Abstract
Introduction: Long non-coding RNAs (lncRNAs) have been investigated as a new class of regulators of cellular processes, such as cell growth, apoptosis, and carcinogenesis. lncRNA GAS5 has recently been identified to be involved in tumorigenesis of several cancers such as breast cancer, lung cancer and renal cancer. However, the regulation of GAS5 in hepatocellular carcinoma (HCC) has not yet been reported before. Methods: Expression of GAS5 in tumor and their normal matched tissues was determined by quantitative real-time PCR in n = 71 HCC patients, and its association with overall survival of patients was analyzed by statistical analysis. Results: The expression level of GAS5 was reduced in HCC in comparison to normal matched tissues (P < 0.05). It is also proved that GAS5 expression was to be associated with HCC tumor size, lymphnode metastasis and clinical stage (P < 0.05). In addition, the Kaplan-Meier survival curves revealed that low GAS5 expression was associated with poor prognosis in HCC patients. GAS5 expression was an independent prognostic marker of overall HCC patient survival in a multivariate analysis. Conclusions: The study proved for the first time that GAS5 down regulated in a majority of HCC patients. Our results indicated that GAS5 expression was an independent prognostic factor for patients with liver cancer, which might be a potential valuable biomarker for HCC.
Keywords: GAS5, hepatocellular carcinoma, quantitative real-time PCR, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third cause of cancer-related deaths [1]. Despite recent advances in cancer treatment with respect to surgery, chemotherapy and biologics, majority of HCC remains incurable once it has become metastatic and has a poor prognosis [2]. The major causes of HCC are viral infections, alcohol and tobacco use. While efforts have been made to identify appropriate prognostic markers for HCC, including primary tumor size, elevated AFP levels, and gene expression markers in the primary tumor, these method have not proven adequate to predict the prognosis of all HCC patients [3,4]. Thus, it is important to find a reliable clinical marker for HCC diagnosis and prediction of clinical outcome.
Long non-coding RNA (LncRNA) is an RNA molecule that is longer than 200 nucleotides and is not translated into a protein [5]. Although these long non-coding transcripts were once considered to be simply tran-scriptional “noise” or cloning artifacts [6]. Recent evidence showed that lncRNAs play important roles in diverse biological processes, such as transcriptional regulation, cell growth and tumorigenesis [7,8]. In this regard, highlighting the potentially widespread functional roles of lncRNAs in human cancer is important. For example, SRA (steroid receptor RNA activator), a coactivator for nuclear receptors (NRs), which Increased expression may alter ER/PR activity, leading to cell proliferation and breast tumorigenesis [9]. In addition to SRA, Geng showed that HOTAIR (HOX transcript antisense RNA) gene was significantly overexpressed in HCC tissues compared with adjacent non-tumor tissues and patients with high HOTAIR gene expression in their tumors had an increased risk of recurrence after hepatectomy [10]. Unfortunately, the emerging functional role of lncRNAs in hepatocellular carcinoma remains largely unknown.
GAS5 (growth arrest-specific transcript 5) was identified using a functional screen through its ability to suppress apoptosis in a mouse thymoma cell line [11]. This gene is encoded at 1q25, a chromosomal locus which has been associated with lymphoma [12]. A large number of studies demonstrate that GAS5 is a tumor-suppressor lncRNA. Qiao indicated that a decrease in GAS5 expression is associated with renal cell carcinoma genesis and progression and overexpression of GAS5 can act as a tumor suppressor for renal cell carcinoma [13]. Shi’s studies showed that GAS5 is a tumor suppressor in non-small-cell lung carcinoma, and the action of GAS5 is mediated by p53-dependent and p53-independent pathways [14]. Williams identified that GAS5 transcript levels were significantly reduced in breast cancer samples relative to adjacent unaffected normal breast epithelial tissues and GAS5 as critical to the control of mammalian apoptosis and cell population growth [15]. However, the clinical and prognostic significance of lncRNA GAS5 expression in hepatocellular carcinoma has not been reported yet.
In this study, we aimed to investigate the expression of lncRNA GAS5 in HCC specimens and adjacent normal tissues, and investigated whether GAS5 is a useful prognostic indicator in HCC patients.
Materials and methods
Patients and specimens
Patient data were accessed from the databank of the Department of Oncology of The People’s Hospital of Zhengzhou (Henan, China). 71 consecutive patients (40 male and 31 female) with HCC and who underwent surgery from 2005 to 2009 were included in the study. None of the patients received preoperative chemotherapy or radiation therapy. Cancer tissue specimens were collected from the patients after obtaining informed consent, which was in accordance with the institutional guidelines of The People’s Hospital of Zhengzhou. All tumors were fixed in formalin and embedded in paraffin. Clinical charts were reviewed, and follow-up data were collected. Patients were only included in the study if they had provided written consent to participate in the study after receiving oral and written information regarding its course and purpose. Approval for the study was received from the Ethics Committee of the host institution.
Quantitative real-time PCR assay
Total RNA was isolated tissue using TRIZOL reagent according to the manufacturer’s protocol (Invitrogen). RNA was reverse transcribed using Super Script First Strand cDNA System (Invitrogen) according to the manufacturer’s instructions. The PCR amplification were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, on a Applied Biosystems 7900HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (Takara). Data was collected and analyzed by SDS2.3 Software (Applied Biosystems). The expression level of each candidate gene was internally normalized against that of the GAPDH. The relative quantitative value was expressed by the 2-ΔΔCt method. Each experiment was performed in triplicates and repeated three times.
Statistical analysis
All computations were carried out using the SPSS software version 17.0 for Windows (IBM Corporation, NY, USA). The significance of differences between two groups was estimated using the Student’s t-test. Overall survival curves were plotted according to the Kaplan-Meier method. Variables with a P-value of < 0.05 by univariate analysis were used in subsequent multivariate analysis on the basis of the Cox proportional hazards model. All differences were considered statistically significant when P < 0.05.
Results
Down-regulation of GAS5 in HCC tissues
To ascertain whether lncRNA GAS5 was differentially expressed in the HCC tissues, a total of 71 paired clinical HCC tissues and adjacent normal tissues were analyzed for GAS5 expression using real-time quantitative PCR. GAS5 expression was significantly down-regulated in clinical HCC specimens compared to adjacent normal liver tissues (P < 0.05, Figure 1A). The results indicated that GAS5 might play an tumor suppressor role in HCC. HCC patients who expressed GAS5 at levels less than the cutoff value were assigned to the low expression group (mean expression value 0.19, n = 51), and those with expression above than the cutoff value were assigned to the high expression group (mean expression value 0.68, n = 20).
Figure 1.
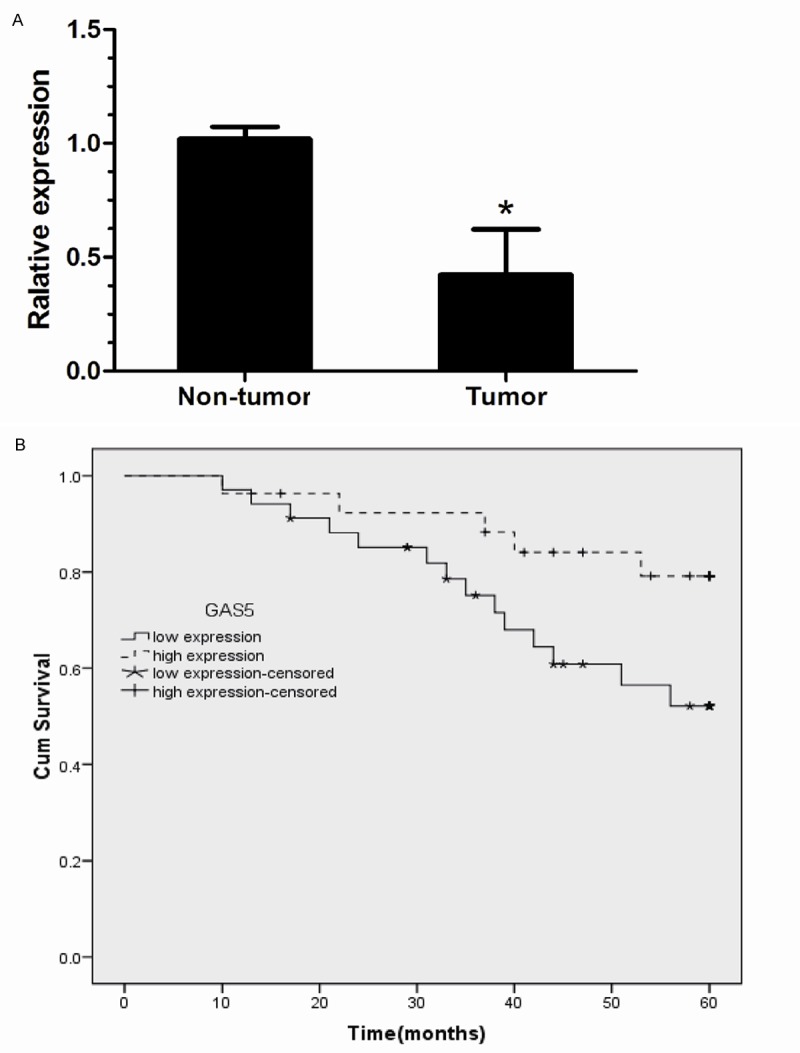
Clinical significance of expression of lncRNA GAS5 in the human hepatocellular carcinoma (HCC). A. Lower relative GAS5 lncRNA levels were detected in tumours than in adjacent normal tissues in patients with HCC (*P < 0.05). B. Kaplan-Meier curves estimating the 5-year recurrence-free survival rates according to the level of expression of the GAS5 in patients with HCC who underwent hepatic resection.
Relationship between GAS5 expression and the clinicopathologic features of HCC
Table 1 summarizes the association between GAS5 expression and clinicopathologic features in HCC. Low expression of GAS5 was found to significantly correlate with tumor size (P = 0.003), lymphnode metastasis (P = 0.008), and clinical stage (P = 0.001). However, GAS5 expression was not significantly related to gender, age, tumor number, HBsAg and AFP (P > 0.05).
Table 1.
Association of GAS5 with clinicopathological characteristics of HCC patients
| Parameters | Group | Total | GAS5 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Gender | Male | 40 | 28 | 12 | 0.697 |
| Female | 31 | 23 | 8 | ||
| Age (years) | < 60 | 42 | 30 | 12 | 0.928 |
| ≥ 60 | 29 | 21 | 8 | ||
| Tumor size (cm) | < 5 cm | 29 | 15 | 13 | 0.003 |
| ≥ 5 cm | 42 | 33 | 5 | ||
| Tumour number | Solitary | 47 | 34 | 13 | 0.894 |
| Multiple | 24 | 17 | 7 | ||
| HBsAg | Postive | 45 | 32 | 13 | 0.859 |
| Negative | 26 | 19 | 7 | ||
| AFP | < 20 | 22 | 12 | 10 | 0.030 |
| > 20 | 49 | 39 | 10 | ||
| Lymphnode metastasis | Absence | 39 | 23 | 16 | 0.008 |
| Presence | 32 | 28 | 4 | ||
| Clinical stage | I-II | 43 | 25 | 18 | 0.001 |
| III-IV | 28 | 26 | 2 | ||
Low GAS5 expression predicts poor prognosis in patients with HCC
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. First, to confirm the representativeness of the HCC in our study, we analyzed established prognostic predictors of patient survival. Kaplan-Meier analysis demonstrated a significant impact of well-known clinical pathological prognostic parameters, such as tumor size, lymphnode metastasis and clinical stage on patient survival (P < 0.05, Table 2). Assessment of survival in HCC patients revealed that lower expression of GAS5 was correlated with adverse survival of HCC patients (P < 0.001, Table 2, Figure 1B).
Table 2.
Prognostic factors in Cox proportional hazards model
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | 1.042 | 0.874-1.611 | 0.674 | |||
| Male vs Female | ||||||
| Age (years) | 1.294 | 0.574-1.814 | 0.513 | |||
| < 60 vs ≥ 60 | ||||||
| Tumour number | 2.024 | 1.476-4.113 | 0.089 | |||
| Solitary vs Multiple | ||||||
| HBsAg | 1.486 | 0.971-2.481 | 0.407 | |||
| Postive vs Negative | ||||||
| AFP | 2.174 | 1.365-5.118 | 0.107 | |||
| < 20 vs > 20 | ||||||
| Tumor size | 2.342 | 1.784-3.921 | 0.006 | 1.787 | 1.169-3.274 | 0.035 |
| < 5 cm vs ≥ 5 cm | ||||||
| Lymphnode metastasis | 4.428 | 2.714-7.625 | 0.007 | 3.548 | 2.169-4.783 | 0.011 |
| Absence vs Presence | ||||||
| Clinical stage | 3.164 | 1.761-8.131 | 0.003 | 2.614 | 1.114-5.628 | 0.006 |
| I-II vs III-IV | ||||||
| GAS5 | 0.297 | 0.149-0.518 | < 0.001 | 0.417 | 0.244-0.617 | 0.002 |
| low vs high | ||||||
Abbreviation: CI, confidence interval.
Independent prognostic factors for HCC: multivariate Cox regression analysis
Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of GAS5 and those clinicalopathological parameters that were significant in univariate analysis (clinical stage, lymphnode metastasis and tumor size) were further examined in multivariate analysis. Our results showed a significant correlation between low expression levels of GAS5 and adverse disease-specific survival (relative risk: 0.417, CI: 0.244-0.617, P = 0.002, Table 2). With regard to other parameters, the clinical stage, lymphnode metastasis and tumor size were also shown to be an independent prognostic factor for overall survival (P < 0.05, Table 2).
Discussion
Hepatocellular carcinoma (HCC) is one of the most common malignant diseases with a poor prognosis. Although several clinicopathologic features have been the standard for determining the clinical outcome of HCC patients, this classification scheme is probably an imprecise predictor of the prognosis of an individual patient [16]. Therefore, it is necessary to identify novel and effective biologic markers which are associated with advanced tumor progression for the early diagnosis and the discovery of a therapeutic target.
In 2012, the GENCODE lncRNAs catalog consists of 14,880 transcripts grouped into 9,277 gene loci in the human genome [17]. Dysregulated expression of lncRNAs in cancer marks the spectrum of disease progression and may serve as an independent predictor for patient outcomes. Ge’s study suggested that high expression of lncRNA PCAT-1 is involved in colorectal cancer progression and could be a novel biomarker of poor prognosis in patient with colorectal cancer [18]. Kogo found that lncRNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers [19]. However, the role of lncRNAs in HCC is yet to be fully elucidated. In the present study, our attention focused on the lncRNA GAS5. Pickard found that GAS5 promotes the apoptosis of prostate cancer cells and abnormally low levels of GAS5 expression may reduce the effectiveness of chemotherapeutic agents [20]. Shi reported for the first time that GAS5 down-regulation is involved in non-small-cell lung carcinoma tumorigenesis and progression, and GAS5 overexpression can dramatically induce apoptosis and growth arrest in vitro and reduce tumor growth in vivo, In addition, although they found that p53 and E2F1 are key downstream mediators of GAS5 [14]. Mourtada indicated that GAS5 plays a crucial role in normal growth arrest in both T-cell lines and non-transformed lymphocytes and GAS5 overexpression results in both an increase in apoptosis and a reduction in the rate of progression through the cell-cycle, whereas GAS5 knockdown inhibits apoptosis and maintains a more rapid cell cycle [21]. Other studies showed that the genetic aberrations of GAS5 have relationship with many other types of tumors including melanoma, glioblastoma and renal cancers [22].
However, there have been no reports on the clinical relevance of lncRNA GAS5 to HCC, In the present study, we sought to determine whether there was any difference in GAS5 expression between HCC specimens and adjacent normal tissues which had not been studied previously. This study showed that GAS5 were down-regulated in the HCC, and explored available evidence of close correlation of GAS5 expression and the total patients’ survival during a 5-year follow-up survey.
To directly address the potential roles for GAS5 in the occurrence and development of HCC, an elaborate experiment was conducted and a rigorous analysis was performed of human GAS5 expression on a HCC samples. Our results revealed that the GAS5 expression in HCC specimens was remarkably lower than that in adjacent normal tissues (P < 0.05). In the present study, we found the expression level of GAS5 was significantly associated with tumor size (P = 0.003), lymphnode metastasis (P = 0.008) and clinical stage (P = 0.001). It is suggested that GAS5 may play important roles in liver cancer carcinogenesis and progression.
Ultimately, A total of 71 patients histologically proven liver cancer with follow-up information were conducted a systematically analysis to confirm the relationship of GAS5 and outcome of patient initially. Our finding demonstrated that patients with lower expression of GAS5 in tumor tissue had a worse overall survival than patients with higher expression (P < 0.05, respectively), providing an evidence that reduced expression of GAS5 in hepatocellular cancer might facilitate an increased malignant and worse prognostic phenotype. It is noteworthy that by multivariate Cox analysis combining expression of GAS5 with other parameters, GAS5 was found as an independent prognostic factor (P = 0.002) for patient survival. The aberrant expression of GAS5 linked to a poor prognosis of patients has never been investigated in hepatocellular cancer before.
In conclusion, our data indicated that GAS5 expression was decreased in human HCC and was associated with advanced tumor progression. Furthermore, GAS5 expression was demonstrated for the first time to be an independent marker for predicting the clinical outcome of patients with HCC. Apparently, a further understanding of the molecular mechanism by GAS5 in human HCC would help in the discovery of novel targeted agents and might also lead to the development of new approaches for effective therapy of human HCC.
Disclosure of conflict of interest
None.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan K, Liang X, Zhang H, Zhao J, Wang D, Li J, Lian Q, Chang AE, Li Q, Xia J. Characterization of bridging integrator 1 (BIN1) as a potential tumor suppressor and prognostic marker in hepatocellular carcinoma. Mol Med. 2011;18:507–518. doi: 10.2119/molmed.2011.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 6.Costa FF. Non-coding RNAs: Meet thy masters. Bioessays. 2010;32:599–608. doi: 10.1002/bies.200900112. [DOI] [PubMed] [Google Scholar]
- 7.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113:1868–1874. doi: 10.1002/jcb.24055. [DOI] [PubMed] [Google Scholar]
- 9.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of the steroid receptor RNA activator in human breast tumors. Cancer Res. 1999;59:4190–4193. [PubMed] [Google Scholar]
- 10.Geng Y, Xie S, Li Q, Ma J, Wang G. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39:2119–2128. doi: 10.1177/147323001103900608. [DOI] [PubMed] [Google Scholar]
- 11.Williams GT, Hughes JP, Stoneman V, Anderson CL, McCarthy NJ, Mourtada-Maarabouni M, Pickard M, Hedge VL, Trayner I, Farzaneh F. Isolation of genes controlling apoptosis through their effects on cell survival. Gene Ther Mol Biol. 2006;10:255. [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y, Takahashi N, Kakegawa E, Yoshida K, Ito Y, Kayano H, Niitsu N, Jinnai I, Bessho M. The GAS5 growth arrest-specific transcript 5) gene fuses to BCL6 as a result of t (1;3)(q25;q27) in a patient with B-cell lymphoma. Cancer Genet Cytogenet. 2008;182:144–149. doi: 10.1016/j.cancergencyto.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Qiao HP, Gao WS, Huo JX, Yang ZS. Long Non-coding RNA GAS5 Functions as a Tumor Suppressor in Renal Cell Carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–82. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2013 doi: 10.1002/mc.22120. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 16.Gish RG. Hepatocellular carcinoma: overcoming challenges in disease management. Clin Gastroenterol Hepatol. 2006;4:252–261. doi: 10.1016/j.cgh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge XS, Chen YB, Liao XY, Liu DQ, Li FF, Ruan HL, Jia WH. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Medical Oncology. 2013;30:588. doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 19.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long Noncoding RNA HOTAIR Regulates Polycomb-Dependent Chromatin Modification and Is Associated with Poor Prognosis in Colorectal Cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 20.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochimica Et Biophysica Acta. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5) J Cell Sci. 2008;121:939–946. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 22.Tani H, Torimura M, Akimitsu N. The RNA Degradation Pathway Regulates the Function of GAS5 a Non-Coding RNA in Mammalian Cells. PLoS One. 2013;8:e55684. doi: 10.1371/journal.pone.0055684. [DOI] [PMC free article] [PubMed] [Google Scholar]


