Abstract
Melatonin is a powerful antioxidant. Decreased melatonin excretion has been reported to be associated with several oxidative stress-related diseases. The urinary metabolite of melatonin, 6-sulfatoxymelatonin (aMT6s), has proved to be a very reliable index of melatonin production. The present study aims to evaluate the level of urinary aMT6s in patients with type 2 diabetes mellitus and diabetic retinopathy. Urine samples were collected from 10 patients with diabetes and no diabetic retinopathy (NDR), 19 patients with nonproliferative diabetic retinopathy (NPDR), 38 patients with proliferative diabetic retinopathy (PDR), and 16 subjects without diabetes mellitus, who served as controls. The level of aMT6s in specimens was assayed by a commercial aMT6s ELISA kit, creatinine levels were also measured for each sample to get urinary aMT6s/creatinine ratio. Creatinine-adjusted urinary aMT6s values were compared among four groups. The urinary aMT6s (mean ± SD) levels were 9.95 ± 2.42, 9.90 ± 2.28, 8.40 ± 1.84 and 5.58 ± 1.33 ng/mg creatinine in the controls and in patients with NDR, NPDR, or PDR, respectively. The urinary aMT6s level of the PDR group was significantly lower than that of the control, NDR and DR groups. No significant difference was found among the control, NDR and DR groups. After adjustment for various factors (age, smoking, cancer, and coronary heart disease) that may influence the aMT6s level, the odds-ratio of urinary aMT6s comparing PDR patients to controls was 0.246 (95% confidence interval = 0.108-0.558, P = 0.001). Therefore, the urinary aMT6s level is significantly decreased in diabetic patients with PDR but not in diabetic patients without PDR, which indicates that decreased urinary aMT6s level may be associated with the pathogenesis of PDR.
Keywords: 6-sulfatoxymelatonin, melatonin, diabetic retinopathy
Introduction
Melatonin is a neurohormone secreted into the blood primarily by the pineal gland during the dark phase of the light-dark cycle in humans. It was first found to be a part of the system that regulates the circadian rhythm and then its antioxidant function had been reported in 1993 by Poeggeler B, et al [1]. Since then, more and more studies discovered that melatonin is a powerful and pervasive antioxidant [2]. Several epidemiologic studies have described that low melatonin levels lead to an increase in some cancers and cardiovascular diseases risk [3-7], which is associated with reduced protection ability from oxidative damage occurred in these chronic diseases.
Diabetic retinopathy (DR) is a severe sight-threatening complication of diabetes and the main cause of blindness in working-aged adults all over the world. The pathogenesis of DR is not completely clear, but oxidative stress mediated cellular dysfunction may play a central role in the occurrence and development of DR [8,9]. Pooled evidence suggests that melatonin is a good antioxidant and can increase the expression of various endogenous antioxidant enzymes by binding to melatonin receptors, exerting a strong protective effect on oxidative stress-induced damage [10,11]. There is very likely that reduced melatonin level can not exert its antioxidant effect to protect retinas from oxidative damage in diabetes patients, which ultimately leads to DR over time.
Serum melatonin is rapidly metabolized and excreted as urinary metabolite, 6-sulfatoxymelatonin (aMT6s). Nocturnal excretion of aMT6s is a reliable reflection of serum melatonin [12-14], provides an overview of peak level of serum melatonin secreted during last night’s sleep, which makes it possible and very convenient to measure aMT6 level as a marker in epidemiologic studies. Hence, in this paper we studied urinary 6-sulfatoxymelatonin level in diabetic retinopathy patients and comparison with age and gender matched controls. Additionally, some factors that may affect melatonin secretion (age, smoking, cancer and coronary artery disease) in these groups were assessed carefully to exclude the possible influence caused by these factors.
Materials and methods
Subjects
All subjects enrolled in this study were randomly selected patients aged 50-75 hospitalized at Shanghai First People’s Hospital to undergo eye surgery. This research was conducted according to the principles of the Declaration of Helsinki and written informed consent was obtained from all participants. After biochemical and physiologic tests (fasting blood sugar, serum creatinine, HbA1c levels, lipid profile, liver enzymes, dipstick urine test and electrocardiography) and careful retinal examination or fluorescein angiography followed by pupillary dilation with tropicamide, all the 83 patients were divided into 2 groups: diabetic group (27 males and 40 females with type 2 diabetes) and nondiabetic group (6 males and 10 females). The nondiabetic group severed as controls. According to the severity of retinopathy, the diabetic group was further divided into 3 subgroups: no apparent diabetic retinopathy (NDR) group (4 males and 6 females), non-proliferative diabetic retinopathy (NPDR) group (7 males and 12 females) and proliferative diabetic retinopathy (PDR) group (19 males and 19 females). The nondiabetic group, DR group and the NPDR group consisted of those admitted to hospital for operation on cataract, while the PDR group consisted of those admitted to undergo vitreous surgery because of massive vitreous hemorrhage combined with cataract.
Demographic information and the history of smoking, cancer and coronary artery disease were collected from all participants. Exclusion criteria included sleep disorders, systemic disease, use of drugs affecting the autonomic nervous system or parasympathetic nervous system, depression, and kidney or liver disease. Smoking was not allowed during hospitalization. The lights in the hospital were turned off at 10 pm and turned on at 6 am.
Specimen collection
Patients were instructed to collect their urine over the previous night. The last void was discarded at 7 pm and urine during the night up to 7 am was collected and kept at room temperature. On the day after overnight collection samples of urine were refrigerated before transport to the laboratory. They were kept frozen at -80°C until test. This study was approved by the Ethics Committee of the hospital.
Measurement of 6-sulphatoxymelatonin and creatinine
The levels of aMT6s in urine samples were assayed in duplicate using commercially available enzyme-linked immunosorbent assay (ELISA). The ELISA kit was purchased from IBL-Germany (IBL International GmbH, Hamburg, Germany). The assay sensitivity was 1.0 ng/ml; and the inter-assay and intra-assay coefficients of variation (CV) were 10.2% and 11.4%, respectively. The test procedure was carried out according to the manufacturer’s instructions. In brief, 50 μl of each diluted standard, control and patient sample was pipetted into the respective wells of the plate and next 50 μl of freshly prepared enzyme conjugate and 50 ul of melatonin sulfate antiserum were added into each well in order. The plate was incubated on an orbital shaker (500 rpm) for 2 h at room temperature. Incubation solution was discarded and all wells were washed 4 times with wash buffer provided in the kit. Then 100 μl of substrate solution was pipetted into each well and incubated for 30 min at room temperature. Stop solution was added and the absorbance at 450 nm of each well was measured by a microplate reader. The results were calculated following the manufacturer’s instructions. Assays were performed by testers who were masked to the sample information.
As urinary aMT6s is often normalized to urine creatinine concentration, urine creatinine was also measured in this study by the Jaffe method [15]. The standard creatinine solution and picric acid were purchased from Sigma-Aldrich (St. Louis, MO). To adjust for variation in the urinary dilution, aMT6s was expressed as urinary aMT6s/urine creatinine ratio.
Statistics
One-way ANOVA was used for statistical analysis of age (expressed as mean ± standard deviation) among all the four groups. Chi-square test was performed on gender difference, and Fisher’s exact test was used to analyze the difference of the rate of smoking history, various cancers history and coronary artery disease history of the participants. A P value less than 0.05 indicated statistical significance. The amount of urinary aMT6s and HbA1c level were expressed as mean ± standard deviation, comparisons of means were conducted using the Kruskal-Wallis test since variances were heterogeneous, and the Mann-Whitney test was used to further compare means from two independent groups, a P value of less than 0.003 (0.05/C 4 2) was considered statistically significant. Logistic regression analysis was also performed to determine the odds ratios and 95% confidence intervals (CI) of urinary aMT6s levels between the PDR patients and the controls. Data were analyzed using the statistical software program SPSS for Windows, version 17.0 (SPSS, Inc., Chicago, Illinois, USA).
Results
Demographic and clinical characteristics of diabetic patients and the controls are summarized in Table 1. There were no statistically significant age and gender differences among the control, NDR, DR and PDR groups. The rate of smoking history, various cancers history and coronary artery disease history of the participants was found no significant difference among the four groups. The mean amount of aMT6s production was 9.95 ± 2.42, 9.90 ± 2.28, 8.40 ± 1.84 and 5.58 ± 1.33 ng/mg creatinine in the four groups, respectively (Figure 1). Significant difference was found in urinary aMT6 level between PDR patients and other three groups when the data were analyzed (P < 0.003). Logistic regression analysis showed that the level of nocturnal urinary aMT6s excretion in PDR patients was significantly lower than that in the controls (adjusted odds ratio = 0.169 and 95% CI = 0.050-0.579, P = 0.005) (Table 2).
Table 1.
Demographical and clinical data of diabetic patients and controls
| Factors | Control | Diabetes Mellitus | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| NDR | NPDR | PDR | |||
| Age | 63.00 ± 8.595 | 63.10 ± 5.567 | 65.84 ± 9.884 | 63.00 ± 5.327 | 0.534 |
| Gender (male/female) | 6/10 | 4/6 | 7/12 | 16/22 | 0.987 |
| HbA1c (%) | 4.97 ± 0.41 | 8.38 ± 1.37* | 7.61 ± 1.46* | 8.46 ± 1.28* | < 0.003 |
| Prior smoking | 2/16 (12.5%) | 1/10 (10%) | 4/19 (21.1%) | 5/38 (13.2%) | 0.831 |
| Cancer history | 1/16 (6.3%) | 0/10 (0.0%) | 2/19 (10.5%) | 2/38 (5.3%) | 0.838 |
| Coronary heart disease | 1/16 (6.3%) | 0/10 (0.0%) | 1/17 (5.9%) | 2/38 (5.3%) | 1.000 |
| Urinary aMT6s amount | 9.95 ± 2.42** | 9.90 ± 2.28** | 8.40 ± 1.84** | 5.58 ± 1.33 | < 0.003 |
HbA1c = glycosylated hemoglobin; NDR = nondiabetic retinopathy; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy.
P < 0.001 vs control.
P < 0.001 vs PDR.
Figure 1.
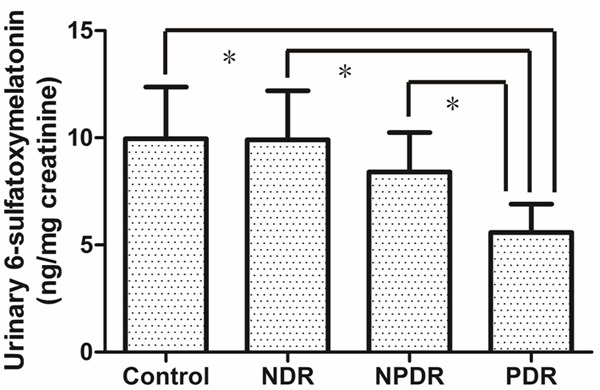
Mean urinary 6-sulfatoxymelatonin/creatinine levels in the control group (Control) and in patients with no diabetic retinopathy (NDR), nonproliferative diabetic retinopathy (NPDR), or proliferative diabetic retinopathy (PDR). *P < 0.003.
Table 2.
Urinary 6-sulfatoxymelatonin/creatinine level in PDR patients and controls
| Statistical adjustment | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Not adjusted for any factor | 0.238 | 0.102-0.557 | 0.001 |
| Adjusted for age and gender | 0.236 | 0.098-0.569 | 0.001 |
| Adjusted for smoking history only | 0.240 | 0.103-0.559 | 0.001 |
| Adjusted for cancer and coronary heart disease history only | 0.251 | 0.112-0.560 | 0.001 |
| Adjusted for all the above factors | 0.246 | 0.108-0.558 | 0.001 |
Logistic regression was used to calculate odds ratios and 95% confidence intervals of urinary 6-sulfatoxymelatonin/creatinine levels between proliferative diabetic retinopathy (PDR) and the controls. Several potential confounders were adjusted, including age, gender, smoking history, cancer history and coronary heart disease.
Discussion
We found a strong inverse relationship between overnight aMT6s output and PDR risk in diabetic patients even when adjusted by several confounding factors. To our knowledge, this is the first report of decreased urinary aMT6s level in PDR patients. Measurement of plasma melatonin concentration requires drawing the patient’s blood at different times during the night, which is less desirable when compared with the method of morning urinary aMT6s measurement. The major metabolic pathway of circulating melatonin is hepatic biotransformation and the metabolites are excreted into urine. There is extensive evidence for good correlations between nocturnal urinary aMT6s and plasma melatonin level during the night [12-14]. Since different dilution of urine samples are generally not suitable for direct analysis, urine samples were normalized by urinary creatinine for comparison.
In the present study, the nocturnal urinary aMT6s level of PDR group was significantly less than that of age- and gender-matched control, NDR and NPDR groups, indicating that PDR is associated with remarkably decreased melatonin. This is in accordance with another plasma melatonin evaluation study, in which the authors found that the nocturnal plasma melatonin level is decreased only in patients with PDR but not in diabetic patients without PDR [16].
There are two possibilities for the relationship between lower nocturnal urinary aMT6s level and PDR: either PDR can interfere with the melatonin production process or a melatonin deficiency plays a role in the pathogenesis of PDR. The pineal gland is the main source of melatonin, but it is not the only melatonin synthesis location. Ocular tissues have also been shown to be capable of synthesizing melatonin [17]. Nevertheless, the local production of melatonin in ocular tissues does not seem to contribute significantly to plasma concentrations [18]. However, the phenomenon that some blind persons with lack of perception of the environmental light-dark cycle have atypical melatonin secretory patterns must be considered [19]. In current study, none of the participants had lost light perception. Therefore, it does not seem that PDR is the reason that leads to a decrease in urinary aMT6s amount.
There is increasing evidence that oxidative stress plays an important role in the pathogenesis of diabetic retinopathy. Diabetic retinopathy is associated with alterations in the structure and function of retinal microvasculature. Oxidative stress can induce biochemical changes to mediate microvasculature damage. For example, increased expression of VEGF [20] and inflammatory mediators [21], accumulation of advanced glycation end products which results in basement membrane thickening [22] and modulation of vasoactive effector molecules which leads to changes in vascular permeability and blood flow [23]. Several experimental animal and clinical studies suggest that antioxidant supplementation has beneficial effects on the prevention, treatment and control of diabetic retinopathy [24-27].
As an antioxidant, the protective effects of melatonin against the oxidative damage are well confirmed [28-31]. A major distinguishing feature of melatonin is that its metabolites also have the ability to scavenge reactive oxygen species (ROS) and reactive nitrogen species (RNS) when comparing most other antioxidant, which makes melatonin highly effective in scavenging the free radical even at low concentrations. N 1-acetyl-N 2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK) are two metabolites of melatonin; both have been found to exhibit protective effects against oxidative stress. Apart from its direct antioxidant activity, melatonin also may enhance the expression of various antioxidant enzymes via activation of melatonin receptors, such as glutathione reductase, glutathione peroxidase, γ-glutamylcysteine synthase, hemoperoxidase/catalase, glucose-6-phosphate dehydrogenase, Cu, Zn- and Mn-superoxide dismutases [32-34].
Decreased production of melatonin in PDR compared to other three groups in this study might indicate a role for melatonin deficiency in the occurrence of PDR. A relatively melatonin deficiency may cannot exert its direct or indirect antioxidant effects. Ocular cells are also able to synthesized melatonin. Therefore, melatonin levels in the eye depend on both circulating melatonin level and local melatonin production. However, the amount of melatonin produced in the eye is far less than that produced in pineal body [35]. It has been reported that melatonin protects RPE cells [36] and photoreceptors [37] from oxidative damage, whether melatonin can also prevent diabetes-induced blood-retinal barrier breakdown requires further investigation. The limitation of our study is that our sample size is not sufficiently large, further studies are needed to determine the exact relationship between melatonin and diabetic retinopathy.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (81271032).
Disclosure of conflict of interest
None.
References
- 1.Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 2.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 3.Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 4.Masue T, Wada K, Hayashi M, Takeda N, Yasuda K, Deguchi T, Nagata C. Associations of urinary 6-sulfatoxymelatonin with biomarkers related to cardiovascular disease in Japanese women. Metabolism. 2012;61:70–75. doi: 10.1016/j.metabol.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Schernhammer ES, Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer. 2004;90:941–943. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schernhammer ES, Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:74–79. doi: 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan AN, Schernhammer ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281:1–7. doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu D, Mandal T, Nandi M, Osta M, Bandyopadhyay U, Ray D. Oxidative stress in diabetic patients with retinopathy. Ann Afr Med. 2014;13:41–46. doi: 10.4103/1596-3519.126951. [DOI] [PubMed] [Google Scholar]
- 9.Naruse R, Suetsugu M, Terasawa T, Ito K, Hara K, Takebayashi K, Morita K, Aso Y, Inukai T. Oxidative stress and antioxidative potency are closely associated with diabetic retinopathy and nephropathy in patients with type 2 diabetes. Saudi Med J. 2013;34:135–141. [PubMed] [Google Scholar]
- 10.Ekmekcioglu C, Thalhammer T. Melatonin and Melatonergic Drugs in Clinical Practice. Springer; 2014. Melatonin Receptors and Their Role in Human Diseases; pp. 1–15. [Google Scholar]
- 11.Zlotos DP, Jockers R, Cecon E, Rivara S, Witt-Enderby PA. MT and MT Melatonin Receptors: Ligands, Models, Oligomers, and Therapeutic Potential. J Med Chem. 2014;57:3161–85. doi: 10.1021/jm401343c. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs J, Brodner W, Kirchlechner V, Arif T, Waldhauser F. Measurement of urinary melatonin: a useful tool for monitoring serum melatonin after its oral administration. J Clin Endocrinol Metab. 2000;85:666–670. doi: 10.1210/jcem.85.2.6349. [DOI] [PubMed] [Google Scholar]
- 13.Pääkkönen T, Mäkinen TM, Leppäluoto J, Vakkuri O, Rintamäki H, Palinkas LA, Hassi J. Urinary melatonin: a noninvasive method to follow human pineal function as studied in three experimental conditions. J Pineal Res. 2006;40:110–115. doi: 10.1111/j.1600-079X.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24:230–238. doi: 10.1111/j.1600-079x.1998.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 15.Pardue HL, Bacon BL, Nevius MG, Skoug JW. Kinetic study of the Jaffe reaction for quantifying creatinine in serum: 1. Alkalinity controlled with NaOH. Clin Chem. 1987;33:278–285. [PubMed] [Google Scholar]
- 16.Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:655–660. doi: 10.2147/OPTH.S19559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–522. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan GM, Reiter RJ. Pineal dependence of the Syrian hamster’s nocturnal serum melatonin surge. J Pineal Res. 1986;3:9–14. doi: 10.1111/j.1600-079x.1986.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewy AJ, Newsome DA. Different types of melatonin circadian secretory rhythms in some blind subjects. J Clin Endocrinol Metab. 1983;56:1103–1107. doi: 10.1210/jcem-56-6-1103. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–224. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications. 2012;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21:637–643. doi: 10.5301/EJO.2010.6212. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Horikawa C, Ohashi Y, Araki A, Ito H, Akanuma Y, Yamada N, Yamashita H, Sone H. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;24:204–211. doi: 10.1097/EDE.0b013e318281725e. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Zhang XL, Zhu BF, Ding YN. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol Biol Rep. 2012;39:3727–3735. doi: 10.1007/s11033-011-1148-9. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev. 2002;123:1007–1019. doi: 10.1016/s0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Li L, Zhao M, Chen YH, Zhang ZH, Zhang C, Ji YL, Meng XH, Xu DX. Melatonin alleviates lipopolysaccharide-induced placental cellular stress response in mice. J Pineal Res. 2011;50:418–426. doi: 10.1111/j.1600-079X.2011.00860.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 31.Bonnefont-Rousselot D, Collin F, Jore D, Gardes-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res. 2011;50:328–335. doi: 10.1111/j.1600-079X.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 32.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 33.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 34.Hardeland R. Melatonin and Melatonergic Drugs in Clinical Practice. Springer; 2014. Melatonin’s Antioxidant Properties: Molecular Mechanisms; pp. 17–26. [Google Scholar]
- 35.Chiquet C, Claustrat B, Thuret G, Brun J, Cooper HM, Denis P. Melatonin concentrations in aqueous humor of glaucoma patients. Am J Ophthalmol. 2006;142:325–327. e321. doi: 10.1016/j.ajo.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 2004;78:1069–1075. doi: 10.1016/j.exer.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Marchiafava PL, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res. 1999;26:184–189. doi: 10.1111/j.1600-079x.1999.tb00582.x. [DOI] [PubMed] [Google Scholar]


