Abstract
The pathogenesis of severe human monkeypox, which causes systemic and fulminant infections, is not clear. This study presents a case repot of fulminant monkeypox with bacterial sepsis after experimental infection with monkeypox virus in a cynomolgus monkey (Macaca fascicularis). In our previous study (Saijo et al., 2009, J Gen Virol), two cynomolgus monkeys became moribund after experimental infection with monkeypox virus Liberia strain, West African strain. One exhibited typical monkeypox-related papulovesicular lesions. The other monkey presented fulminant clinical symptoms with a characteristic flat red rash similar to that found in smallpox, which is associated with extremely high fatality rates. In this study, we found that the monkey with flat red rash had high levels of viremia and neutropenia, as well as high plasma levels of pro-inflammatory cytokines and chemokines compared with the other monkey. Monkeypox virus replicates in epithelial cells and macrophages in various organs. Sepsis due to Gram-positive cocci was confirmed histopathologically in the monkey with flat red rash. The lack of inflammatory response in the lesion suggested that the monkey with sepsis experienced strong immune suppression during the viral infection. The neutropenia and excessive inflammatory cytokine responses indicate that neutrophils play key roles in the pathogenesis of systemic and fulminant human monkeypox virus infections with sepsis.
Keywords: Animal model, cynomolgus monkey, cytokine, monkeypox, neutropenia
Introduction
Human monkeypox, i.e., monkeypox virus infection of humans, is the most important orthopoxvirus infection at present now that smallpox, which is caused by Variola virus, has been eradicated. Monkeypox virus is a zoonotic agent in rodents, nonhuman primate species, and humans. Outbreaks of human monkeypox occur occasionally in remote villages in central and western Africa near tropical rainforests [1-6]. In 2003, an outbreak of human monkeypox with fever and skin eruptions occurred in Midwestern USA. The patients were found to be infected with a West African strain of monkeypox virus caused by close contact with sick pet prairie dogs (Cynomys spp.), which may have been in contact with wild rodents imported from Ghana [7,8]. There were no deaths during the USA outbreak, but some pediatric patients developed serious complications that could have resulted in death [8]. The disease course is often milder than that of smallpox. However, the mortality rate with monkeypox infection has been reported as 1.5-17% in Africa [3].
The clinical features of classical smallpox with a high mortality rate, i.e., Variola major, are classified as ordinary type, modified type, Variola sine eruptione, hemorrhagic type, and flat type [9,10]. The hemorrhagic type, with widespread hemorrhages in the skin and mucous membranes, and the flat type, where the pustules remain flat, were usually fatal. The pathophysiological processes of these fulminant types of smallpox are not well understood. Cynomolgus (Macaca fascicularis) and rhesus macaques have been used as nonhuman primate models of smallpox and orthopoxvirus infections [11-17].
The virulence and pathophysiology of monkeypox two strains, Zr-599 and Liberia (Congo Basin and West African monkeypox virus strains, respectively), were evaluated in cynomolgus monkeys [18], which showed that Zr-599 was more virulent than Liberia. The clinically advanced stage was characterized by poxviral papulovesicular rashes on days 7-9 post-inoculation (p.i.). Three Zr-599-inoculated monkeys and one Liberia-inoculated monkey were moribund during days 13-18 p.i. The other monkeys recovered and survived for the observational period of 22 days p.i. However, one Liberia-inoculated monkey did not exhibit typical rashes, although it was debilitated. A postmortem pathological examination on day 10 p.i. showed that the monkey had sepsis with Gram-positive cocci. The present study investigated the clinical, immunological, and histopathological features of the monkey with fulminant monkeypox to elucidate the pathogenesis and risk factors related to this severe orthopoxvirus infection with sepsis.
Materials and methods
Animal experiments
This study used four adult cynomolgus monkeys (Tsukuba Primate Research Centre, National Institute of Biomedical Innovation, Tsukuba, Japan), which were infected with monkeypox virus Liberia strain at a dose of 106 plaque-forming units via subcutaneous injection, as reported previously [18]. After experimental infection, the animals were examined until 21 days p.i. to assess their clinical manifestations and hematological parameters. During the clinical observation period, two moribund animals (#4567 and #4625) were sacrificed under anesthesia and autopsied to perform pathological examinations on days 10 and 18 p.i. The complete blood cell counts in the peripheral blood collected in sodium heparin-treated tubes were measured using an autoanalyzer (Cell Tuck, Nihon Koden, Tokyo, Japan), and the neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts were determined by microscopic analysis. To evaluate the immune responses of the monkeys infected with monkeypox virus, the cytokine and chemokine levels in the plasma were measured using a Luminex 100TM (Luminex Co., Austin, TX) with a Human Cytokines 25-plex Antibody Bead Kit (BioSource Invitrogen, Camarillo, CA), according to the manufacturer’s instructions. All of the infection experiments using cynomolgus monkeys were conducted under biosafety level 3 conditions according to the guidelines for animal experiments at the National Institute of Infectious Diseases. All of the procedures used in this study were approved as biosafety level 3 and 2 by the Committees for Biosafety, Animal Experiments, and Handling, and the Ethical Regulations of the National Institute of Infectious Diseases, Tokyo, Japan.
Histopathology, immunohistochemistry, and double-immunofluorescence staining
Paraffin-embedded tissues, including skin, lymph node, spleen, thymus, lung, heart, liver, kidney, intestine, testis, and brain, were used for the histopathological examinations and for the immunohistochemical detection of monkeypox virus antigen using anti-Vaccinia virus rabbit antibody, which was prepared as described previously [19]. The antigen was retrieved by autoclaving for 15 min at 121°C in citric acid solution at pH 6.0. Formalin-fixed lung tissue was subjected to electron microscopic analysis using Epon 812-embedded ultra-thin sections. To characterize the virus-infected cells, paraffin-embedded tissues were subjected to a double-immunofluorescence staining procedure using a rabbit antiserum against Vaccinia virus, cytokeratin monoclonal mouse antibody (clone MAB1611, Chemicon, CA), and a monocyte monoclonal mouse antibody (clone Mac387). The antigens were retrieved by autoclaving in the retrieval solution at 121°C for 15 min, at pH 9.0 (Nichirei, Tokyo, Japan). The sections were incubated with the first monoclonal antibody against cytokeratin or monocytes, followed by incubation with antiserum against the Vaccinia virus antigen. The virus antigen-binding sites, and cytokeratin and monocytes were detected using goat anti-rabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR) and goat anti-mouse Alexa Fluor 586 (Molecular Probes), respectively. The sections were incubated with the antibodies for 30 min at 37°C, before mounting in SlowFade Gold antifade reagent with 4’,6-diamidino-2-phenylindole (DAPI) (Molecular Probes) and the images were captured using a fluorescence microscope (IX71; Olympus, Tokyo, Japan) equipped with a Hamamatsu high-resolution digital B/W CCD camera (ORCA2; Hamamatsu Photonics, Hamamatsu, Japan).
Results
Clinical features of fulminant monkeypox
All four monkeys exhibited clinical illness after subcutaneous inoculation with Liberia, including low activity, lethargy, and rash. Three monkeys had typical poxviral papulovesicular rashes on days 7-9 p.i. (Figure 1A, left panel). One monkey (#4625) was moribund on day 18 p.i. but two monkeys (#4639 and #4650) survived until the end of the 3-week clinical observation period. However, one monkey (#4567) had a red rash without the typical papulovesicular rash evolution, which is generally observed in ordinary type monkeypox, and it was moribund on day 10 p.i. (Figure 1A, right panel). Analysis of the virus genome in the serum using quantitative real-time PCR, as reported previously [19], showed that the peak viremia level of the recovered monkeys was <107 gene copies/ml on day 7 p.i., whereas that of the moribund monkey (#4567) was >108 gene copies/ml on day 10 p.i. (Figure 1B). In addition, the peak viremia remained continuous in another moribund monkey (#4625) after 7 days p.i. The total blood cell count showed that moribund monkey #4567 had counts of <5,000 neutrophils/µl during the observation period (Figure 1B). By contrast, the other three monkeys had counts >5,000 neutrophils/µl, which peaked at around 15,000 neutrophils/µl at day 15 p.i. The neutrophil level decreased suddenly in #4652 monkey on day 14 p.i. and the animal was moribund. Interestingly, the lymphocyte counts increased in all of the monkeys at day 10 p.i., including the moribund monkey.
Figure 1.

Clinical course, including the rash, viremia, and hemograms, of cynomolgus monkeys (#4639 and #4650, recovered monkeys; #4567 and #4625, moribund monkeys) after subcutaneous inoculation with monkeypox virus. A: Clinical images showing the rash on a surviving monkey (#4650, left panel) and a moribund monkey (#4567, right panel) on days 9 or 10 post-inoculation. B: The viral loads (number of copies/ml) in the whole blood of monkeys were measured using quantitative real-time PCR. Time course of the hematological changes in the white blood cell, neutrophil, lymphocyte, and monocyte counts (number of cells/μl) of the monkeys.
Plasma cytokine levels
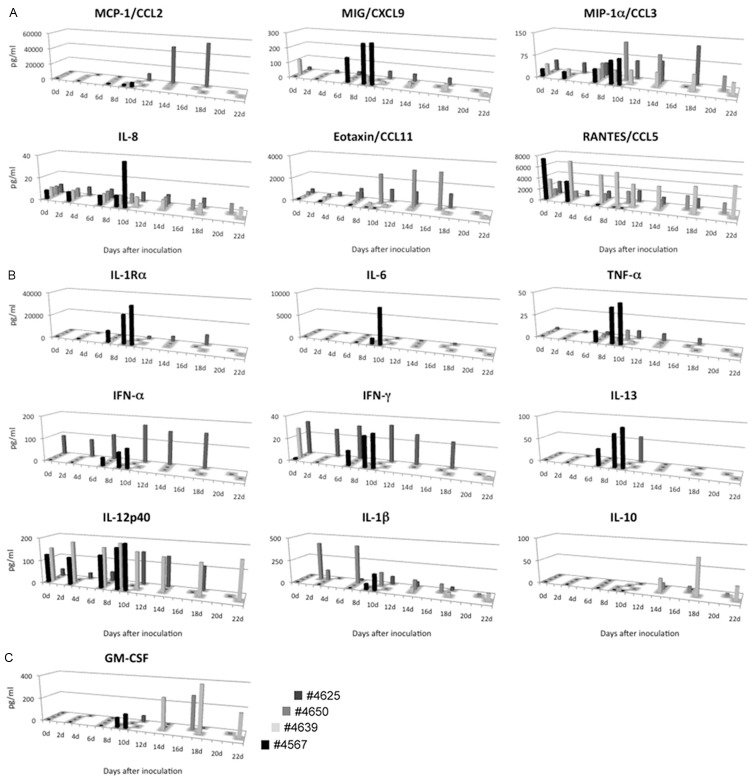
Moribund monkey #4567 had high concentrations of monocyte chemoattractant protein 1 (MCP-1/CCL2), IL (interleukin)-1-receptor antagonist (IL-Ra), and IL-6 in its plasma at the moribund endpoint on days 9 and 10 p.i. (Figure 2A, 2B). Moribund monkey #4625 had extremely high concentrations of MCP-1/CCL2 and macrophage inflammatory protein-1 α (MIP-1α/CCL3) in the plasma at the moribund endpoint on days 10, 14, and 18. The concentrations of monokine-induced interferon-γ (MIG/CXCL9), IL-8, tumor necrosis factor-α (TNF-α), interferon (IFN)-α, IFN-γ, and IL-13 in moribund monkey #4567 were at least three times higher than those in the surviving monkeys at days 9 or 10 p.i. (Figure 2A, 2B). The levels of IFN-α increased in moribund monkey #4625 after 12 days p.i. The levels of anti-inflammatory cytokine IL-10 were undetectable within 10 days p.i. in all monkeys, but they increased after 14 days p.i. in the surviving monkeys (Figure 2B). The high levels of MIP-1α/CCL3 and IL-12 p40 in the plasma were the same as those found in the surviving monkeys after the infection, whereas the level of regulated on activation normal T cell expressed and secreted (RANTES/CCL5) was lower in monkey moribund #4567 (Figure 2A, 2B). These results show that the levels of macrophage-, neutrophil-, and IFN-related pro-inflammatory chemokines and cytokines were elevated significantly at the moribund endpoint in monkey #4567.
Figure 2.
Changes in the chemokine (A), cytokine (B), and colony-stimulating factor (C) responses of cynomolgus monkeys after subcutaneous monkeypox inoculation.
Histopathological features of fulminant monkeypox
Moribund monkey #4625 had a typical poxviral papulovesicular rash, where the histological findings indicated that the rash was swollen with necrotic epithelial layers and severe inflammatory reactions (Figure 3A, 3B). Numerous neutrophils, mononuclear cells, and poxvirus antigen-positive cells were detected in the lesion (Figure 3B, 3C). The histopathology detected swollen keratinocytes (ballooning degeneration) in the skin of moribund monkey #4567 (Figure 3D, 3E). The inflammatory response was very slight or lacking in the skin lesions of moribund monkey #4567. The degenerate epithelial cells were positive for the virus antigen according to the immunohistochemistry (Figure 3F). Stratified and degenerated bronchiolar epithelial cells with very slight inflammatory infiltration were also present in the lungs of moribund monkey #4567 (Figure 3G, 3H). The pathological findings for the two monkeys (#4639 and #4650) were described in a previous study [18]. The immunohistochemical analysis showed that the degenerate epithelial cells in the bronchi were positive for the virus antigen (Figure 3J, 3K). There were no obvious changes in the alveolar area, such as inflammatory responses or detached cells (Figure 3I). However, there were large amounts of virus antigen-positive cells in the alveoli according to the immunohistochemistry (Figure 3L). Surprisingly, virus antigen-positive cells were found in all of the lung lobes in moribund monkey #4567. Formalin-fixed lung tissue was subjected to electron microscopic analysis using Epon 812-embedded ultra-thin sections to confirm viral replication in the lungs. The sequential stages of virus particle development, including immature and mature virions, were observed in the cytoplasm of alveolar macrophages (Figure 3M-O).
Figure 3.

Histopathological features of the skin and lungs of the two moribund monkeys, #4625 (A-C) and #4567 (D-O), on days 18 and 10 post-inoculation (p.i.), respectively. (A-C) Pock lesion from the skin showing necrosis with severe inflammatory infiltration in the epidermis and dermis (A). Diffuse infiltration of mononuclear cells and neutrophils (B). Virus antigen-positive cells detected in the lesion (C). (D-F) Skin: spongiform degeneration (D, asterisks) and ballooning degeneration (E, higher magnification of D). The degenerate epithelial layer was positive for virus antigen (F, sequence section of E). (G-L) Lung: poxvirus infections in the bronchi and alveoli. Stratified and degenerate bronchiolar epithelial cells (G, H). Lack of inflammatory responses in the alveolar area (I). Virus antigen-positive cells detected in the bronchi and alveoli (J-L). (A, B, D, E, G-I) Hematoxylin-eosin staining. (C, F, J-L), Immunoperoxidase test using a polyclonal antibody against Vaccinia virus. (G, J) Original magnification ×10. (A, D, G, J, bar = 200 μm), ×100. (B, C, E, F, H, I, K, L, bar = 20 μm). (M-O) Electron microscopic analysis of alveolar macrophages in the lungs of moribund monkey #4567 on day 10 p.i. Virus particles are visible in the degenerate alveolar macrophages (M). (N), bar = 2 μm. Immature (M inset, bar = 200 nm) and mature (O, bar = 200 nm) virions in the cytoplasm of alveolar cells.
The histopathological analysis detected swollen monocytes in the sinus of the lymph node in moribund monkey #4625, which were virus antigen-positive cells (Figure 4A). The liver did not exhibit any histopathological changes, although the Kupffer cells were positive for virus antigen whereas the hepatocytes were not (Figure 4B). The pock lesions in the testis comprised necrosis and granulomatous inflammatory reactions, including virus antigen-positive cells (Figure 4C). The lack of inflammatory responses in the skin and lung lesions, and the severe lymphocyte depletion in the lymphoid tissues, including the lymph nodes, spleen, thymus, and bone marrow, suggest that moribund monkey #4567 experienced severe immune suppression (Figure 4D-F). Hemorrhage and necrosis were also observed in the lymphoid tissues (Figure 4D-F). Edema and slight inflammatory infiltration with mononuclear cells were observed in the epicardium and visceral pleura membrane of moribund monkey #4567 (Figure 4G, 4H). Antigen-positive cells were detected in the lymphoid tissues and serous membrane by immunohistochemistry. Amphophilic cytoplasmic inclusion bodies were found in the swollen hepatocytes of the liver in moribund monkey #4567, which were positive for the viral antigen (Figure 4I). Intralesional bacterial colonies were detected in the lungs, bone marrow, and brain (data not shown). These results suggest that moribund monkey #4567 suffered from a systemic monkeypox infection with Gram-positive coccal sepsis.
Figure 4.

Histopathological features of the lymph node (A), liver (B), and testis (C) of moribund monkey #4625 on day 18 post-inoculation (p.i.), and those of the lymph node (D), spleen (E), bone marrow (F), epicardium (G), visceral pleura (H), and liver (I) of moribund monkey #4567 on day 10 p.i. Sinus enlargement and virus antigen-positive cells were present in the lymph node (A). Kuppfer cells were positive for virus antigen in the liver (B). Pock lesion in the testis (C). Severe inflammatory cell infiltration occurred in the surrounding focal necrosis (C, plus). Hemorrhage and necrosis were observed in the lymph node and spleen (D and E, asterisks). Lymphocytes were reduced in the follicular area (D and E). Myeloblasts and megakaryocytes were reduced and necrotic substances were observed in the bone marrow (F). Edema and slight inflammatory infiltration were observed in the epicardium (G) and visceral pleura (H). Virus antigen-positive cells in the lesion (D-H, insets). Swollen hepatocytes and amphophilic cytoplasmic inclusion bodies were detected with virus antigens in the liver (I, inset, arrows). (A–I) Hematoxylin-eosin staining. Insets, immunoperoxidase test using a polyclonal antibody against Vaccinia virus. Original magnification: ×10 (A, C, D, E; bar = 200 μm); ×100 (B, F, G, H, I, and all insets; bar = 20 μm).
Double-immunofluorescence staining was conducted to detect monkeypox virus-susceptible cells in moribund monkey #4567 (Figure 5). The cytokeratin-positive epithelial cells in the skin (Figure 5), bronchi in the lung, intestine, and liver (data not shown), but not those in the alveoli, were also positive for virus antigen (Figure 5). The Mac387-positive macrophages in the lung alveoli, lymph node, bone marrow (Figure 5), spleen, and liver (data not shown) were virus antigen-positive.
Figure 5.
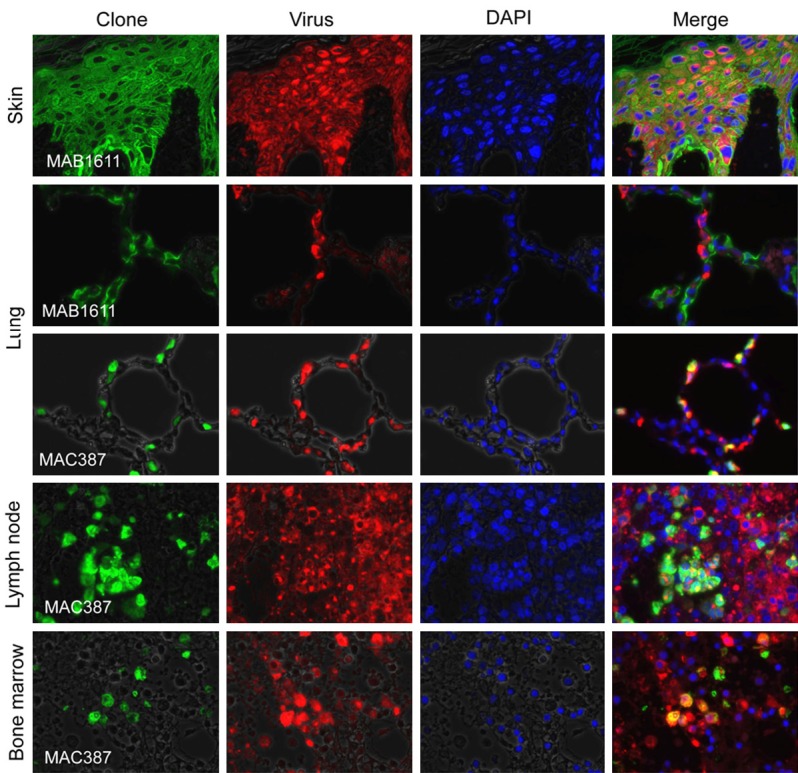
Double-immunofluorescence staining to detect monkeypox-infected cells in moribund monkey #4567 on day 10 post-inoculation. The monkeypox virus antigen (red) colocalized with MAB1611-positive epithelial cells (green) in the skin, liver, and intestine. MAC387-positive monocytes (green) and monkeypox virus antigen (red) colocalized in the same cells.
Discussion
Previous analyses of the histopathological appearance of vaccination-inoculation sites in biopsies have shown that papulovesicular dermatitis comprises vacuolated epithelium, edema of the papillae, hemorrhage, mononuclear and polymorphonuclear cells, and perivascular infiltration [10]. The histopathological features of ordinary type monkeypox virus infections comprise fibrinonecrotic inflammatory reactions with macrophage and neutrophil infiltration in the viral replication sites, including the skin, respiratory tract, gastrointestinal tract, lymphoid organs, mucosal surfaces, and genital organs [11-13,18]. In the present study, however, inflammatory infiltration was observed rarely in the skin, lung, and liver, although abundant virus antigens were detected in these sites in moribund monkey #4567. The absence of inflammatory infiltration in the skin may have caused the general lack of papulovesicular dermatitis in moribund monkey #4567 and it was also the cause of the widespread systemic viral infection. In another study, 1/15 monkeys inoculated with aerosolized Zaire strain monkeypox virus appeared to exhibit secondary bacterial septicemia, whereas the others died of bronchopneumonia [13]. The septic monkey had preexisting subacute-to-chronic portal hepatitis, bacterial septicaemia, and terminal differential interference contrast [13].
The presence of neutropenia in moribund monkey #4567 after infection might explain the development of the systemic and fulminant monkeypox infection, including sepsis. Chemotherapy-induced neutropenia occurs in some cancer patients and is caused by bacterial infections [20]. In septic shock syndrome, bacterial lipopolysaccharide induces high levels of the pro-inflammatory cytokine IL-6 and pro-inflammatory chemokine IL-8 [21,22]. Septic patients also have high levels of TNF-α, IL-1 receptor antagonists, and IL-2 receptors. Moribund monkey #4567 had high levels of IL-6 and IL-8 in its serum. Thus, the high expression levels of IL-6 and IL-8 were probably inflammatory responses to bacterial shock syndrome rather than the monkeypox infection. In addition, the increased levels of MIG/CXCL-9 and macrophage- and/or T cell-related cytokines (TNF-α, IFN-γ, and IL-13) from day 7 p.i. appeared to be related to the bacterial co-infection. Interestingly, both moribund monkeys (#4567 and #4625) had high levels of macrophage-related chemokines (MCP-1/CCL2 and MIP-1α/CCL-3) and IFN-α from days 6 to 10 p.i., which appeared to be associated with the systemic monkeypox infection. Orthopoxviruses encode multiple immunomodulatory proteins that hijack the host immune system directly [23-26]. Monkeypox virus shares 14 immunomodulatory genes with Variola virus, which affect IFNs, TNFs, interleukins, complement, and chemokines. In addition, monkeypox virus encodes two additional proteins compared with Variola virus, which target the innate immunity pathway [25].
Increased numbers of leukocytes in the blood, including lymphocytes, neutrophils, and monocytes, appear to be crucial for the immune response when surviving monkeypox virus infections. However, moribund monkey #4567 had lower numbers of neutrophils compared with the surviving monkeys. In addition, monkey #4652 exhibited a sudden increase in its neutrophil levels on day 14 p.i. and it was moribund. The hematological and histopathological analyses suggested that the moribund monkey #4567 had myelodysplasia-related neutropenia as an underlying condition. Previously, a Vaccinia virus infection mouse model demonstrated that CD11b+Ly6C+Ly6G+ cells, including neutrophils and macrophages, produce type I IFNs and large quantities of reactive oxygen species [27]. Mice with depleted Ly6G+ cells and an absence of reactive oxygen species also exhibited severe tissue damage after infection. Furthermore, a previous study showed that monocytes and granulocytes were positive for poxvirus antigen after intravenous infection with monkeypox virus in rhesus macaques, according to flow cytometry analysis [28].
In conclusion, the moribund monkey #4567 exhibited neutropenia and excessive inflammatory responses after experimental infection with the Liberia strain of monkeypox and concomitant bacterial sepsis. These reactions might have promoted the development of systemic and fulminant monkeypox. Thus, neutrophils play a key role in the resolution of systemic monkeypox virus infection. This animal model will facilitate a better understanding of the pathogenesis of fulminant orthopoxvirus infections.
Acknowledgements
We thank our colleagues, especially Ms A Harashima and Ms M Fujino. This work was supported in part by research grants from the Ministry of Health, Labor, and Welfare of Japan (H22-Shinko-Ippan-009 and H26-Shinkogyosei-Shitei-002), and from the Japanese Health Sciences Foundation (KHC1204 and KHC1216).
Disclosure of conflict of interest
None.
References
- 1.Arita I, Jezek Z, Khodakevich L, Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 2.Formenty P, Muntasir MO, Damon I, Chowdhary V, Opoka ML, Monimart C, Mutasim EM, Manuguerra JC, Davidson WB, Karem KL, Cabeza J, Wang S, Malik MR, Durand T, Khalid A, Rioton T, Kuong-Ruay A, Babiker AA, Karsani ME, Abdalla MS. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 4.Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer H, Perrichot M, Stemmler M, Emmerich P, Schmitz H, Varaine F, Shungu R, Tshioko F, Formenty P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimoin AW, Kisalu N, Kebela-Ilunga B, Mukaba T, Wright LL, Formenty P, Wolfe ND, Shongo RL, Tshioko F, Okitolonda E, Muyembe JJ, Ryder R, Meyer H. Endemic human monkeypox, Democratic Republic of Congo, 2001-2004. Emerg Infect Dis. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 8.Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, Damon IK, Reynolds MG, Kuehnert MJ. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 9.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. The clinical features of smallpox. Smallpox and Its Eradication. 1988:1–68. [Google Scholar]
- 10.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. The pathogenesis, pathology and immunology of smallpox and vaccinia. Smallpox and Its Eradication. 1988:121–168. [Google Scholar]
- 11.Nalca A, Livingston VA, Garza NL, Zumbrun EE, Frick OM, Chapman JL, Hartings JM. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS 0ne. 2010;5:e12880. doi: 10.1371/journal.pone.0012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman JL, Nichols DK, Martinez MJ, Raymond JW. Animal models of orthopoxvirus infection. Vet Pathol. 2010;47:852–870. doi: 10.1177/0300985810378649. [DOI] [PubMed] [Google Scholar]
- 13.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahon N, Wilson BJ. Pathogenesis of variola in Macaca irus monkeys. Am J Hyg. 1960;71:69–80. doi: 10.1093/oxfordjournals.aje.a120091. [DOI] [PubMed] [Google Scholar]
- 15.Hahon N, Mc GM. Air-borne infectivity of the variola-vaccinia group of poxviruses for the cynomolgus monkey, Macaca irus. J Infect Dis. 1961;109:294–298. doi: 10.1093/infdis/109.3.294. [DOI] [PubMed] [Google Scholar]
- 16.Jahrling PB, Hensley LE, Martinez MJ, Leduc JW, Rubins KH, Relman DA, Huggins JW. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc Natl Acad Sci U S A. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RF, Dyall J, Ragland DR, Huzella L, Byrum R, Jett C, St Claire M, Smith AL, Paragas J, Blaney JE, Jahrling PB. Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. J Virol. 2011;85:2112–2125. doi: 10.1128/JVI.01931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Iizuka I, Shiota T, Sakai K, Ogata M, Fukushi S, Mizutani T, Sata T, Kurata T, Kurane I, Morikawa S. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J Gen Virol. 2009;90:2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- 19.Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Ogata M, Fukushi S, Mizutani T, Sata T, Kurata T, Kurane I, Morikawa S. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80:5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29:490–494. doi: 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 21.Van Zee KJ, DeForge LE, Fischer E, Marano MA, Kenney JS, Remick DG, Lowry SF, Moldawer LL. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991;146:3478–3482. [PubMed] [Google Scholar]
- 22.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Hansen SJ, Rushton J, Dekonenko A, Chand HS, Olson GK, Hutt JA, Pickup D, Lyons CR, Lipscomb MF. Cowpox virus inhibits human dendritic cell immune function by nonlethal, nonproductive infection. Virology. 2011;412:411–425. doi: 10.1016/j.virol.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huemer HP, Lassnig C, Nowotny N, Irschick EU, Kitchen M, Pavlic M. Diazepam leads to enhanced severity of orthopoxvirus infection and immune suppression. Vaccine. 2010;28:6152–6158. doi: 10.1016/j.vaccine.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 26.Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer MA, Davies ML, Reider IE, Heipertz EL, Epler MR, Sei JJ, Ingersoll MA, Rooijen NV, Randolph GJ, Norbury CC. CD11b(+), Ly6G(+) cells produce type I interferon and exhibit tissue protective properties following peripheral virus infection. PLoS Pathog. 2011;7:e1002374. doi: 10.1371/journal.ppat.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H, Janosko K, Johnson RF, Qin J, Josleyn N, Jett C, Byrum R, St Claire M, Dyall J, Blaney JE, Jennings G, Jahrling PB. Poxvirus antigen staining of immune cells as a biomarker to predict disease outcome in monkeypox and cowpox virus infection in non-human primates. PLoS One. 2013;8:e60533. doi: 10.1371/journal.pone.0060533. [DOI] [PMC free article] [PubMed] [Google Scholar]