Abstract
Ectopic cortisol-producing adrenocortical adenomas (CPA) are extremely rare, and only four cases have previously been reported so far but the tumors were not ultrastructurally studied. Presented in this paper is the fifth case with ectopic CPA which was extensively examined to gain deeper insights in terms of the histopathological features and steroidogenic enzyme profile of the tumor. A 53-year-old woman complained of accidental discovery of left renal mass. She had a 5-year history of hypertension, weight gain, moon face, thin skin and systemic edema. These symptoms completely relieved after the tumor removal. Two years later, the above symptoms recurred, and a recurrent tumor was revealed in left renal hilum. The tumor was removed completely with relief of her symptoms of Cushing’s syndrome. Histologically and ultrastructurally, the tumor was composed of compact cells and clear cells, and the former was prominent, suggesting an active secretory function of the tumor. The adenoma tissue showed a strong immunostaining for Melan-A, 3beta-hydroxysteroid dehydrogenase (HSD3B2) and 17alpha-hydroxylase1 (CYP17A1). Expression pattern for 11beta-hydroxylase 1 (CYP11B1), 11beta-hydroxylase 2 (CYP11B2), CYP17 and HSD3B2 mRNA in ectopic CPA was similar to that in the adrenal CPA. In conclusion, in terms of histopathological characteristic and steroidogenic enzyme profile, ectopic CPA is similar to adrenal CPA, suggesting that they are of identical cell origin.
Keywords: Ectopic adrenocortical adenoma, Cushing’s syndrome, ultrastructure, steroidogenic enzyme
Introduction
Cushing’s syndrome falls into two types, ACTH dependent and independent Cushing’s syndrome. The later is often divided into four subtypes, adrenocortical adenoma, adrenocortical carcinoma, primary pigmented nodular adrenal disease (PPNAD), and adrenocorticotropic hormone independent macronodular adrenal hyperplasia (AIMAH). Adrenocortical adenoma is responsible for 10%-20% of all cases of Cushing’s syndrome.
Ectopic cortisol-producing adrenocortical adenomas (CPA) are extremely rare, and to our knowledge, only four cases have been reported till now [1-4]. Although clinical features of the patients have been well delineated, the morphological characteristic, especially ultrastructure of the tumor had not been fully described in these papers. Moreover, whether the ectopic CPA had the same steroidogenic enzyme expression as adrenal CPA was also need to be clarified.
Herein, we reported a 53-year-old woman who had recurrent classical symptom of Cushing’s syndrome with the etiology originated from recurrent ectopic adrenocortical adenoma in left renal hilum. Furthermore, we intensively examined the tumor to gain more insights in terms of the histopathological features and steroidogenic enzyme profile of the tumor.
Materials and methods
Case history
A 53-year-old Chinese woman was admitted with the complaint of accidental discovery of left renal mass. She had a 5-year history of hypertension with the highest blood pressure level of 170/110 mmHg and needed antihypertensive medication. At the same time, she had gained 15 kg companying with moon face, thin skin, easy bruising, systemic edema and generalized weakness. She denied purple striae, headaches or visual field defect. Her family history was noncontributory. A computed tomography (CT) scan (Figure 1A) revealed a nodular structure in left renal hilum and atrophic bilateral adrenals. Serum cortisol and plasma ACTH were not measured and a left renal tumor was diagnosed at that time. The tumor was removed by retroperitoneal laparoscopic operation on July 2nd, 2010. The gross pathological examination demonstrated a well-encapsulated brown solitary lesion measuring 3.5 × 3.0 cm. And histological diagnosis was adrenocortical adenoma. The patient discontinued the antihypertensive treatment postoperatively, and had normal blood pressure and a weight loss of 15 kg.
Figure 1.
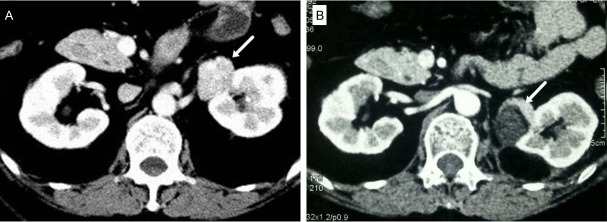
CT scan of the patient. A. A small nodular lesion in the left renal hilum (arrow) detected before the first operation; B. A recurrent nodule in the left renal hilum (arrow) detected 2 years after the first operation.
Two years later, the patient gained weight again, which was a 10 kg increase, companying with systemic edema, supraclavicular and nuchal fat pads, moon face, and weakness. She denied purple striae on the abdomen and had a mild hypertension (140/90 mmHg). Enhanced CT scan (Figure 1B) revealed a recurrent mass measuring 2.7 cm in diameter in the left renal hilum, and atrophic bilateral adrenals. The morning serum cortisol concentration was 17.6 μg/dl (normal: 4.0-22.3 μg/dl), with a plasma ACTH concentration less than 5.0 pg/ml (normal: 10.0-46.0 pg/ml). The 24-h urinary free cortisol was 158.7 μg (normal: 12.3-103.5 μg), and was 135.9 μg and 200.6 μg after a 2-day low dose (2 mg/day) and high dose (8 mg/day) dexamethasone suppression test, respectively. On the basis of the aforementioned findings, the diagnosis of recurrent ectopic CPA in the renal hilum was established. The tumor was totally resected on May 30th, 2013. Pathological findings revealed that the dissection specimen was an oval nodule measuring 3 g in weight and 2.7 × 2 cm in size. The cut surface was brittle and gray-yellow. And histological diagnosis was adrenocortical adenoma.
Plasma cortisol concentration dropped from 17.6 to 1.7 μg/dl soon after the resection. The patient was given a gradually reduced dose of hydrocortisone, which was discontinued 6 months later. At that time, the plasma cortisol concentration was 13.8 μg/dl. ACTH concentration increased to 47.4 pg/ml, and the 24-h urinary free cortisol decreased to 88.8 μg. She gave her informed consent for the use of tumor material for research purposes. The investigation was approved by the local ethical committee.
Immunohistochemistry
Immunohistochemistry was performed using EnVisionTM Detection Kit (Dako). The antibodies and dilutions were as follows: Melan-A (1:200; Dako), 3 beta-hydroxysteroid dehydrogenase (HSD3B2; 1:100; Proteintech) and 17 alpha-hydroxylase 1 (CYP17A1; 1:400; Proteintech).
Tissue homogenization
Surgically removed tumor tissue (30 mg) was homogenized by homogenizer in 2 ml of 0.9% sodium chloride. The homogenate was collected and centrifuged for measurement of cortisol. Another three CPA located in adrenal and three aldosterone-producing adenomas (APA) were conducted for comparison.
Real-time quantitative PCR
Real-time quantitative PCR was conducted to analyze the expression of the 11β-hydroxylase (CYP11B1) aldosterone synthase (CYP11B2), CYP17A1 and HSD3B2 genes in different tissues. Briefly, total RNA was extracted with Trizol reagent (Invitrogen). cDNA was synthesized from 1 mg total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). PCR amplification assays were performed with the FastStart Universal SYBR Green Master mix with Rox (Roche) on an ABI 7500 Real-Time PCR System (Applied Biosystems). Relative quantification was determined by normalizing the expression for each gene toβ-actin gene. All real-time PCR were performed in triplicate. The primers used were as follows: CYP11B1, 5’-AATGCGGAACTGTCGCC-AGATG-3’ (forward) and 5’-TCAGCAAGGGAAACACCGTC 3’ (reverse); CYP11B2, 5’-ACTCGCTGGGTCGCAATG-3’ (forward) and 5’-AGTGTCTCCACCAGGAAGTGC-3’ (reverse); CYP17A1, 5’-CCACCTTTGCCCTGTTCAAG-3’ (forward) and 5’-GCCAGCATATCACACAATGTACTG-3’ (reverse); HSD3B2, 5’-AGGACCAAGCTGACTGTACTT-3’ (forward) and 5’-TAGATGA-AGACTGGCACACTGG-3’ (reverse); and β-actin, 5’-TCCCTGGAGAAGAGCTACG-3’ (forward) and 5’-GTAGTTTCGTGGATGCCACA-3’ (reverse).
Results
Histologically, the tumor was composed of compact cells and clear cells. The compact cells were prominent, which had abundant eosinophilic cytoplasm and round or oval nuclei. There was minute quality of clear cells scattered in the tumor (Figure 2A). Immunohistochemical analysis of the adenoma tissue revealed a strong immunostaining for Melan-A, HSD3B2 and CYP17A1 (Figure 2B-D).
Figure 2.
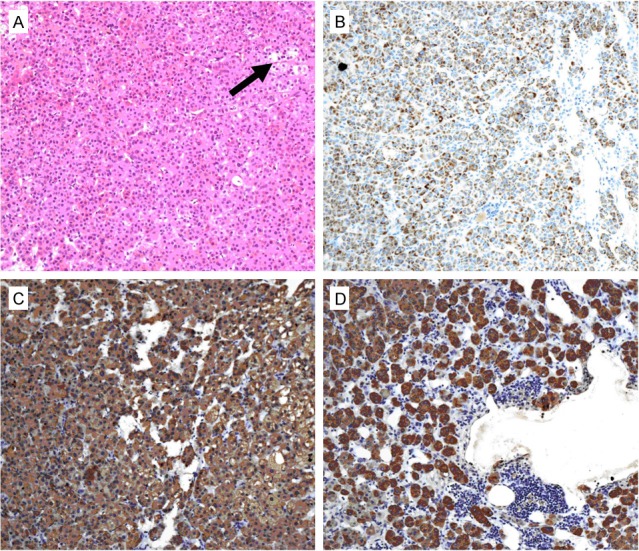
Microscopic feature of the ectopic adrenocortical adenoma. (A) The tumor was composed of compact cells and clear cells (arrow) (× 100); (B-D) Positive immunostaining for Melan-A (B), CYP17A1 (C) and HSD3B2 (D) (× 100).
Two types of cells were also detected in the tumor tissue by electron microscopy. The clear cells, which only accounted for a small proportion of total cells, were surrounded by compact cells. Compact cells were apparently devoid of lipid droplets and had numerous organelles such as mitochondria, smooth endoplasmic reticulum (SER), rough endoplasmic reticulum (RER) and lysosomes (Figure 3A). At high magnification, compact cells possessed parallel disposed RER and exceedingly well developed SER. A conspicuous Golgi apparatus and numerous relatively well developed lysosomes were also observed. Mitochondria were polymorphic and filled with numerous cristae with a tubulovesicular shape (Figure 3B). As for clear cells, the cytoplasm contained numerous lipid droplets of various sizes, which were oval or round in shape, as well as less lysosomes and mitochondria with the characteristics similar to those observed in the compact cells. Abundant polysomes were observed in both clear cells and compact cells (Figure 3C).
Figure 3.
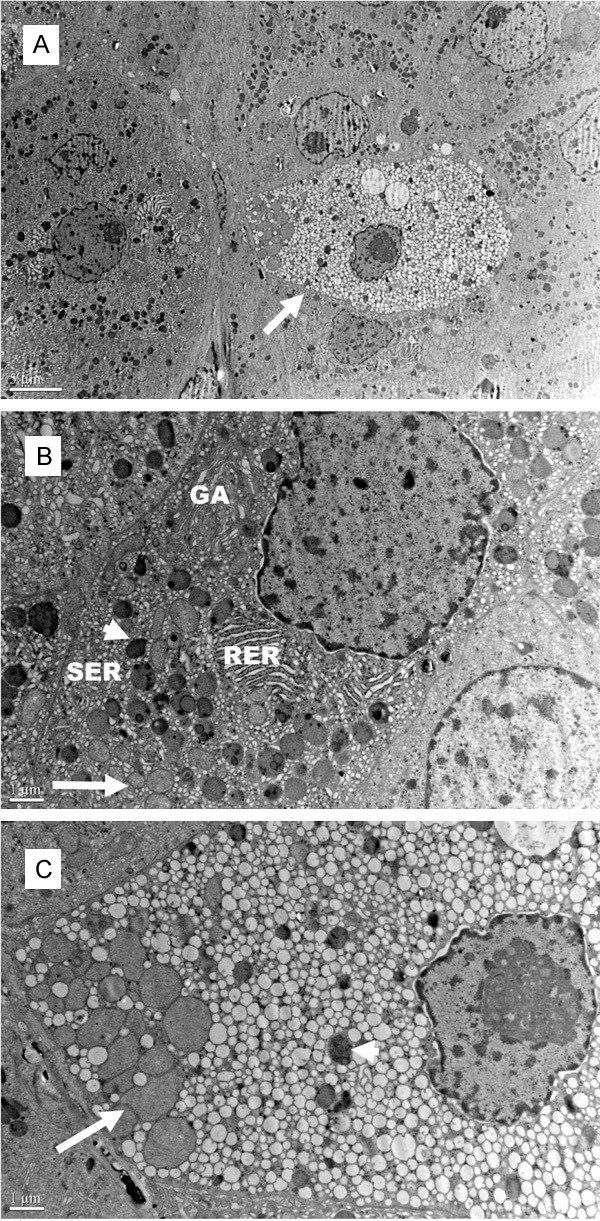
Electron micrograph of the ectopic adrenocortical adenoma. A. The clear cells (arrow), which only accounted for a small proportion of total cells, were surrounded by compact cells; B. Parallel disposed RER, exceedingly well developed SER, numerous lysosomes (arrowhead), abundant mitochondria with replete tubulovesicular cristae (arrow), Golgi apparatus and polysomes in the cytoplasm of compact cells; C. Numerous lipid droplets, mitochondria (arrow), lysosomes (arrowhead) and polysomes in the cytoplasm of clear cells. RER: Rough endoplasmic reticulum; SER: Smooth endoplasmic reticulum; GA: Golgi apparatus.
Cortisol level in tissue homogenate was much higher in the three adrenal CPA and the ectopic CPA tissues than in the APA tissues (Figure 4A). Expression pattern for CYP11B1, CYP11B2, CYP17A1 and HSD3B2 mRNA in the ectopic CPA was similar to that in the adrenal CPA (Figure 4B-E). Compared with the mRNA expression in APA, both CYP11B1 and CYP17A1 mRNA were detected significantly higher, and CYP11B2 mRNA was markedly lower in the adrenal CPA and the ectopic CPA. As for HSD3B2 expression, it was detected in all adenomas, and distinctly high in one APA.
Figure 4.
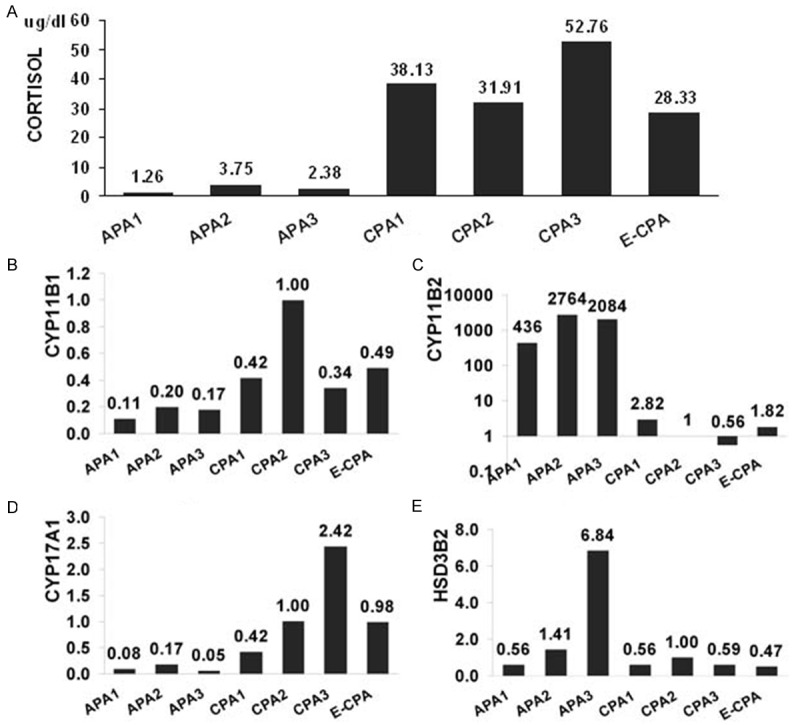
Analysis of cortisol secretion and steroidogenic enzyme expression in adrenocortical tumors. (A) Cortisol level in tumor homogenate; (B-D) Real-time polymerase chain reaction quantification of CYP11B1 (B), CYP11B2 (C), CYP17 (D) and HSD3B2 (E) transcript levels in adrenocortical tumors. Expression of the CYP11B1, CYP11B2, CYP17 and HSD3B2 mRNA is normalized against that of β-actin, used as an internal control. The mRNA expression in CPA2 was set as 1. APA, aldosterone-producing adrenocortical adenoma; CPA, cortisol-producing adrenocortical adenoma; E-CPA, ectopic cortisol-producing adrenocortical adenoma.
Discussion
On the origin of embryo, adrenal cortex originates from the intermediate mesoderm. On 5th week of gestation, mesoblastema begins to proliferate and differentiate, and eventually forms adrenocortical cells and sex gland cells. And then these two kinds of cells gradually separate and migrate. Fragments of adrenocortical tissue are scattered along the migration path, forming ectopic adrenal glands [5]. Most of them remain nearby the adrenal gland, but they are also found in the celiac lymph nodes, ovary, testis, kidney, even abdominal viscera and nerve system [6].
Ectopic adrenocortical neoplasmas are very rare. Some can lead to Cushing’s syndrome, hyperaldosteronism, as well as virilization, while others are nonfunctioning. Most of them have been described in the vicinity of the adrenal gland or in the kidney, and some appeared in spinal canal, liver, gastric wall and placenta [7-9]. Taking 4 cases described before and this one, there are only 5 ectopic CPA reported till now (Table 1) [1-4]. All the ectopic tumors located near the adrenal gland or in the renal hilum. Although these ectopic CPA shared some similar profiles, such as typical characteristics of Cushing’s syndrome, an increased serum cortisol level with a suppressed ACTH, medium-size adenomas measuring 3-5 cm in diameter, they were different in some aspects. Leibowitz J et al. Described a patient who developed ectopic CPA 4 years after a complete removal of an adrenal CPA together with the entire surrounding adrenal gland [1]. Ayala et al. reported a patient who had an ectopic adenoma with abundant lipofuscin deposited in the cytoplasm [2]. Louiset et al. described a patient who bore both PPNAD and ectopic CPA [3]. Although clinical features of the patients had been fully delineated, whether ectopic CPA had the same steroidogenic enzyme expression and morphological characteristic, especially ultrastructure, as those in adrenal CPA has not been well clarified.
Table 1.
Case reports of ectopic cortisol-producing adrenocortical adenoma
| Case | Publishing time | Sex/Age (y/o) | Largest diameter of the tumor (cm) | Tumor location | Cortisol level in tissue homogenate | Enzyme profile | Ultrastructure | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Immunostaining | mRNA expression | |||||||
| 1 | 1998 [1] | F/33 | 3.0 | Next to left adrenal | ND | ND | ND | ND |
| 2 | 2000 [2] | F/63 | 3.5 | Left renal hilum | ND | ND | ND | ND |
| 3 | 2010 [3] | F/35 | 3.8 | Left pararenal | ND | CYP17A1 (+) CYP21 (+) | CYP11B1 mRNA is higher in ectopic CPA than that in normal adrenal | ND |
| 4 | 2012 [4] | M/38 | 5.3 | Anterior of left renal hilum | ND | ND | ND | ND |
| ours | 2014 | F/53 | 3.5 | Left renal hilum | Similar to adrenal CPA | CYP17A1 (+) HSD3B2 (+) | Expression pattern for CYP11B1, CYP11B2, CYP17A1 and HSD3B2 mRNA in the ectopic CPA was similar to that in adrenal CPA | The tumor was composed of compact cells which had numerous organelles, as well as clear cells which contained large clumps of lipid droplets and less organelles |
ND: Not described.
By virtue of the characteristic enzymes present in each zone, the zones in adrenal cortex produce and secrete distinct hormones. CYP11B2, the key enzyme for aldosterone synthesis, is only expressed in zona glomerulosa, whereas CYP17A1 and CYP11B1, which are important for cortisol production, mainly exist in zona fasciculata. HSD3B2, involving in early stages of both aldosterone and cortisol synthesis, is expressed in both zona glomerulosa and fasciculata cells [10-13]. The present ectopic CPA shared the same expression pattern of the steroidogenic enzymes with adrenal CPA, such as expressing CYP11B1, CYP17A1 and HSD3B2, the three characteristic enzymes in zona fasciculate and adrenal CPA, thus suggesting all these tumors had identical cell derivation, and also supporting the assumption that ectopic tumors arise along the migration path of adrenal cortex.
Histologically, the ectopic adenoma was composed of clear cells and compact cell, which was consistent with the characteristic of adrenal CPA [14,15]. It is generally considered that clear cells mainly function in storage of precursors of steroid hormones, while compact cells actually synthesize these [14,15]. In this ectopic adenoma, the proportion of compact cell was prominent, which suggested an active secretory function of the tumor.
Ultrastructural finding also revealed two kinds of cells in this ectopic adenoma, compact cells which were apparently devoid of lipid droplets but had high density organelles, and clear cells which contained large clumps of lipid droplets and less organelles. Abundant SER, RER and mitochondria with predominantly tubulovesicular cristae, which are the electron microscopical characteristics of fasciculata cells and adrenal CPA, was also detected in this ectopic adenoma [14].
In summary, we described a patient who had typical characteristics of hypercortisolism, which relieved completely after the tumor resection. And the biological and radiological data were highly suggestive of Cushing’s syndrome due to ectopic adrenocortical adenoma. Its histology, ultrastructure and steroidogenic enzyme profile were similar to those of adrenal CPA, suggesting all these tumors had identical cell derivation.
Disclosure of conflict of interest
None.
References
- 1.Leibowitz J, Pertsemlidis D, Gabrilove JL. Recurrent Cushing’s syndrome due to recurrent adrenocortical tumor—fragmentation or tumor in ectopic adrenal tissue? J Clin Endocrinol Metab. 1998;83:3786–9. doi: 10.1210/jcem.83.11.5260. [DOI] [PubMed] [Google Scholar]
- 2.Ayala AR, Basaria S, Udelsman R, Westra WH, Wand GS. Corticotropin-independent Cushing’s syndrome caused by an ectopic adrenal adenoma. J Clin Endocrinol Metab. 2000;85:2903–6. doi: 10.1210/jcem.85.8.6749. [DOI] [PubMed] [Google Scholar]
- 3.Louiset E, Gobet F, Libé R, Horvath A, Renouf S, Cariou J, Rothenbuhler A, Bertherat J, Clauser E, Grise P, Stratakis CA, Kuhn JM, Lefebvre H. ACTH-independent Cushing’s syndrome with bilateral micronodular adrenal hyperplasia and ectopic adrenocortical adenoma. J Clin Endocrinol Metab. 2010;95:18–24. doi: 10.1210/jc.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XL, Dou JT, Gao JP, Zhong WW, Jin D, Hui L, Lu JM, Mu YM. Laparoscope resection of ectopic corticosteroid-secreting adrenal adenoma. Neuro Endocrinol Lett. 2012;33:265–7. [PubMed] [Google Scholar]
- 5.Okur H, Küçükaydin M, Kazez A, Kontas O. Ectopic adrenal tissue in the inguinal region in children. Pediatr Pathol Lab Med. 1995;15:763–7. doi: 10.3109/15513819509027011. [DOI] [PubMed] [Google Scholar]
- 6.Ventura L, Leocata P, Hind A, Greco I, Ventura T. Ectopic adrenal tissue in the spermatic cord: Case report and review of the literature. Arch Ital Urol Androl. 1998;70:15–8. [PubMed] [Google Scholar]
- 7.Kepes J, O’Boynick P, Jones S, Baum D, McMillan J, Adams ME. Adrenal cortical adenoma in the spinal canal of an 8-year-old girl. Am J Surg Pathol. 1990;14:481–4. doi: 10.1097/00000478-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ren PT, Fu H, He XW. Ectopic adrenal cortical adenoma in the gastric wall: Case report. World J Gastroenterol. 2013;19:778–80. doi: 10.3748/wjg.v19.i5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon JH, Kim SH, Kim MA, Han JK, Choi BI. MDCT and Gd-EOB-DTPA Enhanced MRI Findings of Adrenal Adenoma Arising from an Ectopic Adrenal Gland within the Liver: Radiologic-Pathologic Correlation. Korean J Radiol. 2010;11:126–30. doi: 10.3348/kjr.2010.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–55. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- 11.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 12.Cao C, Yang X, Li L, Sun R, Xian Y, Lv W, Wang J, Xu Y, Gao Y. Increased expression of CYP17 and CYP11B1 in subclinical Cushing’s syndrome due to adrenal adenomas. Int J Urol. 2011;18:691–6. doi: 10.1111/j.1442-2042.2011.02836.x. [DOI] [PubMed] [Google Scholar]
- 13.Enberg U, Hennings J, Volpe C, Hellman P, Höög A, Hamberger B, Thorén M. Increased ratio of mRNA expression of the genes CYP17 and CYP11B1 indicates autonomous cortisol production in adrenocortical tumors. J Endocrinol Invest. 2009;32:810–5. doi: 10.1007/BF03345750. [DOI] [PubMed] [Google Scholar]
- 14.Hirano D, Okada Y, Ishida H, Okada K. Electron microscopic study to compare preclinical Cushing’s syndrome with overt Cushing’s syndrome. Int J Urol. 2002;9:193–9. doi: 10.1046/j.1442-2042.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- 15.Robba C, Bonanni G, Meneghelli V, Ziliotto D, Mazzocchi G, Nussdorfer GG. Ultrastructure of cortisol-secreting adrenal adenomata. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33:245–55. doi: 10.1007/BF02899185. [DOI] [PubMed] [Google Scholar]


