Abstract
Mucinous tubular and spindle cell carcinoma (MTSCC) is a rare and recently recognized subtype of renal cell carcinoma (RCC). Apart from the classic morphology comprising conventional three components, there exist a large number of non-classic morphological variants of MTSCC, which make it necessity to differentiate from other RCC. Herein, we report two non-classic morphological variants of MTSCC. Case 1, a 85 years old man, showed numerous vacuoles among inherent components and cytoplasmic pallor/clearing within tubules mimicking conventional clear cell RCC with a 8.5 years follow-up, while Case 2 indicated a “mucin-poor” MTSCC associated with simultaneous conventional clear cell RCC at her age of 73 years. Until now Case 1 carries the longest disease-free survival reported in literature since MTSCC was defined and ranks the oldest since reported in literature, while Case 2 is the first report of “mucin-poor” MTSCC associated with simultaneous conventional clear cell RCC. Now, since no biomarkers or imagining tools but pathological examination can confirm the diagnosis of MTSCC, the management is always following the guideline of RCC in clinical practice. Generally, most reports consider it as a good prognosis disease, but sarcomatoid variant, even classic subtype can progress rapidly to life-threatening disease.
Keywords: Mucinous tubular and spindle cell carcinoma of the kidney, clear cell carcinoma, therapy, prognosis
Introduction
Mucinous tubular and spindle cell carcinoma, initially described (the first series) in 1998 [1], is a recently recognized subtype of renal cell carcinoma (RCC) and the diagnostic criteria of which is now well defined in the latest 2004 WHO classification of renal carcinomas [2]. Histologically, the tumor is classically composed of cords and tubules of cuboidal cells and areas of spindle cell configuration separated by a mucinous stroma. However, there are a large number of non-classic morphological variants of MTSCC, some of which are required to differentiate from other non clear RCC [3-5]. Meanwhile, owning to the rarity and recent description, the prognosis and treatment of MTSCC have not been fully elucidated, despite the fact that this tumor is generally considered to be a low-grade carcinoma having a favorable prognosis [6]. Herein, this study shows two novel cases of MTSCC. Case 1 showed numerous vacuoles among inherent components and cytoplasmic pallor/clearing within tubules mimicking conventional clear cell RCC with a 8.5 years follow-up, while Case 2 presented a “mucin-poor” morphological variants of MTSCC associated with hybrid conventional clear cell carcinoma of kidney. To date, Case 1 carries the longest disease-free survival reported in literature since MTSCC was defined and ranks the oldest since reported in literature, while Case 2 is the first report of “mucin-poor” MTSCC associated with simultaneous conventional clear cell RCC. Meanwhile, we have a literature review about the management and the prognosis of MTSCC.
Case reports
Case 1
A 85 years old man who presented with flank discomfort and hematuria was referred to performed a Computed tomography (CT). CT scan showed a solid mass and a small cystic mass in the retroperitoneum arising from the left kidney. Plain CT showed a low homogeneous density lesion (30 HU, 3.5 * 3.28 cm) relative to renal parenchyma, without any cystic change and calcification (Figure 1A). Contrast-enhanced CT showed slight homogeneous enhancement (50 HU) of the mass, without a clear boundary with adjacent renal parenchyma (Figure 1B). Few signs of vascular, adrenal or perinephric fat invasion were detected. The patients underwent left open radical nephrectomy, suffering a chronic obstructive pulmonary disease (COPD) and chronic iron-deficiency anemia (CIDA) deriving from peptic ulcer (Hb 95 g/l). The specimen received consisted of a left kidney with a 3 × 3.5 cm, predominantly cortical-based poorly circumscribed tumor arising from the upper part of the kidney. The tumor was variegated tan, yellow, and gray-white, focally extending into renal hilum and compressing the Gerota’s fascia and pelvis but not invading them. The calyces and the ureter were not involved by the tumor. The tumor was extensively sampled. Sections were fixed in 10% neutral-buffered formalin and processed for light microscopy via conventional methods. Routine processing for histologic examination included paraffin embedding, sectioning, and staining with hematoxylin and eosin. Sections prepared from the formalin-fixed, paraffin-embedded tissue blocks were also used for immunohistochemical analysis. Generally, the renal tumor had a uniform microscopic appearance composed of cuboidal tubular tissue and spindle cells embedded by mucinous stroma Figure 2A). The tubules and cords were lined by round to cuboidal cells of relatively bland morphology. The low-grade cell was associated with amphophilic cytoplasm and small uniform nuclei (Figure 2B). There were abrupt transitions between tubular structures and spindled areas composed of similar cells (Figure 2A), which were banal-appearing and often arranged in short fascicles (Figure 2C). Mitoses were very rare and numerous small vacuoles mimicking clear cells (Figure 2C). Prominent aggregates of foamy macrophages around spindle cells were found between cuboidal cells (Figure 3A). Focal cytoplasmic pallor or deletion conveyed a clear cell change, with some morphologically identical to conventional RCC, which were found in no more than one-eighth of this series (Figure 3B and 3C). The tumor cells in the more typical MTSCC areas showed strong and diffuse positivity for CK19, CK7 (Figure 4A), CAM5.2, Vimentin (Figure 4B), E–Cadherin, AMACR (P540S) (Figure 4C), CD10. The tumor cells were diffusely and weakly positive for 34βE12. In addition, the Ki-67 stain was less than 5%. The RCC antibody, CK20, CD15 stains were negative. Primary antibodies were applied as described in Table 1 and the complete immunohistochemistry results were present in Table 2. The patient received regular outpatient follow-up, and no local recurrence or distant metastasis has been found 8.5 years after resection.
Figure 1.
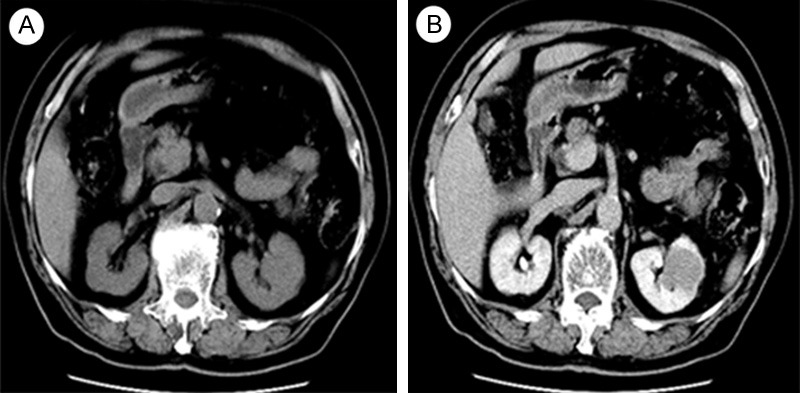
A: Plain CT image shows a solid tumor in the upper pole of left kidney with homogeneous density, measuring 3.5 * 3.28 cm in size. B: Contrast-enhanced CT images show slight enhancement of the mass but was less enhanced compared with normal renal parenchyma, without any cystic change or calcification.
Figure 2.
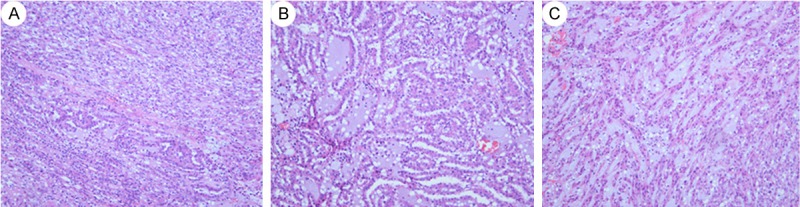
A: Typical MTSCC areas showes tubules, cords and abundant myxoid stroma. There were abrupt transitions between tubular structures and spindled areas composed of similar cells. (H&E, 40). B: The tubules and cords was lined by round to cuboidal cells of relatively bland morphology. The low–grad cell was associated with amphophilic cytoplasm and small uniform nuclei (H&E, 100). C: The spindled areas composed of banal-appearing cells, which were often arranged in short fascicles. Numerous small vacuoles mimicking clear cells. Mitoses were very rare. (H&E, 100).
Figure 3.
A: Prominent aggregates of foamy macrophages around tubules and cords. (H&E, 100). B&C: Clear cell changes of MTSCC with some partially morphologically identical to conventional renal cell carcinoma (H&E, 200 and 400).
Figure 4.

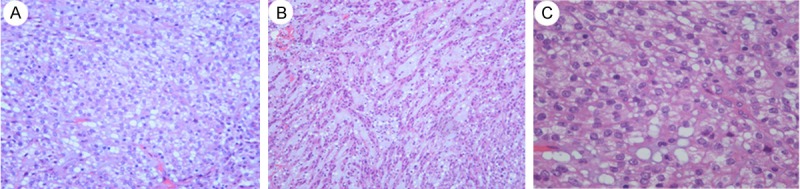
A: Strong and diffuse CK7 positivity in the tumor. B: The positive staining of Vimentin in the kidney tumor. C: Immunopositivity for P504S.
Table 1.
Primary antibodies and concentrations used for immunohistochemistry
| Antibodies | Dilution | Company |
|---|---|---|
| CK7 | 1:100 | Dako, Glostrup, Denmark |
| CK19 | 1:100 | Dako |
| CK20 | 1:100 | Dako |
| P504s | 1:200 | Dako |
| RCC | 1:200 | Dako |
| Ki-67 | 1:150 | Dako |
| 34βE12 | 1:50 | Dako |
| Vimentin | 1:200 | Dako |
| E-Cadherin | 1:100 | Dako |
| CAM5.2 | pre-diluted | GeneTech, Shanghai, China |
| CD10 | 1:50 | Leica |
| CD15 | 1:30 | Novocastra |
Table 2.
Immunohistochemical characteristics of the two case
| Antibodies | Case 1 | Case 2 |
|---|---|---|
| CAM5.2 | ++ | ++ |
| 34βE12 | + | ++ |
| CK7 | ++ | ++ |
| CK19 | ++ | ++ |
| CK20 | -- | -- |
| Vimentin | ++ | ++ |
| CD15 | -- | -- |
| CD10 | ++ | ++ |
| RCC | -- | -- |
| P5O4s | ++ | ++ |
| E-Cadhesin | ++ | ++ |
| Ki-67 | <5% | <5% |
++ strong positive; + positive; -- negative.
Case 2
A 63-year-old woman was referred for an abdominal ultrasonography when going for a medical examination. Ultrasound examination showed a retroperitoneal mass arising from the middle part of the left kidney, with no significant vascularity demonstrable on Doppler ultrasound. There were no symptoms of hematuria, voiding dysfunction, flank pain, or weight loss. Subsequently, MR imaging was performed and confirmed the presence of a 2.9 × 2.2 × 2.8 cm well-marginated mass on her left kidney, with a homogeneous low signal on T1-weighted imaging (Figure 5A) and an intermediate to high signal on T2-weighted imaging (Figure 5B). After intravenous (IV) injection of the contrast medium, there was a diffuse enhancement and most of the lesion was hypovascular compared to the adjacent cortex (Figure 5C). On T1-weighted imaging, before and after contrast, a central small scar could be defined (Figure 5A and 5C). There were no signs of vascular, adrenal or perinephric fat invasion. The patient underwent a left retroperitoneal laparoscopic radical nephrectomy. The left radical nephrectomy specimen measured 11 × 4 × 8.4 cm with a 3 * 2.5 * 2.5 cm, fleshy tan-yellow tumor located near the middle part of the kidney and extending to the medulla and focally into the renal sinus. The renal pelvis, ureter and hilar vessels were identified and showed no direct involvement by tumor. The tumor was extensively sampled, routine processed for histologic examination and immunohistochemical analysis as Case 1. The renal tumor has a uniform microscopic appearance. Nearly two thirds of the tumor was composed of small elongated and occasionally angulated tubules, bland spindle cells and limited myxoid stroma Figure 6A-C). There was abrupt transition between the main two components and the tubular structures were lined by low-grade of cell of flattened, sparse cytoplasm and regular small elongated nuclei, which merge gradually to banal-appearing spindle-shaped with lightly eosinophilic cytoplasm (Figure 6D). Meanwhile, a clear cell component morphologically identical to conventional clear cell RCC was found (Figure 7A and 7B). It comprised approximately one-third of the tumour bulk and the tumour cell was of grade II-III of Fuhrman’s grading system (Figure 7C). The tumor cells in the more typical MTSCC areas showed strong positivity for CK19, CK7 (Figure 8A), CAM5.2, Vimentin (Figure 8B), E-Cadherin, AMACR (P540S) (Figure 8C), 34βE12. The tumor cells were diffusely and weakly positive for CD10. In addition, the Ki-67 stain was less than 5%. The RCC, CK20, CD15 stains were negative. The immunohistochemical analysis was recorded in Tables 1 and 2. The patient is currently alive and well, 8 months after surgery. The imagining and biochemical tests show no evidence of recurrence or metastasis.
Figure 5.
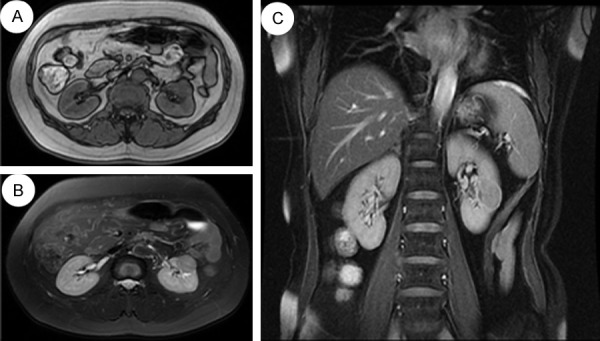
A: MR imaging confirmed the presence of a 2.9 × 2.2 × 2.8 cm well-marginated mass on left kidney, with a homogeneous low signal on T1-weighted imaging. B: An intermediate to high signal on T2-weighted imaging. C: After intravenous (IV) injection of the contrast medium, there was a diffuse enhancement and most of the lesion was hypovascular compared to the adjacent cortex. On T1-weighted imaging, pre- and postcontrast, a central small scar could be defined.
Figure 6.
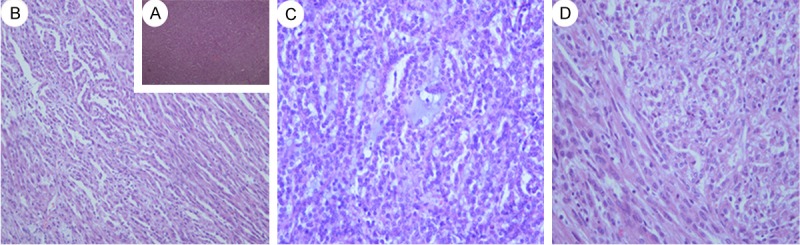
A: “mucin-poor” MTSCC exhibits tubules, cords and limited myxoid stroma. (H&E, 40); B: “mucin-poor” MTSC with elongated to serpentine tubules. The tubular structures were lined by low-grad of cell of flattened, sparse cytoplasm and regular elongated nuclei, which merge gradually to banal-appearing spindle-shaped with lightly eosinophilic cytoplasm. The nuclei were small, spherical nd regular (H&E, 100). C: Limited myxoid stroma was observed in the center of the picture (H&E, 200). D: The transitions between the two components were abrupt (H&E, 200).
Figure 7.
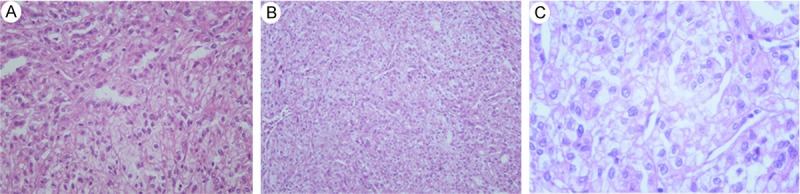
A: Tumor cells containing clear cytoplasm that exhibited histological features similar to clear cell carcinoma adjacent to the inherent components of MTSCC in the right of the picture. (H&E, 100). B&C: Nuclei were generally round and centrally located. Single distinct nucleoli were occasionally seen. Nuclear pleomorphism or abnormal mitotic figures were occasionally identified. The renal cell carcinoma component was of grade II-III of Fuhrman’s grading system (H&E, 100 and 400).
Figure 8.
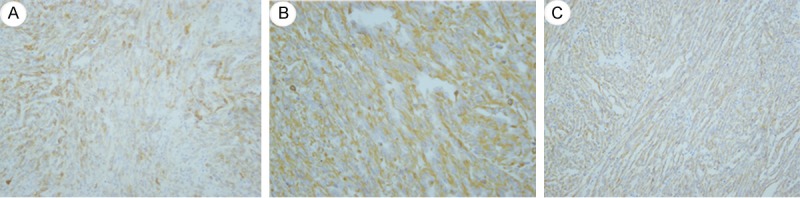
A: Strong and diffuse CK7 positivity in the tumor. B: Positive staining of Vimentin in the kidney tumor. C: Immunopositivity for P504S.
Discussion
MTSCC accounts for no more than 1% of all the renal neoplasms with less than 100 cases reported so far in the literature [7-9]. It affects patients from 1 to 82 years old with a mean age of 58 years and has a strong female predominance (with a male to female ratio of 1:4) [10]. Our first case was 85 years old when confirmed pathologically and is still alive at almost 94 in spite of suffering COPD. The majority of these tumors are accidentally detected when abdominal imaging studies were performed due to other indications [11]. Occasionally, when the mass are large enough, the patients may present with flank pain or hematuria [2]. The size of MTSCC may range from less than 1.0 cm in diameter to more than 18.0 cm, the majority of which measure 2.0 to 4.0 cm in the longest axis [12].
The diagnosis of MTSCC is mainly based on histological and morphological grounds, and immunohistochemical studies may show mostly specifically positive for RCC marker antigen, Vimentin, CK7, and AMACR [7,13,14]. Histologically, the MTSCC are characterized by admixture of low-grade tubular cuboidal cells with array of banal-appearing spindle cells embedded in the mucinous background. There are abrupt transitions between this component and spindled areas composed of similar cells. Non-classic morphological variants of MTSCC include foamy macrophages, papillations or well formed papillae focal clear cells in tubules, necrosis, oncocytic tubules, numerous small vacuoles, heterotopic bone, psammomatous calcification, nodular growth with lymphocytic cuffing and neuroendocrine differentiation [3-5]. Besides the inherent components, Case 1 showed numerous small vacuoles and focal cytoplasmic pallor or deletion within tubules morphologically identical to conventional clear cell RCC, while Case 2 indicated a “mucin-poor” change associated with simultaneous conventional clear cell RCCs. Actually, cells with optically clear cytoplasm are most commonly associated with clear cell RCC. Despite the cytoplasm in MTSCC is typically minimal and eosinophilic [2,15], cytoplasmic pallor/clearing within tubules (no less than four cases [3,16]) and numerous small vacuoles (no less than three cases [3,6]) may focally present a “clear cell” appearance in these MTSCC. Therefore, several reports [3,15,17,18] claimed that it is not sufficient to cause diagnostic confusion with clear cell RCC. The clear cell appearance of Case 1 had not been described before 2004 in our clinical center and it was considered not to be clear cell RCCs but small vacuoles and cytoplasmic pallor/clearing within tubules. However, apart from the typical morphologic characteristics of the clear cell RCCs, the neoplasm of clear cells account for a large part of the mass in Case 2, which in all lead to the diagnosis of a hybrid tumor of MTSCC. Until now 2 cases of MTSCC associated with extensive clear cells either resembling [4] or morphologically identical to [19] clear cell RCC have been reported. Hes O and colleagues described it as a hybrid tumor [19], while Kuroda N and colleagues [4] claimed that foci of clear cells should be added to the histological spectrum of MTSCC. But, some researchers [3] believed that cases showing overall architecture of MTSC, but with definitive areas typical of clear cell RCC are currently best designated as “unclassified”, until the origin and long-term biologic potential of the tumors is better understood. However, it is possible that other diseases (including other renal mass) may coexist with MTSCC, as evidenced by 2 case with angiomyolipoma [3,16], 5 cases with renal cysts [6,16,20] including ours, 1 case of papillary RCC [20], no less than 5 cases with nephrolithiasis including one case in our report [16,19]. The real connection between MTSCCs and coinstantaneous other RCCs has not been interpreted clearly.
Meanwhile, Case 2 presented a “mucin-poor” appearance (apart from the “clear cell” area). To date no less than 8 cases of MTSCC of this variation have been reported [3,9]. Fine SW and colleagues [3] reported the morphology of 7 mucin poor of 17 MTSCC cases, with equal tubular and spindled areas (n = 4), spindle cell predominance (n = 2), or tubular predominance (n = 1). Farghaly H and colleagues [9] described that approximately 95% of their case was composed of spindle cell carcinoma cells. However, despite it is spindle cell predominance in Case 2, the mucin is quite poor (<1% of all components of MTSCC) in all slices. As most regard the typically abundant extracellular matrix as a key to MTSCC diagnosis [18,19,21], its absence makes accurate diagnosis of this entity more challenging. Nevertheless, it is indicated that the three components are present in variable proportions, with one or two predominance and individual cases of MTSCC may show uniform spindle cells with bland nuclear features, but lack in cords/tubules and mucinous background [22], so we are inclined to consider it as “mucin-poor” change of MTSCC in spite of being concomitant other non-classic variants. It is, therefore, critical for pathologists to recognize that “mucin-poor” variants of MTSCC exist.
Because of the rarity and recent description of MTSCC, few reports have focused on the treatment. Meanwhile, there is no biomarkers or imagining tools but pathological examination can confirm the diagnosis of MTSCC. Therefore, we used to follow the guideline of RCCs to deal with this kind of tumor in clinical practice [23]. Partial nephrectomy or radical nephrectomy is mostly performed via open approach, few cases are done with laparoscopic or coelioscopic robot-assisted approaches. Herein, we present two cases of MTSCC resected by open radical nephrectomy (Case 1) or by laparoscopic radical nephrectomy (Case 2), respectively. Case 1 is still well and alive without any evidence of recurrence or metastasis when following for 8.5 years which may rank first related to followups reported in the literature since 2004, while Case 2 is well after 8 months of resection of tumor. Although Case 2 has not given any medical intervention except active surveillance, Case 1 had received Proleukin (a recombinant human IL-2) for three months. As is indicted in the ESMO guideline of RCCs [23], partial nephrectomy can be performed via open, laparoscopic or coelioscopic robot assisted approaches, especially for patients with compromised renal function, solitary kidney or bilateral tumors, with no tumor size limitation (imperative indication). MacLennan S and colleagues [24] declared that laparoscopic radical nephrectomy is recommended if partial nephrectomy is not technically feasible. The two patients in our research were fully informed consent and received the strategy, which took the guideline [23], the general condition of patients and patients’ will into consideration. Simon RA and colleagues [14] report one case of MTSCC with two other identified lesions in the thoracic vertebral bodies, which received high-dose steroids with minimal neurological response and underwent a CT-guided biopsy of the mass. Then, the patient received radiotherapy followed by tumor embolization and a radical nephrectomy with vertebral body resection. However, follow-up imaging studies showed additional vertebral body lesions, a parietal bone lesion, liver lesions, and possible malignant pleural effusions and the patient died three weeks after surgery. Radio frequency or cryoablative treatments are alternative approaches for RCC, which have not been reported for MTSCC in literatures. Meanwhile, Larkin J and colleagues [25] founded that tumor shrinkage occurred in response to sunitinib in the biopsy-proven MSTCC retroperitoneal lymph node lesion.
Despite that MTSCC is a rare renal lesion, the majority of MTSCC have a favorable prognosis [6]. However, to date no less than 8 cases have been went though recurrence [18], metastasis to regional lymph nodes [6,13,19,25], or distant metastasis [10,13,25,26]. About six case have been dead since reported [6,10,13,14,19,26] with a follow up from 3 week to 5 years, four of them dying from distance metastasis [10,13,14,26], one case dying from unrelated condition [19], and one case dying from unknown reason [6]. Sarcomatoid transformation reflects areas of high-grade dedifferentiation in renal epithelial tumors and generally indicates a worse prognosis with shorter disease-free survival and earlier, more frequent metastasis [27]. Four [10,13,14,18] of the six reported cases [10,13,14,18,28] of sarcomatoid change in RCC have been described to be outgo through recurrence, regional lymph nodes metastasis or distance metastasis, three [10,13,14] of which have been dead since reported, with a follow up of 3 weeks to 9 month. In spite of the case alive reported by us, the prognosis is still unclear for clear cell change of MTSCC or MTSCC with clear cell morphologically identical to conventional clear cell RCC. Hes O and colleagues [19] reported a case of MTSCC with clear cell morphologically identical to conventional RCC. Although no regional lymph nodes or distance metastasis before and after resection, the patient died of unrelated condition two years after resection. So the long-term biologic potential of clear cell change of MTSCC requires further study. colleagues [26] reported a common subtype of MTSCC which developed multiple metastatic pulmonary nodules 2 months after resection, then was found to have the left supraclavicular, right hilar and right subcarinal lymph nodes metastasis four months after the surgery and the patient died from respiratory failure 13 months after the operation. The prognosis data of other variants of MTSCC is relatively poor.
In summary, we have reported two cases non-classic morphological variants of MTSCC. Case 1 showed numerous vacuoles among inherent components and cytoplasmic pallor/clearing within tubules mimicking conventional clear cell RCC with the longest follow-up since MTSCC was defined, while Case 2 was the first report of a “mucin-poor” MTSCC associated with simultaneous conventional clear cell RCC. The current study also enriches the information of the management and the prognosis of MTSCC.
Acknowledgements
Thanks to Xiao-Chun Fei for the outstanding immunohistochemical work of these cases.
Disclosure of conflict of interest
None.
References
- 1.He Q, Ohaki Y, Mori O, Asano G, Tuboi N. A case report of renal cell tumor in a 45-year-old female mimicking lower portion nephrogenesis. Pathol Int. 1998;48:416–420. doi: 10.1111/j.1440-1827.1998.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 2.Srigley JR. Mucinous tubular and spindle cell carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 3.Fine SW, Argani P, DeMarzo AM, Delahunt B, Sebo TJ, Reuter VE, Epstein JI. Expanding the histologic spectrum of mucinous tubular and spindle cell carcinoma of the kidney. Am J Surg Pathol. 2006;30:1554–1560. doi: 10.1097/01.pas.0000213271.15221.e3. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda N, Nakamura S, Miyazaki E, Hayashi Y, Taguchi T, Hiroi M, Yamasaki Y, Shuin T, Enzan H. Low-grade tubular-mucinous renal neoplasm with neuroendocrine differentiation: a histological, immunohistochemical and ultrastructural study. Pathol Int. 2004;54:201–207. doi: 10.1111/j.1440-1827.2004.01608.x. [DOI] [PubMed] [Google Scholar]
- 5.Jung SJ, Yoon HK, Chung JI, Ayala AG, Ro JY. Mucinous tubular and spindle cell carcinoma of the kidney with neuroendocrine differentiation: report of two cases. Am J Clin Pathol. 2006;125:99–104. [PubMed] [Google Scholar]
- 6.Ferlicot S, Allory Y, Comperat E, Mege-Lechevalier F, Dimet S, Sibony M, Couturier J, Vieillefond A. Mucinous tubular and spindle cell carcinoma: a report of 15 cases and a review of the literature. Virchows Arch. 2005;447:978–983. doi: 10.1007/s00428-005-0036-x. [DOI] [PubMed] [Google Scholar]
- 7.Shen SS, Ro JY, Tamboli P, Truong LD, Zhai Q, Jung SJ, Tibbs RG, Ordonez NG, Ayala AG. Mucinous tubular and spindle cell carcinoma of kidney is probably a variant of papillary renal cell carcinoma with spindle cell features. Ann Diagn Pathol. 2007;11:13–21. doi: 10.1016/j.anndiagpath.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Shanbhogue AK, Vikram R, Paspulati RM, MacLennan G, Verma S, Sandrasegaran K, Prasad SR. Rare (<1%) histological subtypes of renal cell carcinoma: an update. Abdom Imaging. 2012;37:861–872. doi: 10.1007/s00261-011-9810-1. [DOI] [PubMed] [Google Scholar]
- 9.Farghaly H. Mucin poor mucinous tubular and spindle cell carcinoma of the kidney, with nonclassic morphologic variant of spindle cell predominance and psammomatous calcification. Ann Diagn Pathol. 2012;16:59–62. doi: 10.1016/j.anndiagpath.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Arafah M, Zaidi SN. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid transformation. Saudi J Kidney Dis Transpl. 2013;24:557–560. doi: 10.4103/1319-2442.111066. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Breyer BN, Weiss DA, MacLennan GT. Mucinous tubular and spindle cell carcinoma of the kidney. J Urol. 2010;183:738–739. doi: 10.1016/j.juro.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srigley JR, Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol. 2009;22(Suppl 2):S2–S23. doi: 10.1038/modpathol.2009.70. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon J, Amin MB, Selbs E, Turi GK, Paner GP, Reuter VE. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid change. Am J Surg Pathol. 2009;33:44–49. doi: 10.1097/PAS.0b013e3181829ed5. [DOI] [PubMed] [Google Scholar]
- 14.Simon RA, di Sant’agnese PA, Palapattu GS, Singer EA, Candelario GD, Huang J, Yao JL. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid differentiation. Int J Clin Exp Pathol. 2008;1:180–184. [PMC free article] [PubMed] [Google Scholar]
- 15.Parwani AV, Husain AN, Epstein JI, Beckwith JB, Argani P. Low-grade myxoid renal epithelial neoplasms with distal nephron differentiation. Hum Pathol. 2001;32:506–512. doi: 10.1053/hupa.2001.24320. [DOI] [PubMed] [Google Scholar]
- 16.Hussain M, Ud Din N, Azam M, Loya A. Mucinous tubular and spindle cell carcinoma of kidney: a clinicopathologic study of six cases. Indian J Pathol Microbiol. 2012;55:439–442. doi: 10.4103/0377-4929.107776. [DOI] [PubMed] [Google Scholar]
- 17.Aubert S, Duchene F, Augusto D, Llinares K, Lemaitre L, Gosselin B, Leroy X. Low-grade tubular myxoid renal tumors: a clinicopathological study of 3 cases. Int J Surg Pathol. 2004;12:179–183. doi: 10.1177/106689690401200216. [DOI] [PubMed] [Google Scholar]
- 18.Rakozy C, Schmahl GE, Bogner S, Storkel S. Low-grade tubular-mucinous renal neoplasms: morphologic, immunohistochemical, and genetic features. Mod Pathol. 2002;15:1162–1171. doi: 10.1097/01.MP.0000031709.40712.46. [DOI] [PubMed] [Google Scholar]
- 19.Hes O, Hora M, Perez-Montiel DM, Suster S, Curik R, Sokol L, Ondic O, Mikulastik J, Betlach J, Peychl L, Hrabal P, Kodet R, Straka L, Ferak I, Vrabec V, Michal M. Spindle and cuboidal renal cell carcinoma, a tumour having frequent association with nephrolithiasis: report of 11 cases including a case with hybrid conventional renal cell carcinoma/spindle and cuboidal renal cell carcinoma components. Histopathology. 2002;41:549–555. doi: 10.1046/j.1365-2559.2002.01515.x. [DOI] [PubMed] [Google Scholar]
- 20.Paner GP, Srigley JR, Radhakrishnan A, Cohen C, Skinnider BF, Tickoo SK, Young AN, Amin MB. Immunohistochemical analysis of mucinous tubular and spindle cell carcinoma and papillary renal cell carcinoma of the kidney: significant immunophenotypic overlap warrants diagnostic caution. Am J Surg Pathol. 2006;30:13–19. doi: 10.1097/01.pas.0000180443.94645.50. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan GT, Bostwick DG. Tubulocystic carcinoma, mucinous tubular and spindle cell carcinoma, and other recently described rare renal tumors. Clin Lab Med. 2005;25:393–416. doi: 10.1016/j.cll.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Lloreta J, Corominas JM, Munne A, Dominguez D, Bielsa O, Gelabert A, Serrano S. Low-grade spindle cell carcinoma of the kidney. Ultrastruct Pathol. 1998;22:83–90. doi: 10.3109/01913129809032262. [DOI] [PubMed] [Google Scholar]
- 23.Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii65–71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 24.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, Royle P, Stewart F, MacLennan G, MacLennan SJ, Canfield SE, McClinton S, Griffiths TR, Ljungberg B, N’Dow J. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Larkin J, Fisher R, Pickering L, Thway K, Livni N, Fisher C, Gore M. Metastatic mucinous tubular and spindle cell carcinoma of the kidney responding to sunitinib. J. Clin. Oncol. 2010;28:e539–540. doi: 10.1200/JCO.2010.30.1457. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Oh MH, Cho HD, Kim YS. Mucinous Tubular and Spindle Cell Carcinoma of the Kidney with Aggressive Behavior: An Unusual Renal Epithelial Neoplasm-A Case Report. Korean Journal of Pathology. 2010;44:211–215. [Google Scholar]
- 27.Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pillay N, Ramdial PK, Cooper K, Batuule D. Mucinous tubular and spindle cell carcinoma with aggressive histomorphology--a sarcomatoid variant. Hum Pathol. 2008;39:966–969. doi: 10.1016/j.humpath.2007.10.006. [DOI] [PubMed] [Google Scholar]


