. Thus, p21 is often not expressed in various human tumors and its expression depends on the cell environment, in which p21 may act as a tumor suppressor gene, or an oncogene [10,15]. In laryngeal squamous cell carcinoma, p21 protein expression may be in p53-independent pathway, which increased the stability of p21 protein and accumulated protein rather than increasing transcription.
Cde2 is one of the most important regulated kinase of cell cycle and its activity is not only regulated positively by cyclin B1, but also by CKI negatively [16]. The main function of Cdc2 is to monitor spindle microtubule assembly and kinetochore’s proper connection of DNA in the G2/M checkpoint. If an error occurs in adjustment mechanism of Cdc2, it will lead directly to cell differentiation disorders, disorder of cell cycle progression and induce abnormal cell proliferation or malignant transformation, thus promoting the occurrence and progression of tumor [17-19]. Cdc2 overexpressed in many cancers, such as oral squamous cell carcinoma [20], tongue squamous cell carcinoma [21], supraglottic cancer [22], esophageal squamous cell carcinoma, esophageal adenocarcinoma [23], gastric cancer [24], liver cancer [25], colorectal cancer [26], breast cancer [27], ovarian cancer [28] and so on. This study found that positive rate of Cdc2 protein in laryngeal cancer and cancer adjacent surgical margin tissues was 70.6% (60/85) and 29.4% (25/85), and in laryngeal recurrent group and non-recurrent group, the positive rate of Cdc2 in the cutting edge of cancer is 57.1% (8/14) and 23.9% (17/71), respectively. Therefore we believe that expression of Cdc2 protein is closely related to recurrence and poor prognosis of laryngeal cancer.
HNSCC patients with positive pathological margins has high postoperative recurrence rate, about 75% of local recurrence. While for patients with negative pathological margins there are still 25% of patients with local recurrence ultimately [29]. Therefore, some researchers conducted molecular studies for the negative pathological margin to clarify the underlying mechanism. It is considered that two mechanisms could explicate the local recurrence with the negative pathological margin after surgery: one is the possible presence of residual tumor cells, which means the incomplete cutting edge. That histological technology nowadays could not find the residual disease is called minimal residual cancer (MRC). Another is the presence of the lesion that may have changed at the molecular level (precancerous lesions), and eventually turn into invasive cancer. Therefore we used various techniques including immunohistochemistry, RT-PCR, FISH and other molecular studies to identify potential molecular markers of local recurrence. Studies found that local recurrence of tumor was related to the expression of p53, CCDN1, p16, etc [30]. But little about the combined detection of protein expression in laryngeal peripheral tissues have reported yet.
In conclusion, the expression of p53, p21 and Cdc2 protein in laryngeal squamous cell carcinoma was higher than that in tumor adjacent tissues, while the local recurrence of laryngeal squamous cell carcinoma was significantly correlated with positive expression of proteins in tumor adjacent tissue, suggesting that expression change of p53, p21 and Cdc2 protein is an important factor affecting the occurrence of laryngeal carcinoma. p53, p21, and Cdc2 are expected to become important biomarkers for evaluation of prognosis and treatment of laryngeal squamous cell cancer. For patients with positive expression of p53, p21 and Cdc2 should be closely followed up, shorten the interval of follow-up time and once potential recurrence suggested, prompt surgical should be made to remove of the tumor again. Immunohistochemistry is a simple, sensitive and reliable method to detect the expression of p53, p21 and Cdc2 jointly in early laryngeal cancer and its surgical margins. Combined detection of the three proteins may be a useful reference for screening patients at high risk of recurrence which is worthy of further molecular studies.
Disclosure of conflict of interest
None.
References
- 1.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21 (Cip1), p27 (Kip1) and p57 (Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu N, Chang DC. Different thresholds of MPF inactivation are responsible for controlling different mitotic events in mammalian cell division. Cell Cycle. 2007;6:1639–1645. doi: 10.4161/cc.6.13.4385. [DOI] [PubMed] [Google Scholar]
- 4.Lucioni M, Bertolin A, Rizzotto G, Accordi D, Giacomelli L, Marioni G. CO(2) laser sur gery in elderly patients with glottic carcinoma: univariate and multivariate analyses of results. Head Neck. 2012;34:1804–1809. doi: 10.1002/hed.22907. [DOI] [PubMed] [Google Scholar]
- 5.Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, Zhou H, Smith M, Kimberly D, Glass J. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10:5820–5827. doi: 10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 6.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 7.Nathan CO, Sanders K, Abreo FW, Nassar R, Glass J. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: prognostic implications. Cancer Res. 2000;60:3599–3604. [PubMed] [Google Scholar]
- 8.Allegra E, Puzzo L, Cutrona D, Trichini A, Garozzo A, Serra A. p53 overexpression on the resection margins as a marker of local recurrence in glottic T1a carcinoma. Acta Otorhinolaryngol Ital. 2003;23:454–458. [PubMed] [Google Scholar]
- 9.Jalali MM, Heidarzadeh A, Zavarei MJ, Sarmast H. p53 overexpression impacts on the prognosis of laryngeal squamous cell carcinomas. Asian Pac J Cancer Prev. 2011;12:1731–1734. [PubMed] [Google Scholar]
- 10.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapranos N, Stathopoulos GP, Manolopoulos L, Kokka E, Papadimitriou C, Bibas A, Yiotakis J, Adamopoulos G. p53, p21 and p27 protein expression in head and neck cancer and their prognostic value. Anticancer Res. 2001;21:521–528. [PubMed] [Google Scholar]
- 12.Bandoh N, Hayashi T, Takahara M, Kishibe K, Ogino T, Katayama A, Imada M, Nonaka S, Harabuchi Y. Loss of p21 expression is associated with p53 mutations and increased cell proliferation and p27 expression is associated with apoptosis in maxillary sinus squamous cell carcinoma. Acta Otolaryngol. 2005;125:779–785. doi: 10.1080/00016480410023056. [DOI] [PubMed] [Google Scholar]
- 13.Fischer CA, Jung M, Zlobec I, Green E, Storck C, Tornillo L, Lugli A, Wolfensberger M, Terracciano LM. Co-overexpression of p21 and Ki-67 in head and neck squamous cell carcinoma relative to a significantly poor prognosis. Head Neck. 2011;33:267–273. doi: 10.1002/hed.21440. [DOI] [PubMed] [Google Scholar]
- 14.Caffo O, Doglioni C, Veronese S, Bonzanini M, Marchetti A, Buttitta F, Fina P, Leek R, Morelli L, Palma PD, Harris AL, Barbareschi M. Prognostic value of p21 (WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res. 1996;2:1591–1599. [PubMed] [Google Scholar]
- 15.Gartel AL. p21 (WAF1/CIP1) and cancer: a shifting paradigm? Biofactors. 2009;35:161–164. doi: 10.1002/biof.26. [DOI] [PubMed] [Google Scholar]
- 16.Kaldis P, Aleem E. Cell cycle sibling rivalry: Cdc2 vs. Cdk2. Cell Cycle. 2005;4:1491–1494. doi: 10.4161/cc.4.11.2124. [DOI] [PubMed] [Google Scholar]
- 17.Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH. Prognostic implications of cyclin B1, p34cdc2, p27 (Kip1) and p53 expression in gastric cancer. Yonsei Med J. 2007;48:694–700. doi: 10.3349/ymj.2007.48.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27:4733–4744. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 20.Chang JT, Wang HM, Chang KW, Chen WH, Wen MC, Hsu YM, Yung BY, Chen IH, Liao CT, Hsieh LL, Cheng AJ. Identification of differentially expressed genes in oral squamous cell carcinoma (OSCC): overexpression of NPM, CDK1 and NDRG1 and underexpression of CHES1. Int J Cancer. 2005;114:942–949. doi: 10.1002/ijc.20663. [DOI] [PubMed] [Google Scholar]
- 21.Wada S, Yue L, Furuta I. Prognostic significance of p34cdc2 expression in tongue squamous cell carcinoma. Oral Oncol. 2004;40:164–169. doi: 10.1016/s1368-8375(03)00146-5. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Itoh T, Inoue I, Takahashi H. Expression of p34cdc2 protein kinase and p53 in supraglottic carcinomas. Auris Nasus Larynx. 1996;23:105–110. doi: 10.1016/s0385-8146(96)80016-2. [DOI] [PubMed] [Google Scholar]
- 23.Hansel DE, Dhara S, Huang RC, Ashfaq R, Deasel M, Shimada Y, Bernstein HS, Harmon J, Brock M, Forastiere A, Washington MK, Maitra A, Montgomery E. CDC2/CDK1 expression in esophageal adenocarcinoma and precursor lesions serves as a diagnostic and cancer progression marker and potential novel drug target. Am J Surg Pathol. 2005;29:390–399. doi: 10.1097/00000478-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH. Prognostic implications of cyclin B1, p34cdc2, p27 (Kip1) and p53 expression in gastric cancer. Yonsei Med J. 2007;48:694–700. doi: 10.3349/ymj.2007.48.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, Takeda T, Sakon M, Monden M, Tsujimoto M, Matsuura N. Expression and prognostic role of cyclin-dependent kinase 1 (cdc2) in hepatocellular carcinoma. Oncology. 2000;59:68–74. doi: 10.1159/000012140. [DOI] [PubMed] [Google Scholar]
- 26.Nozoe T, Honda M, Inutsuka S, Korenaga D. p34cdc2 expression is an independent indicator for lymph node metastasis in colorectal carcinoma. J Cancer Res Clin Oncol. 2003;129:498–502. doi: 10.1007/s00432-003-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourea HP, Koutras AK, Scopa CD, Marangos MN, Tzoracoeleftherakis E, Koukouras D, Kalofonos HP. Expression of the cell cycle regulatory proteins p34cdc2, p21waf1, and p53 in node negative invasive ductal breast carcinoma. Mol Pathol. 2003;56:328–335. doi: 10.1136/mp.56.6.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrette BA, Srivatsa PJ, Cliby WA, Keeney GL, Suman VJ, Podratz KC, Roche PC. Overexpression of p34cdc2 protein kinase in epithelial ovarian carcinoma. Mayo Clin Proc. 1997;72:925–929. doi: 10.1016/S0025-6196(11)63362-4. [DOI] [PubMed] [Google Scholar]
- 29.Bradley PJ, MacLennan K, Brakenhoff RH, Leemans CR. Status of primary tumour surgical margins in squamous head and neck cancer: prognostic implications. Curr Opin Otolaryngol Head Neck Surg. 2007;15:74–81. doi: 10.1097/MOO.0b013e328058670f. [DOI] [PubMed] [Google Scholar]
- 30.de Carvalho AC, Kowalski LP, Campos AH, Soares FA, Carvalho AL, Vettore AL. Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol. 2012;48:240–248. doi: 10.1016/j.oraloncology.2011.10.018. [DOI] [PubMed] [Google Scholar]


